Abstract
Introduction
BCG vaccination is currently recommended for all newborns in Ireland except where specifically contraindicated. This paper describes a marked increase in the number of referrals of patients with localised complications after vaccination to two Dublin paediatric hospitals. This increase coincided with the introduction of a new strain of BCG vaccine
Methods
A population surveillance study was undertaken to determine the frequency and spectrum of complications associated with the new strain of BCG vaccine introduced in Ireland. Patients were identified though review of the infectious disease service case records and microbiology laboratory culture reports for the two year period from August 2002 to July 2004. Prospectively gathered data were supplemented by retrospective chart review. All infants who had inoculation site abscesses, suppurative adenopathy, or non‐suppurative adenopathy with nodes ⩾2 cm were included.
Results
Fifty eight patients presented a median of 13 weeks post‐inoculation: 32 with suppurative adenitis, 17 with inoculation site abscess, three with both inoculation site abscess and suppurative adenitis, and six with non‐suppurative adenopathy. The overall complication rate was estimated at 1/931 vaccinees with 1/1543 developing suppurative adenitis. Twenty six infants required surgery.
Discussion
This series illustrates the role of hospitals in sentinel surveillance and highlights the importance of having a well functioning and responsive system of adverse event reporting. These events raise a serious question as to the suitability of this vaccine strain for use in a national immunisation programme in a country where the prevalence of tuberculous disease is 10.4/100 000.
Keywords: BCG, abscess, adenitis, vaccination
Bacille Calmette‐Guérin (BCG), a live attenuated Mycobacterium bovis vaccination, has been provided in Ireland since 1937.1 It is currently recommended for all newborns except where specifically contraindicated.2 It is given to infants in maternity hospitals and local health centres by trained public health doctors and nurses. Studies have supported its effectiveness in the Irish context.3,4 Worldwide, the World Health Organisation reports that as many as 100 million children are given the vaccination each year. Normal inoculation site reactions include up to 5 mm of erythematous induration, progressing to a bluish‐red pustule 2–3 weeks post‐vaccination, subsequent ulceration, drainage, exudative crust formation after 4–6 weeks, and full healing 10–12 weeks post‐vaccination, leaving a small residual scar. Non‐suppurative involvement of regional or local lymph nodes is also part of the normal process. In Lotte and colleagues' review of approximately ten thousand such cases, suppurative adenitis, keloid formation, and abscesses comprised the vast majority (84%) of complications reported, with the rate of BCG suppurative adenitis being approximately one per 2800 vaccinations.5 There is no consensus regarding the management of BCG suppurative complications.
We report a case of severe protracted complications in an otherwise healthy infant. This case heralded a marked increase in the number of referrals of patients with localised complications to two Dublin paediatric hospitals: Our Lady's Hospital for Sick Children and the Children's University Hospital. The timing of this increase coincided with the withdrawal from use of the Evans BCG vaccine (M bovis Copenhagen substrain 1077) on 3 July 2002 and the introduction of the SSI vaccine (M bovis, Danish strain 1331) which first became available in August 2002 and has been in continuing use since. This report details the result of a population surveillance study describing complications incurred by children inoculated in the two years following the introduction of the SSI vaccine—that is, August 2002 to July 2004. This was initiated prompted by the index case (described below) and the hospitalisation in quick succession of two additional cases of suppurative adenitis. This unusual occurrence led to immediate notification to the Department of Health and the Irish Medicines Board by the Infectious Diseases Service of the potential problem and prospective surveillance of all cases of BCG related complication referred to the Infectious Diseases service. Retrospective cases were identified though review of the infectious disease service case records, day surgery admission lists, “keyword” searches on the correspondence files of the surgical and infectious disease departments' computers, and microbiology laboratory culture reports. All cases in the series, therefore, were either seen in the outpatients department or were admitted for surgical treatment of the complication. The referral source for those who attended the outpatients department was either the patient's general practitioner or the public health service. Some of the referrals for surgery came from paediatric consultants, in addition to the sources listed above.
