Abstract
Aim
To identify the clinical and biochemical risk factors associated with outcome of paracetamol induced significant hepatotoxicity in children.
Methods
Retrospective case notes review of those with paracetamol overdose admitted from 1992 to 2002. Patients were analysed in two groups: group I recovered after conservative treatment and group II developed progressive liver dysfunction and were listed for liver transplantation.
Results
Of 51 patients (6 males, 45 females, aged 0.8–16.1 years), 6 (aged <7 years) received cumulative multiple doses, and 45 a single large overdose (median 345 mg/kg, range 91–645). The median (range) interval to hospital at presentation post‐ingestion was 24 hours (4–65) and 44 hours (24–96) respectively in groups I and II. Patients received standard supportive treatment including N‐acetylcysteine. All children in group I survived. In group II, 6/11 underwent orthotopic liver transplantation (OLT) and 2/6 survived; 5/11 died awaiting OLT. Cerebral oedema was the main cause of death. Children who presented late to hospital for treatment and those with progressive hepatotoxicity with prothrombin time >100 seconds, hypoglycaemia, serum creatinine >200 μmol/l, acidosis (pH <7.3), and who developed encephalopathy grade III, had a poor prognosis or died. Although hepatic transaminase levels were markedly raised in both groups, there was no correlation with necessity for liver transplantation or death.
Conclusion
Accidental or incidental paracetamol overdose in children may be associated with toxic liver damage leading to fulminant liver failure. Delayed presentation and/or delay in treatment, and hepatic encephalopathy ⩾grade III were significant risk factors, implying poor prognosis and need for OLT. Prompt identification of high risk patients, referral to a specialised unit for management, and consideration for liver transplantation is essential.
Keywords: paracetamol, fulminant liver failure, orthotopic liver transplantation, hepatotoxicity
Paracetamol is a widely used medication which has a good safety profile,1,2,3 although large doses may lead to severe hepatic necrosis and fatal hepatic failure.4 Paracetamol poisoning is treated effectively with intravenous N‐acetylcysteine if started early enough,5 but once hepatic encephalopathy develops, the risks of complications and death increase significantly.6 Orthotopic liver transplantation (OLT) is a therapeutic option for liver failure following paracetamol overdose.7 It is important to define prognostic factors for outcome in order to develop clear criteria for referral to specialised units for further management and/or liver transplantation.
In 1996, concern about paracetamol overdose led to approximately 10% of all enquiries to the UK National Poisons Information Service and 73 000 (over 113 000 including paracetamol containing preparations) reports to the Toxic Exposure Surveillance Scheme of the American Association of Poison Control Centres.8 Because of age associated differences in the drug metabolism and detoxification of paracetamol metabolites,9 infants and young children may be less susceptible to paracetamol toxicity after acute ingestion than adults,10 but delayed presentation and treatment markedly increase the risk of clinically significant hepatocellular injury.11 The threshold for severe toxicity in children is variable although doses exceeding 150 mg/kg have been considered hepatotoxic.12 Evidence suggests that although younger children may tolerate doses higher than 150 mg/kg13,14,15 they may develop toxicity after repeated therapeutic or supratherapeutic doses of paracetamol.16,17,18,19,20 Management of paracetamol overdose is based on the detailed history, clinical examination, baseline biochemistry, initial serum paracetamol level at 4 hours post‐ingestion, and treatment with the antidote, N‐acetylcysteine.21 The published nomogram does not provide an estimation of potential severity of hepatotoxicity, which is necessary to identify the group of patients who may require liver transplantation.
A set of simple clinical and laboratory criteria in adults for paracetamol overdose was published by King's College Hospital (KCH)22 to predict patients with a less than 10% chance of survival without a liver transplant. Severe and sustained coagulopathy (prothrombin time >100 seconds) and serum creatinine >300 μmol/l in patients with advanced encephalopathy ⩾grade III or pH <7.30 (irrespective of grade of encephalopathy) were features that identified patients with a poor prognosis for hepatic recovery. These criteria have not been validated in children.
