Abstract
Background
Croup remains a common respiratory problem presenting to emergency departments. A single oral treatment of oral dexamethasone results in improved outcome. Prednisolone has similar pharmacokinetic properties and has a significant advantage in that it is commercially available in liquid preparations.
Objective
To ascertain whether a single oral dose of prednisolone was equivalent to a single oral dose of dexamethasone (matched for potency) in children with mild to moderate croup.
Design
A double blind, randomised, controlled equivalence trial
Setting
Tertiary paediatric emergency department.
Patients
133 children aged 3 to 142 months presenting with mild to moderate croup.
Interventions
Children received either a single oral dose of dexamethasone 0.15 mg/kg or single oral dose of prednisolone 1 mg/kg.
Outcome
The main outcome measure was unscheduled re‐presentation to medical care as determined by telephone follow up at 7 to 10 days. Croup score, adrenaline (epinephrine) use, time spent in the emergency department, and duration of croup and viral symptoms were secondary outcome measures.
Results
Children treated with prednisolone were more likely to re‐present: 19 of 65 children (29%) reattended medical care compared with 5 of 68 (7%) from the dexamethasone group. The confidence intervals around this 22% difference in outcome were 8% to 35%, outside the 0% to 7.5% range of equivalence. There were no significant differences in other outcome measures.
Conclusion
A single oral dose of prednisolone is less effective than a single oral dose of dexamethasone in reducing unscheduled re‐presentation to medical care in children with mild to moderate croup.
Keywords: croup, dexamethasone, prednisolone
Acute laryngotracheobronchitis or croup describes an acute clinical syndrome of hoarse voice, barking cough, and stridor resulting from inflammation of the upper airway. Croup is one of the more common childhood respiratory illnesses and often presents to paediatric emergency departments.1 Steroids are effective in the management of all children presenting to emergency departments with croup, whether mild, moderate, or severe.2,3,4 Studies have found a reduction in both hospital admission and symptoms of airway obstruction from as early as one hour post treatment.4 Oral dexamethasone is as effective as intramuscular dexamethasone;5 and one centre has shown than an oral dose of 0.15 mg/kg is as affective as the traditional 0.6 mg/kg.6,7 The same centre found that a single dose of oral dexamethasone was effective in reducing return to medical care in children with milder croup.8
Oral preparations have an advantage over intramuscular preparations in terms of ease of administration. Dexamethasone is much cheaper and easier to administer than the inhaled steroid, budesonide. Currently oral dexamethasone, if available, is the treatment of choice for croup. Children affected are predominantly infants and young children who will only swallow drugs in liquid form. In Australia, dexamethasone is only commercially available in intravenous, intramuscular, and tablet form. For our own departmental use, the elixir is made up by the hospital pharmacy. It is not available to general practitioners, and is considerably more expensive than the commercially available preparations of prednisolone. Dexamethasone, however, has recently become commercially available in both the United Kingdom and the USA in liquid form.9,10
Prednisolone as an alternative preparation has not been as extensively studied as dexamethasone, and yet it has an obvious advantage over dexamethasone in Australia, as it is commercially available in two elixir preparations, Redipred and Predmix.11 At least one previous study has shown that it is effective in the management of severe croup.12 While prednisolone is often substituted for dexamethasone when the latter is unavailable, the efficacy of prednisolone has never been directly compared with dexamethasone.
Few children referred by general practitioners receive steroids.13 In addition, neither dexamethasone nor budesonide has product licenses for use for croup in children in Australia. Prednisolone, as Redipred or Predmix, may be used “wherever corticosteroid therapy is indicated.”11 Knowing prednisolone was a reasonable alternative to dexamethasone might be expected to promote management in the community or hospitals without oral dexamethasone available.