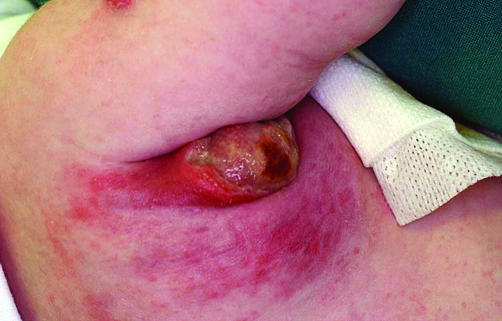
For the purpose of this review, children with inoculation site abscess of greater than 1.0 cm, regional lymphadenopathy with nodes greater than 2.0 cm, or suppurative adenitis were included (figs 1 and 2).
Figure 1 Severe suppurative adenopathy post‐BCG vaccination. Consent was obtained for publication of this figure.
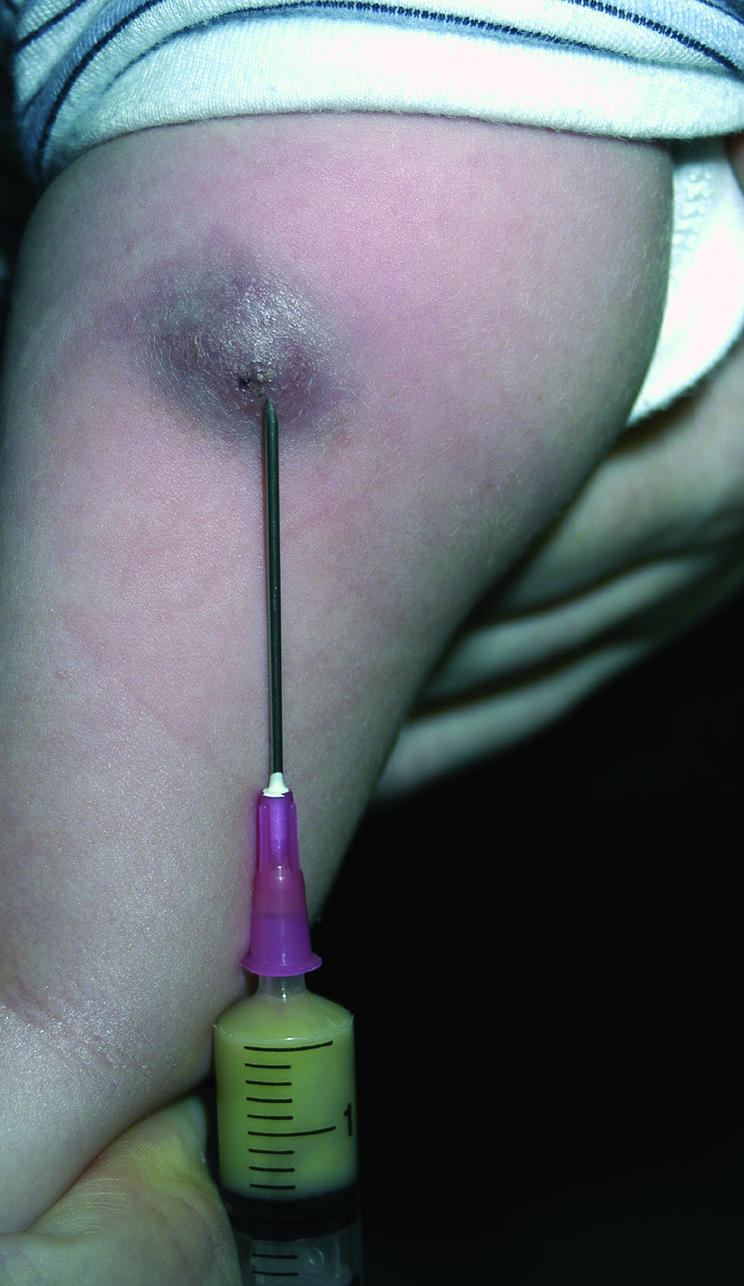
Figure 2 Inoculation site abscess post‐BCG vaccination. Consent was obtained for publication of this figure.