The purpose of this study was to identify the clinical and biochemical risk factors correlated with significant hepatotoxicity following paracetamol overdose in children, in order to define the factors associated with a poor prognosis necessitating a liver transplantation and to compare them with the established KCH criteria for OLT in adults following paracetamol overdose.
Subjects and methods
A retrospective review of all medical records of children admitted from 1992 to 2002 with the diagnosis of paracetamol overdose induced significant hepatotoxicity was performed. Significant hepatotoxicity was defined as serum alanine or aspartate transaminase (ALT or AST) level more than 1000 IU/l (normal range 10–40 IU/l). Severe hepatotocellular injury was defined as evidence of hepatic failure with ALT/AST >1000 IU/l, clinical evidence of altered mental status noted in the chart by the clinician, and/or coagulopathy (increase of prothrombin time >50% above the upper limit of normal). The study was conducted at the national liver unit at Birmingham Children's Hospital NHS Trust, Birmingham, UK. All children were referred from district general or tertiary hospitals for further specialist management. The patients were divided into two groups based on the following endpoints: group I included children who developed significant hepatotoxicity but made a complete recovery with conservative management, while group II were children who developed fulminant liver failure and required listing for OLT. All received a standard protocol management for acute liver failure.23 All patients received N‐acetylcysteine except 4/51 referred in 1992–93. Patients were listed for liver transplantation if there was progressive deterioration in liver function, coagulopathy (PT>50 seconds), and encephalopathy grade ⩾III. Six children aged <7 years (three in each group), who received multiple overdoses form a separate group because of their age, possible concomitant infection, age related differences in cytochrome P‐450 activity, and inability to estimate the drug doses. They were not included in the analysis of risk factors but were only included in outcome analysis.
The data collected included age, sex, reported dose of paracetamol ingested, time from reported ingestion to presentation at the hospital, and grade of encephalopathy. Laboratory data included serum paracetamol concentration, and peak values of AST, ALT, bilirubin, serum creatinine, blood glucose, pH, and prothrombin time (PT). “Peak” values were defined as the highest recorded value (reached within 48–72 hours of admission to liver unit) after overdose in a given patient. Toxic serum levels were determined with a nomogram based on single ingestion of paracetamol.24 A well established KCH criterion22 for liver transplantation in fulminant hepatic failure in adults following paracetamol overdose was assessed in the study population.
Continuous variables were expressed as median and range. The Mann‐Whitney test was used to compare those who recovered with conservative treatment and those listed for liver transplantation. Sensitivity, specificity, and predictive probabilities were calculated for possible discriminants. Logistic regression was used to identify possible best discriminators between groups.
Results
Clinical features
The clinical characteristics of 51 children (6 males) with paracetamol induced significant hepatotoxicity are shown in table 1. The median age was 14.7 years (range 0.8–16.1) with a bimodal distribution; 6/51 (11.7%) children were less than 7 years (range 0.8–7 years), 4/6 were male. In adolescent patients, overdose was usually an impulsive act with a female predominance (43/45). Three children in each group, all under 7 years old, received multiple cumulative overdoses accidentally or intentionally. The median dose for single paracetamol ingestion was 345 mg/kg (range 91–645). There was little difference in the dose ingested between groups. Two patients in group I who took supratherapeutic doses (97 and 127 mg/kg) showed significant hepatotoxicity without encephalopathy and survived. There was no significant difference in the median serum paracetamol level measured in either group. Twenty seven per cent with significant hepatotoxicity received N‐acetylcysteine treatment despite the paracetamol level being less than the nomogram treatment line. The median time from ingestion to presentation at hospital was 24 hours (range 4–72) in group I compared with 44 hours (range 24–96) in group II (p = 0.03).
