Abstract
Several aspects of body composition, in particular the amount and distribution of body fat and the amount and composition of lean mass, are now understood to be important health outcomes in infants and children. Their measurement is increasingly considered in clinical practice; however, paediatricians are often unsure as to which techniques are appropriate and suitable for application in specific contexts. This article summarises the pros and cons of measurement technologies currently available for paediatric application. Simple techniques are adequate for many purposes, and simple regional data may often be of greater value than “whole body” values obtained by more sophisticated approaches.
Keywords: fat mass, lean mass, fat distribution
Body composition and growth are key components of health in both individuals and populations. The ongoing epidemic of obesity in children and adults has highlighted the importance of body fat for short term and long term health.1 However, other components of body composition also influence health outcomes, and its measurement is increasingly considered valuable in clinical practice.
The gold standard for body composition analysis is cadaver analysis, so no in vivo technique may be considered to meet the highest criteria of accuracy. As discussed below, only multicomponent models are now considered sufficiently accurate to act as reference or criterion methods for the molecular approach to measuring body composition (distinguishing fat and fat‐free masses), against which other methods should be evaluated. Several techniques are available, varying in complexity and ease of use, and each making assumptions that may affect its suitability for different conditions. A single technique is unlikely to be optimal in all circumstances. A further important issue is that of the difficulty of validating techniques in humans. In vivo techniques do not measure body composition directly, but rather predict it from measurements of body properties. Thus all techniques suffer from two types of error: methodological error when collecting raw data, and error in the assumptions by which raw data are converted to final values. The relative magnitude of these errors varies between techniques.
The aim of this review is to discuss the theoretical basis, assumptions, and advantages and disadvantages of available techniques. For more information, readers are strongly encouraged to read the more detailed e‐version of this article on the journal website (http://www.archdischild.com/supplemental).
Techniques for measuring body composition
Simple measurements or indices
Traditionally, skinfold thickness measurements have been used to rank individuals in terms of relative “fatness” or to assess the size of specific subcutaneous fat depots.2 Measurements are quick and simple to obtain in most age groups including young infants. In general, intraobserver and interobserver error are low compared to between‐subject variability, but in obese children accuracy and precision are poorer.
The best use of skinfold thickness data is as raw values, where they act as reliable indices of regional fatness. They can be converted into standard deviation score (SDS) format for longitudinal evaluations. However, publication of contemporary children's skinfold reference data remains a current research priority for assessment of relative fatness in patients. Although the contemporary epidemic of obesity presents challenges for body composition reference data, whether individual patients are becoming more or less fat over time can only be assessed through comparison with a reference population. Such data would therefore represent a “reference” (what exists), but not a “standard” (what should exist).
Body mass index (BMI, calculated as weight/height2) is also widely used as an index of relative weight, often expressed as SDS to take into account age and sex.3 In adults, BMI is predictive of clinical outcomes such as type 2 diabetes; however, its predictive value for children and adolescents is less clear. BMI is a global index of nutritional status—used, for example, to categorise both overweight/obesity,4 and eating disorders in combination with psychological criteria5—but its relation with body composition per se is controversial.
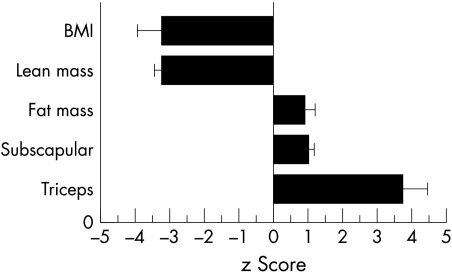
Although correlated with per cent fat,6,7 BMI cannot distinguish fat and lean masses, and there is a twofold range of variation in fatness for a given BMI value in individual children.8 BMI may be particularly misleading in hospital patients, where children apparently “malnourished” in terms of BMI actually have an increase in relative body fat and a severe decrease in lean tissue (fig 1).9 This may be important for their nutritional management, as the low BMI may lead to inappropriate overfeeding.