Case report
A 7 week old female infant was admitted to hospital with a three day history of irritability and poor feeding. Her parents had noted a lump in her left axilla during the previous week. She had received BCG in the maternity hospital on day 2 of life. The vaccination was given by an experienced nurse whose sole responsibility in the hospital was to administer the vaccinations. On examination, she had a tender, erythematous, 2×3 cm fluctuant swelling in her axilla on the left lateral chest wall. Her BCG inoculation site was normal. Ultrasound examination of the axilla revealed a multiloculated swelling with multiple central areas of low echogenicity, consistent with abscess formation. Needle aspiration yielded 3–4 ml of pus. Treatment with intravenous flucloxacillin and gentamicin was initiated. Polymorphonuclear leucocytes and acid fast bacilli were seen on microscopy of the aspirate and Mycobacterium TBv bovis was isolated after three weeks' culture. All immunological investigations on both the mother and child were normal.
Three weeks later, ultrasound examination showed re‐accumulation of the abscess and deeper lymphadenitis. Surgical debridement was performed and mTB complex was again isolated. Despite additional antibiotic treatment at this time, the infant developed cellulitis and a sinus tract three weeks later which required further surgical treatment. This case is an extreme example of the complicated courses encountered.
In the two year period, 58 patients with regional BCG complications were identified: 32 with suppurative adenitis, 17 with inoculation site abscess, an additional three patients with both inoculation site abscess and suppurative adenitis, and six with non‐suppurative adenopathy.
Thirty infants were male and all but one had received BCG vaccination for the first time (one infant received a repeat inoculation due to concerns about reduced potency of the preceding batch of BCG vaccination). Of 49 infants with exact inoculation dates known, the median age at inoculation was 8 days (range 1 day to 114 weeks). Twenty four of 49 infants were inoculated in the first week of life, 11 at 1–6 weeks, three at 7–12 weeks, and 11 at >12 weeks of age. The infants presented with complications 1–42 weeks post‐inoculation (median 13 weeks). There was no difference in time to presentation with site abscess (median 14.0 weeks, range 3.3–33.6) or suppurative adenitis (median 13.6 weeks, range 1.3–41.7)
Twelve infants received antibiotic treatment at some stage during their management. One infant received anti‐TB therapy (rifampicin and isoniazid) following development of supraclavicular caseous lymphadenitis some four months after excision of affected axillary nodes and eight months post‐vaccination. Significantly, 26 infants required surgery, three of whom required more than one procedure. The remaining infants were managed conservatively and are well. Two children have residual adenopathy with calcification.
Mycobacterium tuberculosis complex was isolated from 12 of 26 patients sampled; in five of whom, AFB were seen on microscopy. In one additional patient, AFB were seen but the culture was negative. All isolates were referred for confirmatory testing to national reference laboratories in the UK and Ireland (PHLS Mycobacterial Reference Laboratory at Dulwich, UK, and National TB Reference Laboratory, St James Hospital, Dublin). All isolates were tested for sensitivity to rifampicin (RIF) isoniazid (INH), pyrazinamide (PZA), and ethambutol (ETH). One isolate (inoculation site) was reported sensitive to all agents. Of the remaining 11 isolates, all were resistant to PZA as is typical for M bovis BCG, and sensitive to rifampicin. Four were resistant to INH and one was resistant to ethambutol. No isolate showed resistance to more than two agents.
Standard microbiological cultures were performed on 25 patients. Staphylococcus aureus, isolated from five, was the predominant organism found. Fourteen infants had immunologic evaluations including FBC, lymphocyte subset analysis, serum immunoglobulin levels, and an oxidative burst test. No significant immunological disorder was detected.