Table 1 Demographics and clinical characteristics of 51 patients with paracetamol overdose.
| Group I (n = 40) | Group II (n = 11) | p value | |
|---|---|---|---|
| Age in years | 14.7 (1.0–16.1) | 13.3 (0.8–16.1) | 0.26 |
| Age <7 years (n = 6) | 3 | 3 | 0.11 |
| Sex: M:F | 5:35 | 1:10 | 0.74 |
| Paracetamol dose (mg/kg) | 348* (91–582) | 285† (222–645) | 0.7 |
| Cumulative doses/unreported | 3/1 | 3/2 | – |
| Time from reported ingestion to presentation (h) | 24 (4–72) | 44 (24–96) | 0.03 |
| Encephalopathy | |||
| Nil | 30 (75%) | 0 | |
| Grade I and II | 10 (25%) | 0 | |
| Grade ⩾III | 0 | 11 (100%) | 0.001 |
*n = 36;†n = 6.
Values expressed as median (range).
Outcome
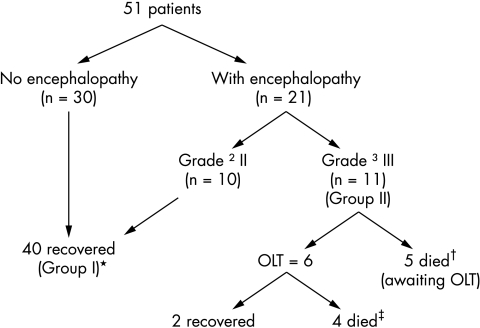
The outcome according to group is summarised in fig 1. A total of 41% (10/40 in group I and 11/11 in group II) had signs of severe hepatocellular injury. By definition, all children in group I recovered with conservative management, while children in group II developed rapidly progressing encephalopathy ⩾grade III and were listed for liver transplantation. Six of 11 children underwent OLT, of whom only 2/6 (33%) survived and 5/11 died awaiting OLT. There was a significant difference in the grade of encephalopathy between the groups (p < 0.001). Survival was 100% in children with grade ⩽II (group I) compared to 18% in those with grade ⩾III encephalopathy (group II) (p < 0.001) in spite of intensive management, suggesting that grade III encephalopathy was a sensitive and specific independent predictor of outcome in children with significant hepatotoxicity. The main cause of death was cerebral oedema in 8/11 (73%) patients (3 post‐transplant and 5 while awaiting transplant), while 1/11 (8%) died of fungal sepsis. The histopathological examination of liver tissue in 9/11 children in group II confirmed drug toxicity in the explanted livers and/or postmortem specimens.
Figure 1 Management and outcome of 51 patients with severe hepatotoxicity. Group I received conservative treatment; group II listed for OLT. *3 younger children <7 years; †1 young child; ‡2 younger children.
Laboratory parameters
Medians (ranges) for each laboratory parameters are reported for single overdose cases (n = 45) in table 2 and sensitivity, specificity, and predictive values in table 3.
Table 2 Laboratory parameters in 45 children with single paracetamol overdose.
| Parameter (normal value) | Group I (n = 37) | Group II (n = 8) | p value |
|---|---|---|---|
| Peak bilirubin (2–14 µmol/l) | 45 (21–496) | 98 (54–184) | 0.01 |
| Peak AST (10–40 IU/l) | 7500 (1025–15542) | 7500 (6780–8564) | 0.394 |
| Peak ALT (10–40 IU/l) | 10000 (1221–17928) | 10000 (7500–10000) | 0.90 |
| PT on admission (10–14 seconds) | 26 (14–98) | 95 (16–124) | 0.001 |
| Peak PT (in seconds) | 48 (17–146) | 120 (65–180) | 0.001 |
| Creatinine on admission (µmol/l) | 73 (48–112) | 141 (92–285) | 0.001 |
| Peak creatinine (µmol/l) | 82 (40–606) | 406(191–660) | 0.001 |
| Minimum pH (7.35–7.45) | 7.44 (7.16–7.52) | 7.29 (7.12–7.5) | 0.002 |
| Lowest glucose (mmol/l) | 4.8 (1.9–9.7) | 2.0 (0.8–4.7) | 0.001 |
Values expressed as median (range).