Figure 1 Data from infant patients with congenital myasthenia, a condition in which the development of connective tissue is impaired. Despite extremely low BMI SDS, the patients have body fat levels higher than the average in healthy children. This paradox can be attributed to extremely low levels of lean mass.9 Though underweight, energy intake is not itself constraining their growth.
Waist circumference (WC) provides a simple measure of central fatness, which may be more predictive of adverse outcomes such as lipid profile or insulin resistance than total fat. In adults, waist–hip ratio is independently associated with morbidity after adjustment for relative weight, such that the use of relative weight and body shape simultaneously gives a better estimate of risk of morbidity than either alone.10,11 Similar findings are now emerging in children.12 Reference data on WC were obtained from a nationally representative sample of United Kingdom children in 1988.13
Studies investigating the relation of WC with measures of abdominal fatness obtained from magnetic resonance imaging (MRI) have shown correlations consistently in the range of 0.5 to 0.8, although the associations with total abdominal fat tend be higher than those with intra‐abdominal fat.14,15,16 In contrast, studies reporting the association the between waist–hip ratio and abdominal fat are inconsistent, and some find no significant relation.14,17
Predictive techniques
Both skinfold thickness and bioelectric impedance measurements can be used to predict body composition. A generic problem with this approach is that it involves not one but two predictions. First, raw measurements are used to predict a body component or property using regression equations; second, this value is converted to final body composition data using further theoretical assumptions. In this section, we address the first of these issues. The second is then addressed in the following section.
Several equations have been derived for the prediction of per cent fat or body density from skinfold thickness measurements.18,19,20,21 Such equations may not be valid in populations other than those from which they were derived. Accuracy in individuals is invariably poor (limits of agreement, ±9% fat), and also varies in relation to body fatness,22,23 making this approach unsuitable for longitudinal comparisons.
Prediction equations inevitably confound accurate raw values with predictive error (standard error of the estimate). Hence, for assessment of fatness, it is better to leave skinfolds in raw form or as SDS, where they act as reliable indices of regional fatness, than to attempt prediction of total fat mass. For assessment of total fat‐free mass (FFM), an approach based on skinfold equations is particularly inappropriate, as no index of this component of weight is directly measured.
Bioelectric impedance analysis (BIA) measures impedance of the body to a small electric current. The generic theoretical model treats the body as a single cylinder, with measurements made between electrodes placed manually on the wrist and ankle. Adjustment of bioelectrical data for height allows estimation of total body water (TBW). In practice, this requires the empirical derivation of regression equations relating height2/impedance to TBW. These equations are then applied subsequently to predict TBW, which is converted to FFM as described below.
BIA incorporates various assumptions. The simplest model, involving hand–foot or foot–foot measurements made at 50 KHz, relies most heavily on these assumptions, and therefore provides the crudest values for body composition. The relation between bioelectrical data and TBW is influenced by the age range investigated and other characteristics of the population. Published BIA equations are population specific and perform poorly in healthy individuals, with errors in individuals typically ±8% fat.23 Paediatric equations have been derived for disease states such as obesity,24 cystic fibrosis,25 and HIV26; however, accuracy in individuals remains poor and measurements may be confounded by clinical status, for example the presence of oedema (see below). Foot–foot measurements, though easier to obtain, have slightly poorer accuracy than whole body measurements.27
Despite these limitations, more sophisticated approaches to BIA have the potential both to improve accuracy and to increase the specificity of outcomes, and such progress is desirable given the ease with which measurements can be made in most age groups. For example, segmental measurements of limbs or torso avoid the confounding effect of variability in body build, while multifrequency measurements provide additional information about water distribution. These are active areas of research but knowledge is currently insufficient to justify routine clinical application.
Conventional BIA analysis is inherently unsuited to the prediction of body fatness in individuals, given that it measures properties of the FFM only. Conventional single frequency BIA does have high precision, providing electrode placement is consistent, and hence could be suitable for assessing short term changes in TBW within individuals. Given that body build is relatively consistent over short time periods even in growing children, such measurements could in turn indicate the direction of changes in FFM, but are unlikely to quantify their magnitude with accuracy. In obesity clinics, BIA might find a role in indicating whether changes in lean mass are in the same direction as body weight, but should not be used to estimate change in fat mass. These issues should be borne in mind as reference data for BIA data become available. At present, therefore, the value of BIA is primarily as an epidemiological technique, where it is the only predictive technique that estimates lean mass.