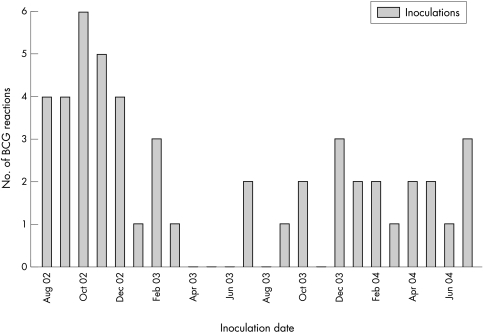
Though it is not possible to calculate the precise rate of these complications, it is possible to estimate their incidence. The cases reported here have all been referred from two Irish health board areas: the Eastern Regional Health Authority which had a birth cohort of approximately 23 000 per year,6 and the South Eastern Health Board with approximately 7000 births per year6 during that time period. As the BCG uptake rate is approximately 90%,6 up to 54 000 infants might have received BCG vaccine in these areas in the two year period. This report includes 35 cases of suppurative lymphadenitis, giving an estimated rate of 1 case per 1543 vaccinees and an overall complication rate of 1/931. The first inoculations that resulted in complications described in this report were administered in August 2002, coincident with the introduction of the SSI vaccine strain. Figure 3 illustrates the numbers of cases per month by inoculation date over the two year period. In the preceding eight years, only one BCG related complication (inoculation site abscess) was seen in the infectious disease clinic. Further supporting evidence that the observed increase in the infectious disease clinic was part of a real phenomenon was the increase in BCG related adverse reaction (ADR) reports to the regulatory authority responsible for drug safety in Ireland, the Irish Medicines Board (IMB): 21 reports from July 2000 to June 2002 compared with 152 reports received between July 2002 and January 2005.7 These ADR figures relate to the entire country of Ireland rather than the regional analysis we have been able to undertake.
Figure 3 Trend in BCG complications in two year period following introduction of SSI vaccine (n = 49 patients with date of inoculation available).
Discussion
BCG complications are well recognised in all regions that routinely administer the vaccination, such that a certain level of mild adverse reactions is considered part of the normal process. The reported incidence of these events is 0.1–17%.8,9,10,11,12 In this paper, the incidence of adverse events is consistent with other reports in the literature. However, the severity of the reactions with 26/58 (45%) requiring surgical intervention is of concern. The incidence of side effects can be affected by a number of factors, including dosage,13,14 age of recipient, and the strain of BCG in use. An increase in the rates of complication after introduction of a new vaccine strain has often been noted, and at times attributed to the use of more reactogenic vaccine strains (for example, Japan,15 Copenhagen,16 and Moscow13 strains), especially if poor inoculation techniques are used.17
In this series, all events were reported to the IMB by the infectious disease team. Some of these cases would have also been previously reported by the physician referring the patient for assessment. This information resulted in an advisory notice being issued in February 2003 which reinforced both the correct dosage and route of administration of the vaccine. In addition, a national meeting of public health staff was urgently convened to further demonstrate the correct procedure in the vaccination of newborn infants. Also, an enhanced nationwide programme of surveillance for adverse events has been initiated and is ongoing. An apparent reduction in BCG complications was subsequently observed but partially coincided with a three month period when no BCG vaccines were administered nationally for other reasons.
Despite the initial downward trend in the numbers seen, as of time of writing, children continue to present with significant complications. An intense education programme is being implemented to ensure correct vaccine administration.
The management of regional BCG complications is controversial. There is no convincing evidence that medical interventions, including use of anti‐tuberculous agents, hasten recovery.18,19 Inherent resistance to PZA, coupled with the recent reports of INH resistance in BCG strains,20,21 as noted for 36% of cases in this series, further complicates the issue. Also worrying has been the reported death of one HIV infected child with disseminated disease where treatment was compromised, not only by the presence of both PZA and INH resistance, but also by the emergence of resistance to RIF during therapy.21
Similarly, optimum surgical management of suppurative adenitis has not been defined. Repeated needle aspiration has been advocated by some as a way of avoiding suppuration and prolonged drainage,22,23 whereas others recommend complete surgical excision once nodes are tense and fluctuant.24
The cause of this reported outbreak of BCG related complications is unclear. It is likely that introduction of a more reactogenic strain coupled with the difficulties of intradermal vaccine administration to newborns may have contributed to this increase. With the change from the Evans vaccine to the SSI vaccine, there was the potential for dosage errors. The dose of the Evans vaccine for infants older than 3 months was 0.1 ml, while the new SSI vaccine dose is only 0.05 ml up to 1 year of age. In these situations, the increase in reactions should be temporary, and return to baseline rapidly as familiarity with the new vaccine increases. As cases continue to present, it is our hypothesis that the inherent potency of the SSI BCG vaccine strain may be the critical factor. This is supported by similar reports from South Africa and the United Kingdom.25,26 In addition to reports from these institutions, there are also reports from regulatory bodies. The Joint Committee on Vaccination and Immunisation of the Department of Health in the UK has reported a marked increase in reactions following administration of the SSI vaccine. The significance of this increase is diluted by the uncertain case definition of “vaccine reaction” and a lack of information regarding the age profile of the patients.27 A subcommittee of this group has determined that there is no immediate concern with this new strain of vaccine.