Table 3 Sensitivity, specificity, and positive and negative predictive value (PPV and NPV) of variables for the identification of patients who were listed for liver transplantation (n = 8) following paracetamol hepatotoxicity.
| Sensitivity % | Specificity % | PPV % | NPV % | |
|---|---|---|---|---|
| Encephalopathy >grade III | 100 (69–100) | 100 (92–100) | 100 (69–100) | 100 (92–100) |
| pH <7.3 | 75 (35–97) | 93 (77–99) | 75 (35–97) | 93 (77–99) |
| PT >100 seconds | 88 (47–100) | 92 (78–98) | 70 (35–93) | 97 (85–100) |
| Creatinine >300 μmol/l | 50 (16–84) | 97 (86–100) | 80 (28–99) | 90 (76–97) |
| Creatinine >200 μmol/l | 88 (47–100) | 92 (78–98) | 70 (35–93) | 97 (85–100) |
| Serum bilirubin >90 μmol/l | 88 (47–100) | 78 (62–90) | 47 (21–73) | 97 (82–100) |
| Glucose <2.6 mmol/l | 62 (24–91) | 97 (85–100) | 83 (36–100) | 92 (78–98) |
Total n = 45.
95% CI in parentheses.
We derived the threshold values from the ROC curves of our data that can be applicable for clinical practice. Although the sensitivity and specificity were high for different variables, encephalopathy grade ⩾III had perfect values followed by PT >100 seconds and bilirubin >90 μmol/l. The sensitivity increased markedly when the threshold for creatinine was adjusted to >200 μmol/l in our cohort.
All patients had raised hepatic transaminase levels, coagulopathy (PT >50% of the upper limit of normal range), and mildly raised serum bilirubin. There was no significant difference (p = 0.39) in peak hepatic transaminase levels between groups, whereas peak serum bilirubin (p = 0.01) and incidence of hypoglycaemia with glucose <2.6 mmol/l (p < 0.001) differed between groups. In group II, patients listed for liver transplant, prolonged PT (p < 0.001) and serum creatinine (p = 0.001) were significantly raised at the time of admission to the liver unit. Logistic regression analysis showed the variables PT >100 seconds, bilirubin >90 μmol/l, glucose <2.6 mmol/l, and creatinine >200 μmol/l to be significant discriminators. Encephalopathy grade III discriminated perfectly. The high overlap of listed cases on these criteria does not allow a sensible multifactor analysis as they are too correlated. Haemofiltration for progressive renal impairment was necessary in one patient in group I and five in group II, and only one in each group survived.
KCH criteria
We applied these criteria to our adolescent population with significant paracetamol hepatotoxicity as shown in table 4. In this study, hepatic encephalopathy ⩾grade III was the best single predictor of poor prognosis as only 18% of children survived despite liver transplantation. The independent parameter pH <7.3 of KCH criteria (in this study pH <7.3 at any time from the time of admission rather than at the time of admission) was noted in 2/40 patients in group I and 8/11 in group II and showed high specificity predicting listing for transplantation. It was noted that absence of metabolic acidosis had a negative predictive value of 93% for listing for transplantation. Applying the three combined variables of the KCH criteria (hepatic encephalopathy ⩾grade III, creatinine >300 μmol/l, and PT >100 seconds) to this cohort had poor sensitivity, although the encephalopathy criterion on its own (table 3) had 100% sensitivity and specificity. However, modifying the serum creatinine level to >200 μmol/l in children and analysing it with encephalopathy ⩾grade III and PT>100 seconds (7/11 in group II) improved sensitivity slightly (table 4). It is of further interest to note that the percentage survival was 97% (34/35) with serum creatinine <200 µmol/l compared with 50% (5/10) otherwise (p = 0.001, Fisher exact test).
Table 4 Sensitivity, specificity, and positive and negative predictive value (PPV and NPV) for the identification of paediatric patients listed for liver transplantation following paracetamol poisoning using KCH criteria22.
| Sensitivity % | Specificity% | PPV % | NPV % | |
|---|---|---|---|---|
| Arterial pH <7.3 (measured anytime from admission) | 75 (35–97) | 93 (77–99) | 75 (35–97) | 93 (77–99) |
| Creatinine >300 μmol/l, Encephalopathy grade III, and PT >100 seconds | 37 (9–75) | 100 (92–100) | 100 (37–100) | 88 (74–96) |
| Modified: Creatinine >200 μmol/l, Encephalopathy grade III, and PT >100 seconds | 75 (35–97) | 100 (92–100 | 100 (61–100) | 95 (83–99) |
95% CI in parentheses.