Two‐component techniques and models
Two‐component methods divide the body into fat mass (FM) and FFM. They avoid two of the difficulties mentioned above, addressing both components of weight and avoiding the need to predict total masses from regional or superficial proxies. However, they remain dependent on theoretical assumptions, such as constancy of the composition of FFM.
Between birth and adulthood, chemical maturation of lean mass occurs, whereby the relative proportions of the three main components (water, protein, and mineral) change with age and pubertal status. In two‐component techniques, these changes are addressed by assuming constant lean mass characteristics for a given age and sex. Such assumptions may largely hold true for healthy subjects but are more problematic in patients who may have deranged body composition or hydration. Various methods are available.
Dual energy x ray absorptiometry
Dual energy x ray absorptiometry (DXA) was developed to measure bone mineral mass, which is calculated from the differential absorption of x rays of two different energies. Because this calculation requires allowance for (and hence quantification of) overlying soft tissue, values of fat and FFM are also calculated for whole body scans, using instrument specific algorithms.
DXA is quick and acceptable for children down to about 4 years, and can also be used in small infants. It uses ionising radiation but the effective dose equivalents of contemporary instrumentation are below background levels. However, although widely used, particularly in the USA, problems with accuracy have received insufficient attention. Bias varies with age and fatness and, in some cases, underlying disease state.28 Comparisons between groups are likely to identify the direction of changes, but fail to quantify the difference accurately, while the accuracy with which changes can be quantified within individuals gaining or losing weight is likewise liable to be confounded by the change in weight status. Thus, although DXA studies increasingly contribute to the evidence base for clinical paediatrics, such studies should be interpreted with caution, and the technique does not represent a reference method.
The limitations of DXA vary according to body shape and outcome. Trunk composition involves substantial prediction rather than measurement, and soft tissue estimation in this area is less accurate than in the limbs. DXA may provide useful information on relative fat and lean masses as a single measurement in an individual, particularly with respect to limb lean mass. However, such assessments are currently hampered by the lack of normal reference data, an issue that is currently being addressed. In contrast to soft tissue outcomes, bone data have good accuracy.
Densitometry
This approach distinguishes FM and FFM, assuming specific densities of these two tissues,29 and therefore requires measurement of total body density (body mass/body volume). While the density of fat is indeed relatively constant, that of FFM varies according to its composition. This variability is partly explained by the process of chemical maturation that occurs before adulthood,30 but interindividual variability is also significant, even in healthy children.23
Traditionally, body volume was measured by hydrodensitometry; however, this approach is clearly unsuitable for many children, especially patients. A new alternative is air displacement plethysmography. The technique measures the volume of air displaced by the subject (fig 2). The technique has better precision than hydrodensitometry in children,31,32 and is acceptable in children as young as 4 years. An infant plethysmograph has also become available, allowing measurement of body volume during the first 6 months of life.33,34