Pharmacokinetics
Both dexamethasone and prednisolone are corticosteroids with predominantly glucocorticoid activity. Regarding anti‐inflammatory potential, dexamethasone is five to six times as potent as prednisolone.14,15 Dexamethasone is traditionally classified as being a long acting corticosteroid with a biological half life between 36 and 72 hours, and prednisolone as intermediate acting with a half life of 12 to 36 hours.15 Suppression of the hypothalamic–pituitary axis by dexamethasone may persist for up to 2.5 days, while the duration of prednisolone is between 1.25 and 1.5 days. Both drugs are rapidly and well absorbed from the gastrointestinal tract, bound to plasma proteins, and excreted in the urine.
Study aims
Our aim was to compare the relative efficacy of a single dose of prednisolone, 1 mg/kg, matched for potency with a single dose of dexamethasone in children with mild to moderate croup. Our primary outcome measure was unscheduled reattendance to medical care. We also measured time spent in the emergency department, use of adrenaline (epinephrine), and the duration of subsequent croup and viral symptoms as secondary outcome measures. The study was undertaken using PMH Emergency Department resources.
Methods
The study design entailed a double blinded, randomised, controlled equivalence trial conducted in the emergency department of Princess Margaret Hospital for Children (PMH), a tertiary paediatric hospital serving the city of Perth, Western Australia. The PMH emergency department sees 43 000 children a year, of whom up to 1500 have croup.
As our hypothesis was that prednisolone would be as effective as dexamethasone, the study was designed as a randomised equivalence trial. In an earlier double blind, placebo controlled trial conducted at PMH comparing oral dexamethasone with placebo, not one of 50 children treated with dexamethasone returned to medical care.8 Thus, expecting a re‐attendance rate of 2% or less for an equivalence range of between 0% and 7.5%, 108 patients would be required to achieve a power of 90%.
Children were eligible for inclusion in the study if they had not received steroids, had mild to moderate croup as defined by clinical symptoms and the modified Taussig croup score (table 1), and were older than 3 months. A report of the interobserver reliability of this score has been published.4 Patients were recruited by emergency department medical staff. Mild to moderate croup was defined as the acute onset of inspiratory stridor and a hoarse voice accompanied by a “barking cough”. Children were excluded if their families did not have a telephone or had limited knowledge of the English language. Informed written consent was obtained from the parents or care givers.
Table 1 Modified Taussig croup score.
| Score* | |
|---|---|
| Stridor | |
| None | 0 |
| Only on crying, exertion | 1 |
| At rest | 2 |
| Severe (biphasic) | 3 |
| Retractions | |
| None | 0 |
| Only on crying, exertion | 1 |
| At rest | 2 |
| Severe (biphasic) | 3 |
*1–2, mild; 3–4, moderate; 5–6, severe.
On recruitment, parents completed a questionnaire administered by emergency department medical staff regarding current and previous episodes of croup and a history of atopy in the child or in first order relatives. At presentation, emergency department nursing staff recorded a modified Taussig croup score, pulse rate, respiratory rate, and arterial oxygen saturation (Sao2) in air (table 2).
Table 2 Baseline characteristics of 133 children with mild to moderate croup.
| Variable | Dexamethasone | Prednisolone |
|---|---|---|
| Number studied | 68 | 65 |
| Male/female | 43/25 | 48/17 |
| GP referral | 45 | 46 |
| Age (months) | 37 (28.8) | 45 (31.6) |
| Weight (kg) | 15.7 (7.3) | 17.8 (7.3) |
| Duration of symptoms (h) | 24 (4) | 19 (3) |
| Pulse rate (beats/min) | 129 (24) | 133 (24) |
| Temperature (°C) | 36.8 (1.1) | 37.5 (0.8) |
| Respiratory rate (/min) | 30 (9) | 30 (9) |
| % Sao2 in air (%) | 97 (1) | 97 (1) |
| Initial croup score | 2.0 (1.3) | 2.0 (1.2) |
| Viral/spasmodic (n) | 52/16 | 55/10 |
| Previous croup (n) | 7 | 11 |
Values are means (SD) or n.