Discussion
Paracetamol overdose leading to toxic liver damage and encephalopathy occurs less frequently in children than in adults,25 but may be fatal.26,27 As liver transplantation is an accepted treatment for this condition it is important to identify those children who might require it early. This is the first retrospective study to assess prognostic risk factors in children with significant paracetamol hepatotoxicity and to consider whether the established KCH criteria for adult transplantation listing following paracetamol overdose are applicable to children.
This study reviewed 10 years' experience from a single major paediatric liver unit. Most patients were adolescent females who took an accidental paracetamol overdose following an impulsive act,11,18,28 whereas some younger children had received cumulative multiple overdoses.17,19 Although paracetamol doses exceeding 150 mg/kg are considered hepatotoxic, and initial serum paracetamol level at 4 hours post‐ingestion suggest who should receive antidote,21 neither the dose nor the level predicts the severity of hepatotoxicity. We did not find a relationship between the median dose ingested (348 v 285 mg/kg) and the severity of liver disease. This confirms previous studies that indicated no significant difference in the ingested dose of paracetamol between children who developed abnormal (345 mg/kg) compared to normal liver function (236 mg/kg)19 and those who developed mild (390 mg/kg) or severe hepatotoxicity (324 mg/kg).28 The dose ingested may not be a useful parameter for assessing severe hepatotoxicity, not only because the dose at presentation may be difficult to estimate, but also because there might have been multiple dosing and/or associated vomiting.15,29 Although the paracetamol level measured was high in 31/51 (61%) patients, it was low or undetectable in 39% who had a definitive history of paracetamol overdose with significant hepatotoxicity. This may be due to late presentation to hospital or multiple small ingestions. A similarly low level was reported in a small series of young children with fulminant liver failure following prodromal illness.20 The Rumack–Matthews nomogram is useful to identify paracetamol levels requiring treatment in the first 24 hours but it does not differentiate between children who subsequently develop severe or significant hepatotoxicity.
This study noted that delayed presentation (24 v 44 hours) to hospital after overdose was a risk factor for severe hepatocellular injury with requirement for OLT, and increased mortality. This confirms results of a previous study29 which showed that delayed initiation of treatment was an important risk factor in developing encephalopathy. In addition, younger age may also be predictive of severe hepatotoxicity.11,19 Although children less than 7 years may be less susceptible to acute paracetamol poisoning,13,14,15,26 we noted that 6/51 (12%) patients less than 7 years developed hepatotoxicity following multiple dosing. Three were listed for liver transplantation; 2/6 died in spite of OLT and 1/6 died awaiting transplant.17,18 Although these children developed similar hepatic toxicity to the adolescents, they were not included in the analysis of risk factors, because of the difference in age and age related cytochrome P‐450 activity.
Hepatic transaminase levels were markedly elevated in both groups following paracetamol overdose, but did not differentiate between groups.17,18 Jaundice was not an apparent clinical feature, although serum bilirubin was significantly elevated in group II (p = 0.01). Miles et al18 and Alonso et al20 noted a distinctive clinical pattern with mildly elevated bilirubin level and disproportionately high transaminase levels. This is probably due to differences in the rapidity and duration of hepatocyte injury together with variations in net hepatic regeneration.
What is already known on this topic
Liver transplantation is a therapeutic option for liver failure following paracetamol overdose
A set of simple clinical and laboratory criteria in adults for paracetamol overdose to predict patients with a less than 10% chance of survival without a liver transplant is available but not validated in children
The potential risk factors for poor prognosis were severe coagulopathy, hypoglycaemia, grade of encephalopathy, raised serum creatinine level, and metabolic acidosis. The association between coagulation and hepatic necrosis with PT >50 seconds was a worse prognostic sign but not necessarily fatal.23 Harrison et al30 found that the severity of coagulopathy had a prognostic value in adults with 80% survival when PT <90 seconds compared to 8% survival in those with peak PT >180 seconds. James et al28 noted that in patients with toxic serum paracetamol levels, an elevation in PT during the first 24 hours was more sensitive and had a higher negative predictive value for hepatotoxicity than hepatic transaminase levels. In this study, although patients with a PT >50 seconds were considered for listing for transplantation, those with a PT >100 seconds had a worse prognosis and increased mortality. PT >100 seconds has 88% sensitivity and 61% positive predictive value of death following paracetamol hepatotoxicity. The blood glucose level was different in the two groups of patients, reflecting the severity of hepatic necrosis; it is a predictive factor for listing for transplant.