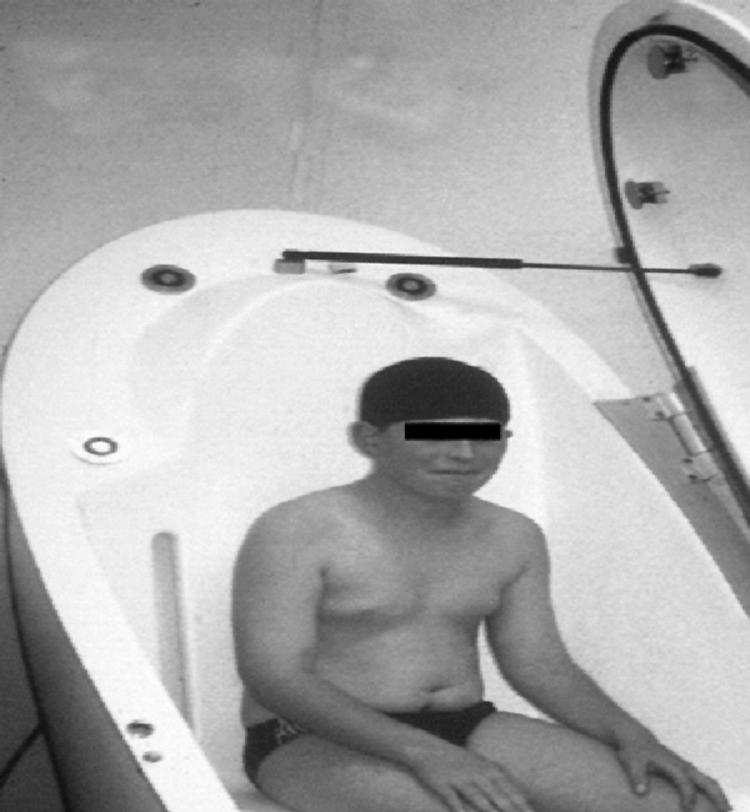
Figure 2 A child sitting inside the measurement chamber of the whole body air displacement plethysmograph, known as the Bodpod (Life Measurements Inc, California). The measurement involves air being gently blown around the subject during two 1 minute measurement periods, while the subject breathes normally. Written permission was obtained from the child's parents for the publication of this photograph.
In general, densitometry is unsuitable for application as a two‐component technique in patients where the composition of lean mass may be abnormal. Typical effects of disease are excess fluid retention and undermineralisation; both decrease the density of lean mass and hence lead to the overestimation of fatness. However, in relative terms, densitometric errors are smaller in larger individuals. Thus, densitometry may prove useful for monitoring changes over time in overweight or obese individuals, and its accuracy is less likely to be confounded by longitudinal changes in fatness than DXA.
Isotope dilution (hydrometry)
Deuterium dilution can be used to measure TBW, allowing estimation of FFM. A dose of water labelled with deuterium is given and, following equilibration, enrichment of the body water pool measured using samples of either saliva, urine, or blood.35 Samples are generally analysed by isotope ratio mass spectrometry; however, clinical services could be based on a substantially cheaper but more labour intensive spectrophotometric technique.36
Estimation of FFM from TBW requires an assumed value for FFM hydration. Published reference values are relatively consistent with measured values in healthy infants and children,37 with between‐individual variability also relatively low.23,38 However, in disease states variability in FFM hydration may be substantially higher, owing to either overhydration or underhydration. In the future, BIA may be used to assess whether hydration is abnormal or not, but for now isotope dilution is best used in populations where the normality of hydration is known or can be assumed.
Isotope dilution is simple to carry out and requires minimal subject cooperation. It has proved particularly valuable in infants and toddlers because of the low compliance required, and can easily be used in field studies. It could prove a useful clinical tool for individuals where normal hydration can be assumed, with rapid provision of results possible using photospectrometric analysis.36
Magnetic resonance imaging
MRI is an imaging technique that estimates the volume rather than the mass of adipose tissue. By analysing the absorption and emission of energy in the radio frequency range of the electromagnetic spectrum, the technique produces images based on spatial variations in the phase and frequency of the energy absorbed and emitted. It primarily addresses hydrogen nuclei, located either in water or fat, and uses these data to discern tissue types in “imaging slices” which can then be summed to calculate regional tissue volumes.
Despite the high quality of imaging data obtained by MRI, there are difficulties in comparing results with those obtained by other techniques. First, in order to derive fat mass, it is necessary to assume the fat content of adipose tissue and the density of fat. While the latter is relatively invariable, the former is not. A second problem is that FM discerned by MRI is only that present in adipose tissue. Thus techniques such as densitometry, hydrometry, or multicomponent models quantify a different entity from MRI, total FM versus adipose tissue mass. Also MRI has relatively high cost and limited availability.