Sao2, arterial oxygen saturation.
Following enrolment patients sequentially received either 0.15 mg/kg of dexamethasone or 1 mg/kg of prednisolone in elixir form based on computer generated orders randomised into blocks of 10. The PMH pharmacy ensured that the two steroid preparations could not be differentiated, the code being held by the pharmacy. Bottles were simply labelled solution A or solution B, and following randomisation, 0.2 ml/kg of either solution was given by a nurse who took no further part in the child's care. The prednisolone elixir used was Redipred. The dexamethasone elixir concentration was manufactured to ensure that the volume of liquid administered was the same and of a similar taste and appearance. Medical notes and drug charts recorded “trial medication” and the volume given to avoid bias. The code was not broken until all data were collected and all follow up completed.
Subsequent management involved repeated clinical observations at 30 minutes after administration of steroid; hourly for the next four hours and four hourly thereafter until discharge. Criteria for discharge home were minimal stridor or chest wall retractions—that is, a croup score of 1 or 0. Nebulised adrenaline was given at any time during the study if clinically indicated as per the Advanced Paediatric Life Support (APLS) manual guidelines on croup.
The principle outcome measure was unscheduled reattendance to medical care. Following discharge the parents of the children were contacted 7 to 10 days later by telephone and asked whether their child had re‐presented to PMH or other medical care with croup symptoms, and if so, whether they had been admitted to hospital. The duration of the “barking cough” was ascertained, as was the duration of viral symptoms (fever and rhinorrhoea) if initially present.
Other outcome measures recorded included duration of time spent in the emergency department and use of nebulised adrenaline.
Data analysis
Data were entered using Microsoft Access specifically designed for this study, and subsequently analysed using SPSS version 11 software. The χ2 test was used for analysis of categorical variables, and Student's t test for analysis of continuous variables. For variables that were highly skewed, such as duration of hospital admission, we carried out a logarithmic transformation before analysis. Expecting a re‐attendance rate of 2% or less, for an equivalence range between 0% and 7.5%, 108 patients were required to achieve a power of 90%.
The study was approved by the PMH ethics committee.
Results
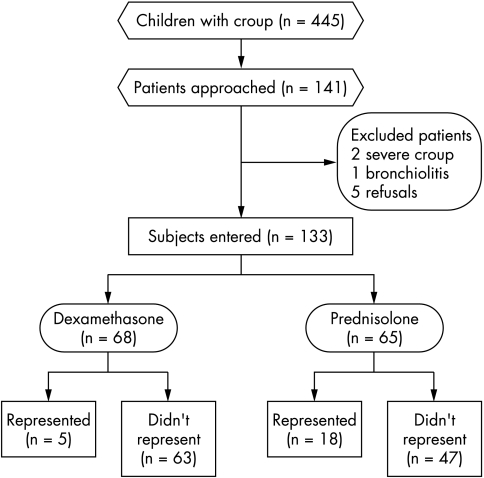
In all, 445 children with croup presented during the study period, of whom 81 were admitted (see fig 1 for details of the presentation of the children during the study period). One hundred and forty one children who had not already been treated with steroids were approached to take part in the trial over a four month period from July to October 2001 inclusive. Many children presenting with croup were not approached owing to pressure on emergency staff over a busy winter period, because they had already received steroids or their croup was too severe. Of those approached, one child was not eligible, being subsequently diagnosed as having bronchiolitis, two were not eligible because they had severe croup requiring intensive care unit admission, and five refused to take part. Sixty five received prednisolone and sixty eight received dexamethasone. Baseline characteristics of the two groups were similar (table 2).
Figure 1 Outcome for children who presented with croup during the study period.
Ninety one of the children (68%) were referred from the general practitioner (GP) or had seen a GP already.
Outcome measures are shown in table 3.