Miles et al18 and Rivera‐Penera et al19 noted that in children with paracetamol overdose, encephalopathy grade II resolved spontaneously, whereas those with grade ⩾III died or required OLT. As was found in this study, all children with grade III were listed for OLT but only 18% survival. The main cause of death was progressive irreversible cerebral oedema following extensive hepatic necrosis and fulminant liver failure, highlighting the need for early referral to a specialist centre for appropriate management and early listing for transplantation in high risk groups with severe hepatocellular injury. OLT has an important therapeutic role in irreversible fulminant liver failure, and outcome depends on severity of encephalopathy.17,18,19,20 In children, the development of hepatic encephalopathy grade ⩾III was the single best prognostic indicator of outcome and listing for transplantation in children with paracetamol overdose.
A number of previous studies have reported the development of nephrotoxicity (8.9%), and oliguric renal failure in 1–2% of all cases and in 11% of severely poisoned patients.31,32,33 In this study, 13.5% patients (7/51) developed nephrotoxicity that required haemofiltration. Adult data showed that a serum creatinine level >300 μmol/l predicted a worse survival rate of 23.2%.22 In this study, survival was 50% in 10 patients with level >200 μmol/l, suggesting that renal dysfunction does influence outcome.
Following a retrospective and prospective study of adult patients at KCH,22 a set of simple criteria was developed to predict patients with less than 10% chance of survival without transplant. O'Grady et al22 found that arterial pH <7.3 on admission after initial resuscitation provides prognostic information irrespective of coma grade, which had a 95% predictive value for subsequent death. We were not able to analyse this parameter as blood gas was not routinely measured in the referral hospital and arterial pH was only measured in those children with worsening liver function. We found mortality was 70% in children with metabolic acidosis pH <7.30 noted at any time from admission regardless of the grade of encephalopathy. We confirm that pH <7.30 has an important prognostic value that may indict early listing for OLT and should highlight the necessity for serial measurement. Although the KCH criteria in adults included elevated serum creatinine >300 μmol/l as a relevant prognostic factor, our results suggest that a lower level of 200 μmol/l with hepatic encephalopathy grade III and PT >100 seconds would be more appropriate in the paediatric population (table 4). This could be related to the height and body mass of adolescents compared to adults in the production of serum creatinine.
What this study adds
Markedly elevated hepatic transaminase levels suggest severe hepatic necrosis but are not predictive for prognostic outcome. A distinctive clinical pattern noted with mildly elevated bilirubin level disproportionate to hepatic transaminase level is characteristic
Hepatic encephalopathy ⩾grade III is an independent risk factor for poor prognosis without liver transplantation
Conclusions
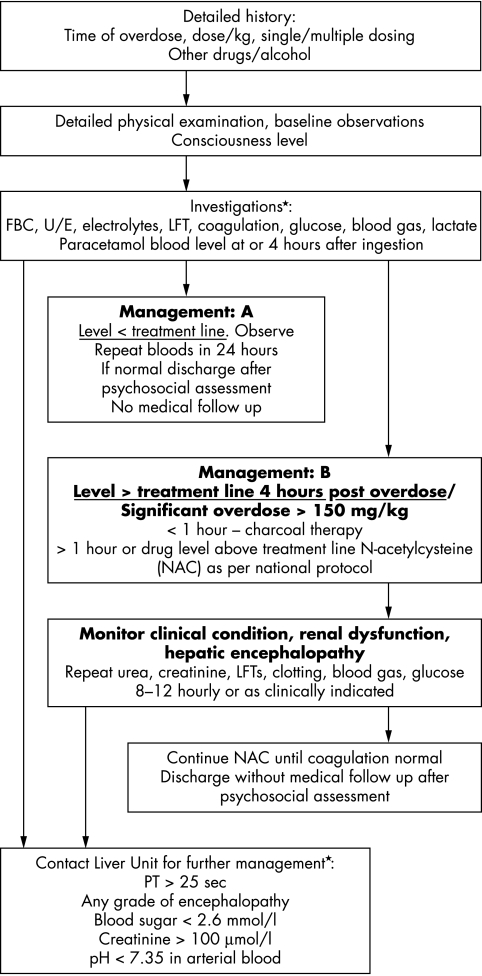