What is already known on this topic
BCG vaccination is part of the recommended primary immunisation schedule in Ireland
Mild BCG reactions are commonly seen among vaccinees
Unfortunately, it is almost impossible to determine with absolute certainty the exact cause of the outbreak. The number of public health centre staff administering the vaccine in this region is greater than one hundred. Therefore, assessment of the injection technique of the staff would have been extremely difficult to perform and analyse. In any case, there was no predominance of patients who had been vaccinated at one health centre compared with other centres, indicating that it is unlikely that poor vaccination techniques alone could account for this outbreak.
This series illustrates the role that hospitals and referral centres must play in sentinel surveillance, and highlights the importance of having a well functioning and responsive system of adverse event reporting. This is particularly important in relation to nationally recommended vaccine use, given the existing parental concerns around vaccine use. Any loss in confidence in a vaccine could potentially damage the remainder of the primary immunisation schedule. These events also raise a serious question as to the suitability of this vaccine strain for use in a national immunisation programme in a country where the prevalence of tuberculous disease is 10.4/100 000.28
What this study adds
Severe BCG reactions have been associated with the introduction of more reactogenic strain in the Irish population
Hospitals and tertiary centres play an important role in the identification and management of an outbreak of complications
Acknowledgements
We would like to acknowledge the assistance of the following in the collection of the clinical data: Prof. M Cafferkey, Dr Dubhfeasa Slattery, Dr W Ferguson (all from TCUH), Dr Niamh O Sullivan (OLHSC), the Departments of Emergency Medicine, Surgery, and Microbiology in both Our Lady's Hospital for Sick Children, Crumlin and the Children's University Hospital, Temple Street). In addition, we are grateful to the National Reference Laboratories in the UK and Ireland for the sensitivity testing of all isolates (PHLS Mycobacterial Reference Laboratory at Dulwich, UK, and National TB Reference Laboratory, St James Hospital, Dublin). We also thank Dr Joan Gilvarry of the Irish Medicines Board for her advice and assistance.
Footnotes
Competing interests: none declared
Consent was obtained for publication of figs 1 and 2
References
- 1.Dunleavy M. The prevention of tuberculosis. Ir J Med Sci 19521–10. [DOI] [PubMed]
- 2.Royal College of Physicians in Ireland National Immunisation Advisory Committee Guidelines. July 2002
- 3.Shannon A, Kelly P. Isoniazid resistant tuberculosis in a school outbreak: the protective effect of BCG. Eur Respir J 19914778–792. [PubMed] [Google Scholar]
- 4.Stinson J, Kelly P. National Tuberculosis Survey (1986). Ir Med J 1988817–10. [PubMed] [Google Scholar]
- 5.Lotte A, Wasz‐Hockert O, Poisson N.et al BCG complications. Estimates of risks among vaccinated subjects and statistical analysis of their main characteristics. Adv Tuberc Res 198421107–193. [PubMed] [Google Scholar]
- 6.National Diseases Surveillance Centre, Ireland Immunisation uptake quarterly reports. www.ndsc.ie
- 7.MIMS Ireland IMB page. February 2005
- 8.Turnbull F M, McIntyre P B, Achat H M.et al National study after vaccination with Bacille Calmette‐Guerin. Clin Infect Dis 200234447–453. [DOI] [PubMed] [Google Scholar]