The main advantage of MRI over other techniques is its capacity for estimation of regional body composition, and it is currently the only accurate and viable approach for the estimation of intra‐abdominal adipose tissue. However, recent studies support the use of waist circumference as a robust index of abdominal fat, and a useful index of visceral fat.39
Other techniques
Other techniques include total body electrical conductivity (TOBEC) and whole body potassium scanning (TBK). Both techniques suffer from three main limitations. First, neither is widely available. Second, their outputs show poor agreement with those of other methods, although this applies less to TOBEC than TBK. Third, there are no UK reference data, hindering their clinical application.
Multicomponent models
Multicomponent models minimise the assumptions made in simpler models and are hence best placed to address variability in the composition of lean mass. TBW and bone mineral are measured by techniques specifically designed for those purposes, improving accuracy of those outcomes. Whereas two‐component models assume key body properties, multicomponent models measure them, and can provide data on the hydration, density, and mineralisation of FFM. This is particularly important in paediatric patients, in whom body composition derangement is often extreme. In the absence of carcass analysis, these models are regarded as the gold standard for in vivo measurement: more importantly, they are the only reference or criterion methods against which less accurate methods may be compared. Because of the diverse equipment required, they are only suitable for research, and along with MRI for fat distribution should be regarded as the optimum approach for acquiring the evidence base to underpin clinical practice.
The three‐component model divides body weight into fat, water, and remaining fat‐free dry tissue, and requires measurements of body weight, body water by hydrometry, and body volume by densitometry. The four component model further divides fat‐free dry tissue into protein and mineral, and requires the same data plus measurement of bone mineral by DXA.23,36
The advantages and disadvantages of the various techniques, their availability, and the existence of reference data are summarised in table 1.
Table 1 Summary of assumptions underlying different techniques, their availability, and their main advantages and disadvantages.
| Technique | Assumptions made | Reference data | Availability* | Advantages/disadvantages |
|---|---|---|---|---|
| Skinfolds – raw | Constant skin protein content | Y | +++ | For: simple measure of regional fat |
| Against: no information on lean mass | ||||
| Skinfolds – equations | Skinfolds ∝ whole body fat | N | +++ | For: simple and quick |
| Against: population specific, poor accuracy in individuals and groups | ||||
| Body mass index | Var weight = var fat | Y | +++ | For: simple and quick |
| Against: measures nutritional status not body composition | ||||
| Waist circumference | Waist ∝ central fat | Y | +++ | For: simple, quick, robust measure of abdominal fat |
| Against: not so accurate as measure of internal visceral fat | ||||
| Impedance | Conductivity ∝ body water | N | ++ | For: simple and quick |
| Against: population specific; poor accuracy in individuals and groups | ||||
| DXA | Constant attenuations of FFM and F | N | ++ | For: accurate for limb lean and fat |
| Against: radiation exposure; whole body bias ∝?size, sex, fatness | ||||
| Densitometry† | Constant Dffm and Df | N | + | For: acceptable two‐component technique |
| Against: effects of disease on lean mass reduce accuracy | ||||
| Isotope dilution | Constant Hffm | N | + | For: only technique acceptable in all age groups |
| Against: delayed results; inaccurate if disease affects Hffm | ||||
| MRI | Electromagnetic properties | N | + | For: accurate for regional AT |
| Against: expensive, limited availability, measures AT not fat | ||||
| TOBEC | Conductivity ∝ body water | N | + | For: acceptable two‐component technique |
| Against: rarely available, accuracy unknown | ||||
| TBK | Constant K in cell mass | N | + | For: measures functional component of body composition |
| Against: rarely available, poor accuracy for fatness | ||||
| Multicomponent models | Constant Dprot and Dmin, | N | + | For: most accurate approach, all measurements acceptable |
| constant mineral composition | Against: expensive, specialist research approach |
*Availability: +, low; ++, medium; +++, high.
†Densitometry by air displacement plethysmography (Bodpod)
AT, adipose tissue; Df, density of fat; Dffm, density of fat‐free mass; Dmin, density of mineral; Dprot, density of protein; F, fat; FFM, fat‐free mass; Hffm, hydration of fat‐free mass; K, potassium; Var, variability.