Substantial liver damage induced by paracetamol overdose in children is a significant problem. The main factors for poor outcome were delayed presentation, and/or delay in establishing treatment, encephalopathy grade ⩾III, severe coagulopathy (PT >100 seconds), bilirubin (>90 μmol/l), metabolic acidosis (pH <7.3), and renal dysfunction (reflected by raised peak serum creatinine >200 μmol/l). A modified KCH criterion may be applicable in the paediatric population. Early referral to a specialised unit for intensive management is essential for this group of children. A further larger multicentre prospective study may be useful to confirm these data. Figure 2 shows an algorithm for paracetamol overdose in children.
Figure 2 Management algorithm for paracetamol overdose in children. The mortality following fulminant hepatitis due to paracetamol overdose in children may be less than in adults, but early referral and/or discussion with a liver unit with access to liver transplant facilities is mandatory.
Abbreviations
ALT - alanine transaminase
AST - aspartate transaminase
KCH - King's College Hospital
OLT - orthotopic liver transplantation
PT - prothrombin time
Footnotes
Competing interests: none declared
References
- 1.American Academy of Pediatrics, Committee on Drugs Acetaminophen toxicity in children. Pediatrics 20011081020–1024. [DOI] [PubMed] [Google Scholar]
- 2.Lesko S M, Mitchell A A. The safety of acetaminophen and ibuprofen among children younger than two years old. Pediatrics 1999104e39. [DOI] [PubMed] [Google Scholar]
- 3.Cranswick N, Coghlan D. Paracetamol efficacy and safety in children: the first 40 years. Am J Ther 20007135–141. [DOI] [PubMed] [Google Scholar]
- 4.Proudfoot A T, Wright N. Acute paracetamol poisoning. BMJ 19703557–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prescott L F, Illingworth R N, Critchley J A.et al Intravenous N‐acetylcysteine: the treatment of choice for paracetamol poisoning. BMJ 197921097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makin A J, Wendon J, Williams R. A 7‐year experience of severe acetaminophen‐induced hepatotoxicity (1987–1993). Gastroenterology 19951091907–1916. [DOI] [PubMed] [Google Scholar]
- 7.Kelly D A. Paediatric liver transplantation. Curr Opin Pediatr 199810493–498. [DOI] [PubMed] [Google Scholar]
- 8.Litovitz T L, Smilkstein M, Felberg L.et al 1996 Annual Report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med 199715447–500. [DOI] [PubMed] [Google Scholar]
- 9.Miller R P, Roberts R J, Fischer L J. Acetaminophen elimination kinetics in neonates, children and adults. Clin Pharmacol Ther 197619284–294. [DOI] [PubMed] [Google Scholar]
- 10.Rumack B H. Acetaminophen overdose in young children: treatment and effects of alcohol and other additional ingestants in 417 cases. Arch Pediatr Adolesc Med 1984138428–433. [DOI] [PubMed] [Google Scholar]
- 11.Alander S W, Dowd M D, Bratton S L.et al Pediatric acetaminophen overdose: risk factors associated with hepatocellular injury. Arch Pediatr Adolesc Med 2000154346–350. [DOI] [PubMed] [Google Scholar]
- 12.Rumack B H, Matthew M. Acetaminophen poisoning and toxicity. Pediatrics 197555871–876. [PubMed] [Google Scholar]
- 13.Anderson B J, Holford N H, Armishaw J C.et al Predicting concentrations in children presenting with acetaminophen overdose. J Pediatr 1999135290–295. [DOI] [PubMed] [Google Scholar]
- 14. 14 Mohler CR, Nordt SP, Williams SR, et al. Prospective evaluation of mild to moderate pediatric acetaminophen exposures. Ann Emerg Med 200035239–244. [DOI] [PubMed] [Google Scholar]