- 9.Stuckey J.Prevalence of BCG related adenitis in healthy children, Maputo City, Mozambique. UNICEF 1988
- 10.Bannon M J. BCG and tuberculosis. Arch Dis Child 19998080–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grange J M. Complications of Bacille Calmette Guerin (BCG) vaccination and immunotherapy and their management. Commun Dis Public Health 1988(2)84–88. [PubMed]
- 12.Praveen K M, Smikle M F, Prabbakar P.et al Outbreak of Bacille Calmette Guerin associated lymphadenitis and abscess in Jamaican children. Pediatr Infect Dis J 19909890–893. [DOI] [PubMed] [Google Scholar]
- 13.Allerberger F. An outbreak of suppurative lymphadenitis connected with BCG vaccination in Austria 1990/1. Am Rev Respir Dis 1991144469. [DOI] [PubMed] [Google Scholar]
- 14.Vitkova E, Galliova J, Krepela K.et al Adverse reactions to BCG. Cent Eur J Public Health 19953138–141. [PubMed] [Google Scholar]
- 15.Karra S, Jain Y, Seth M. BCG associated adenitis. Lancet 1993341970. [DOI] [PubMed] [Google Scholar]
- 16.Millstein J B, Gibson J J. Quality control of BCG vaccine by WHO. Bull WHO 19906893–108. [PMC free article] [PubMed] [Google Scholar]
- 17.Roekel K V, Stapledon R, Gold M. Review of BCG adverse reactions in South Australia [abstract]. 2nd National TB Conference. Sydney, Australia: Public Health Association of Australia, 1997
- 18.Noah P, Pande D, Johnson B.et al Evaluation of oral erythromycin and local isoniazid instillation therapy in infants with Bacille Calmette Guerin lymphadenitis and abscesses. Pediatr Infect Dis J 199312136–139. [DOI] [PubMed] [Google Scholar]
- 19.Goraya J, Virdi V. Treatment of Calmette‐Guerin bacillus adenitis: a meta‐analysis. Pediatr Infect Dis J 200120632–634. [DOI] [PubMed] [Google Scholar]
- 20.Ali S, Almoudaris M. BCG lymphadenitis. Arch Dis Child 200489812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hesseling A C, Schaaf H S, Victor T.et al Resistant Mycobacterium bovis bacillus Calmette‐Guerin disease: implications for management of bacillus Calmette‐Guerin disease in human immunodeficiency virus‐infected children. Pediatr Infect Dis J 200423476–479. [DOI] [PubMed] [Google Scholar]
- 22.Caglayan S, Arikan A, Yaprak I.et al Management of suppuration in regional lymph nodes secondary to BCG vaccination. Acta Paediatr Jpn 199133699–702. [DOI] [PubMed] [Google Scholar]
- 23.Sataynarayana S, Mathur A D, Verma Y.et al Needle aspiration as a diagnostic tool and therapeutic modality in suppurative lymphadentitis following BCG vaccination. J Assoc Physicians India 200250788–791. [PubMed] [Google Scholar]
- 24.Hengster P, Solder B, Fille M.et al Surgical treatment of bacille Calmette Guerin lymphadenitis. World J Surg 199721520–523. [DOI] [PubMed] [Google Scholar]
- 25.Nuttall J, Eley B, Nicol M.et al Adverse events after neonatal BCG immunisation in South African children [abstract 274]. 21st Annual Meeting of the European Society for Paediatric Infectious Diseases, Taromina, Sicily 2003
- 26.Teo S S S, Shingadia S. Cases of BCG‐associated suppurative lymphadenitis [abstract 311]. Annual Meeting of the European Society for Paediatric Infectious Diseases, Tampere, Finland 2004
- 27.Joint Committee on Vaccination and Immunisation Department of Health. Vaccine associated suspected adverse reactions. http://www.dh.gov.uk/assetRoot/04/12/01/96/04120196.pdf
- 28.NDSC Tuberculosis in Ireland, 2002. The Annual Report of the National Diseases Surveillance Centre 2003