Table 3 Outcome measures for 133 children treated with dexamethasone or prednisolone.
| Variable | Dexamethasone | Prednisolone | p Value | |
|---|---|---|---|---|
| Followed up (n) | 68 | 65 | ||
| Reattendance (n) | 5 | 19 | ||
| Admitted (n) | 3 | 2 | 0.7 | |
| Time in emergency dept (min) | 114 (240) | 84 (112) | 0.5 | |
| Duration croup symptoms (h) | 26 (39) | 35 (42) | 0.2 | |
| Duration viral symptoms (d) | 8 (29) | 7 (14) | 0.9 | |
| Received adrenaline (n) | 5 | 5 | 0.9 |
Except where stated values are means (SD). The duration of the viral and croup symptoms date from having received either prednisolone or dexamethasone.
Children who received prednisolone were more likely to represent to medical care. In the prednisolone arm, 19 of 65 children (29%) reattended for medical care compared with five of 68 children (7%) from the dexamethasone arm. The confidence intervals around this 22% difference in outcome were 8% to 35%, outside the 0% to 7.5% range of equivalence. There was no difference in the time spent in the emergency department, use of adrenaline, duration of croup symptoms, or the duration of viral symptoms. No adverse events were noted in either group.
Discussion
Our findings indicate that a single dose of oral prednisolone is not equivalent to a single oral dose of dexamethasone in reducing unscheduled re‐presentation to medical care in children with mild to moderate croup. The overall re‐presentation rate for the prednisolone group was 29%, while for the dexamethasone group it was 7%. The difference of 22% was outside the expected equivalence range of 0% to 7.5%. Although the trial was an equivalence study and was analysed as such, the difference was so pronounced that we did a χ2 test. This showed that the outcome was significantly different, with a probability of 0.01 or less.
The differing duration of suppression of the hypothalamic‐pituitary axis of the two corticosteroids may provide an explanation for this, with the shorter half life of prednisolone allowing more “relapses”. In the initial study done at this hospital comparing dexamethasone with placebo in 100 children with mild croup,8 the re‐presentation rate for children receiving dexamethasone was 0%, and for placebo, 16%. The overall re‐presentation rate in this study was 18%. In the original study, however, the mean croup score was 0.9 compared with 2 in this study, out of a possible 6. We postulate our experience and confidence with dexamethasone, since the earlier study has allowed children with more severe croup to be sent home early. Thus the higher rate of reattendance in the current study in both groups most probably reflects the greater severity of croup in this study.
It could be argued that a more accurate equivalent dose of prednisolone to the 0.15 mg/kg of dexamethasone used would be 0.75 mg/kg rather than 1 mg/kg. Current practice, however, is to round out the dose used to 1 mg/kg. As we did not expect prednisolone to be superior to dexamethasone but either equivalent or inferior we felt this a reasonable approach. It would seem likely that there is no difference in outcome for 0.75 mg/kg or 1 mg/kg of prednisolone, just as we demonstrated no difference between 0.15 mg/kg, 0.3 mg/kg, and 0.6 mg/kg of dexamethasone6,7
Controversy persists over whether patients with mild croup in primary care should receive corticosteroid treatment. Even though many cases of croup are mild and self limiting, it tends to last for a few days and occasionally up to a week. Trials, reviews, and meta‐analyses have repeatedly shown that steroids are beneficial in the management of children with croup. Several studies found that although some children have already seen a general practitioner, very few receive steroids before hospital presentation.13 In Australia and other countries without easy access to oral dexamethasone, this may reflect the lack of evidence about corticosteroids other than dexamethasone.
The major reason for presentation is parental concern resulting from the obvious symptoms of cough and stridor. There is no argument to treat on the basis of parental desire alone, but nor is it justified to withhold a proven, well tolerated treatment from these children. Moreover, as with many respiratory illnesses, there is a nocturnal exacerbation of symptoms, which may disrupt and distress the entire family. The fact that an illness will usually resolve if left untreated in no way infers that it should be left untreated.