- 15.Bond G R, Krenzelok E P, Normann S A.et al Acetaminophen ingestion in childhood: cost and relative risk of alternative referral strategies. J Toxicol Clin Toxicol 199432513–525. [DOI] [PubMed] [Google Scholar]
- 16.Henretig F M, Selbst S M, Forrest C.et al Repeated acetaminophen overdosing. Causing hepatocellular injury in children. Clinical reports and literature review. Clin Pediatr (Phila) 198928525–528. [DOI] [PubMed] [Google Scholar]
- 17.Heubi J E, Barbacci B M, Zimmermann H J. Therapeutic misadventure with acetaminophen: hepatotoxicity after multiple doses in children. J Pediatr 199813222–28. [DOI] [PubMed] [Google Scholar]
- 18.Miles F K, Kamath R, Dorney S A.et al Accidental paracetamol overdosing and fulminant hepatic failure in children. Med J Aust 1999171472–475. [DOI] [PubMed] [Google Scholar]
- 19.Rivera‐Penera T, Gugig R, Davis J.et al Outcome of acetaminophen overdose in paediatric patients and factors contributing to hepatotoxicity. J Pediatr 1997130300–304. [DOI] [PubMed] [Google Scholar]
- 20.Alonso E M, Sokot R J, Hart J.et al Fulminant hepatitis associated with centrilobular hepatic necrosis in young children. J Pediatr 1995127888–894. [DOI] [PubMed] [Google Scholar]
- 21.Peterson R G, Rumack B H. Pharmacokinetics of acetaminophen in children. Pediatrics 197862877–879. [PubMed] [Google Scholar]
- 22.O'Grady J G, Alexander G J, Hayllar K M.et al Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 198997439–445. [DOI] [PubMed] [Google Scholar]
- 23.Alonso E M, Superina R A, Whithington P F. Fulminant hepatitis and acute liver failure. In: Kelly DA, ed. Diseases of the liver and biliary system in children, 2nd edn. Oxford: Blackwell Science, 200377–94.
- 24.Presscot L F. Paracetamol overdosage. Drugs 198325290–314. [DOI] [PubMed] [Google Scholar]
- 25.Rumack B H. Acetaminophen overdose in young children. Am J Dis Child 1984138428–433. [DOI] [PubMed] [Google Scholar]
- 26.Penna A, Buchanan N. Paracetamol poisoning in children and hepatotoxicity. Br J Clin Pharmacol 199132143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kearns G L, Leeder J S, Wasserman G S. Acetaminophen overdose with therapeutic intent. J Pediatr 19981325–8. [DOI] [PubMed] [Google Scholar]
- 28.James L P, Wells E, Beard R H.et al Predictors of outcome after acetaminophen poisoning in children and adolescents. J Pediatr 2002140522–526. [DOI] [PubMed] [Google Scholar]
- 29.Schiodt F V, Bondesen S, Tygstrup N.et al Prediction of hepatic encephalopathy in paracetamol overdose: a prospective and validated study. Scand J Gastroenterol 199934723–728. [DOI] [PubMed] [Google Scholar]
- 30.Harrison P M, O'Grady J G, Keayset al Serial prothrombin time as prognostic indicator in paracetamol induced fulminant hepatic failure. BMJ 1990301964–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prescott L F. The nephrotoxicity and hepatotoxicity of antipyretic analgesics. Br J Clin Pharmacol 19797453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell N R, Baylis B. Renal impairment associated with an acute paracetamol overdose in the absence of hepatotoxicity. Postgrad Med J 199268116–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boutis K, Shannon M. Nephrotoxicity after acute severe acetaminophen poisoning in adolescents. J Toxicol Clin Toxicol 200139441–445. [DOI] [PubMed] [Google Scholar]