At present a one‐off dose of prednisolone is often substituted for dexamethasone. Based on this study, however, if prednisolone is used, a two day course of 1 mg/kg is probably justified. A trial in primary care comparing the effectiveness of a two day course of prednisolone and a one‐off dose of dexamethasone is indicated.
What is already known on this topic
Both dexamethasone and prednisolone are beneficial in the treatment of croup
No direct comparisons of dexamethasone and prednisolone have been made
What this study adds
Dexamethasone and prednisolone seem equally effective when first given but relapse and reattendance to medical care is more common with prednisolone which may reflect its shorter half life
Conclusion
A single oral dose of prednisolone is less effective than a single oral dose of dexamethasone in reducing unscheduled re‐presentation to medical care in children with mild to moderate croup.
Acknowledgements
We would like to thank Max Bulsara and Dr Elizabeth Geelhoed for their guidance with statistical analysis and emergency department staff who helped with the study.
Footnotes
Competing interests: none declared
References
- 1.Denny F W, Murphy T F, Clyde W A.et al Croup: an 11‐year study in a pediatric practice. Pediatrics 198371871–876. [PubMed] [Google Scholar]
- 2.Kairys S W, Olmstead E M, O'Connor G T. Steroid treatment of laryngotracheitis: a meta‐analysis of the evidence of randomised trials. Pediatrics 198983683–693. [PubMed] [Google Scholar]
- 3.Ausejo M, Saenz A, Ba'Phamet al The effectiveness of glucocorticoids in treating croup: meta‐analysis. BMJ 1999319595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geelhoed G C, Macdonald W B G. Oral and inhaled steroids in croup: a randomised, placebo‐controlled trial. Pediatric Pulmonology 199520355–361. [DOI] [PubMed] [Google Scholar]
- 5.Rittichier K K, Ledwith C A. Outpatient treatment of moderate croup with dexamethasone: intramuscular versus oral dosing. Pediatrics 20001061344–1348. [DOI] [PubMed] [Google Scholar]
- 6.Geelhoed G C. Sixteen years of croup in a Western Australian teaching hospital: effects of routine steroid treatment. Ann Emerg Med 199628621–626. [DOI] [PubMed] [Google Scholar]
- 7.Geelhoed G C, Macdonald W B G. Oral dexamethasone in the treatment of croup: 0.15 mg/kg versus 0.3 mg/kg versus 0.6 mg/kg. Pediatr Pulmonol 199520362–368. [DOI] [PubMed] [Google Scholar]
- 8.Geelhoed G C, Turner J, Macdonald W B G. Efficacy of a single small dose of oral dexamethasone for outpatient croup: a double blind placebo controlled clinical trial. BMJ 1996313140–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.British National Formulary London: British Medical Association and Royal Pharmaceutical Association of Great Britain, March 2000316–317.
- 10.American Hospital Formulary Service ( A H F S ) Drug Information 2003 text.
- 11. MIMS Annual 2000. Australian edition. Havas Medimedia, 24th edition, June 20006–449.
- 12.Tibballs J, Shann F A, Landau L I. Placebo‐controlled trial of prednisolone in children intubated for croup. Lancet 1992340745–748. [DOI] [PubMed] [Google Scholar]
- 13.Tillett A J, Gould J D M. Effectiveness of glucocorticoids in treating croup: general practitioners must be ready to treat children [letter]. BMJ 19993191577. [PubMed] [Google Scholar]
- 14.Martindale The complete drug reference, 32nd edition. London: Pharmaceutical Press, 19991037
- 15.Schimmer B P, Parker K L. Adrenocorticotropic hormone: adrenocortical steroids and their synthetic analogs: inhibitors of the synthesis and actions of adrenocortical hormones. Goodman and Gilman's the pharmacological basis of therapeutics, 9th edition. New York: McGraw‐Hill, 20051459–1485.