Recommendations
As highlighted above, measurement of body composition in vivo is an imperfect process, subject to various constraints, yet the outcome has clinical value. We offer the following suggestions for the measurement of body composition in paediatric clinical practice.
Simple techniques should not be rejected because they appear unsophisticated. Skinfold measurements and WC provide a simple, easy, and quick yet highly informative assessment of fatness in most patients. DXA is unlikely to improve substantially on this simple approach unless there are difficulties in obtaining skinfold data, and it has poor accuracy for trunk fatness. For assessment of whole body lean mass, both skinfold measurements and BIA have limitations. Application of the combination of these methods may reduce the likelihood of misdiagnosis of high or low lean mass, but will be least successful when applied to disease states where regional tissue distributions differ from those of healthy children.
Though BIA has potential utility in measuring regional body composition, its value in routine clinical paediatrics is at present limited. Predictive error in individuals is high, even when using population specific equations. If used to monitor individuals over time, it can indicate the direction, but not the magnitude, of changes in lean mass. However, such data are difficult to interpret in the absence of reference data.
Whole body data may appear optimal, but in practice regional data may be more informative about clinical condition, as well as more accurate. Many disease states exert disproportionate effects on particular body regions. In obesity, the main concern is central adiposity, so monitoring of WC may provide a better indication of health risk, and response to treatment, than whole body fatness. Whole body lean mass is difficult to measure with simple methods, but DXA is a relatively accurate technique for quantifying limb lean mass. Its value in this role will increase once reference data for these outcomes are published. Whole body data are useful for estimating energy requirements, or the dose size for drug/therapy treatments and dialysis.
Two‐component models are ideal where the aim is to quantify fat or FFM with greater accuracy than that permitted by the simplest methods. For example, whole body lean mass is sometimes used to calculate the quantity of treatment required. However, this approach is only justified if the technique can be assumed robust to likely derangements in FFM composition. Furthermore, while two component techniques are of great value in research, they often may have less value in routine clinical management as they conceal important regional variability within a global whole body value.
Multicomponent models and MRI are ideal for detailed analyses but remain unfeasible in most contexts. Their main value lies in the quality of their evidence in supporting treatment approaches, rather than in routine practical application.
The value of any approach is greatly enhanced by the availability of reference data. The acquisition of such data is a current priority, being addressed by several research groups.
These recommendations are illustrated using two case studies (tables 2 and 3).
Table 2 Illustration of the advantages and disadvantages of different techniques for measuring body composition in clinical practice. The patient is a 14 year old girl referred for assessment and management of obesity with associated type 2 diabetes. Which measurements might be useful in terms of baseline assessment and monitoring of body composition during weight loss?
| Technique | Advantages | Disadvantages | ||
|---|---|---|---|---|
| Simple measurements | ||||
| Skinfold thickness | – | Poor accuracy and precision in obesity | ||
| BMI | Useful as a simple baseline and longitudinal measurement of relative weight | Will not allow assessment of fat and lean masses and changes in fat which may be more relevant in terms of metabolic risk | ||
| Waist circumference | Useful baseline and longitudinal measurement. Centile charts are available, and central fatness is of greater relevance to metabolic risk | |||
| Predictive measurements | ||||
| Skinfold thickness equations predicting FM and FFM | – | Poor precision and accuracy—any error will be magnified by the use of prediction equations not derived from a comparable (obese) population | ||
| Whole body BIA | Could provide information on the direction of longitudinal changes in lean mass | Poor accuracy in absolute terms. Changes in body weight, the relative proportions of trunk and limbs, or FFM hydration with treatment could introduce further errors | ||
| Two‐component techniques | ||||
| DXA | Could be used to measure regional (limb) lean mass which could provide information on whether weight loss is accompanied by changes in lean mass as well as fat mass | Limited use for measuring baseline fat mass or longitudinal changes in fat mass with weight loss, since measurements are known to be biased by body size (thickness) | ||
| Densitometry (BodPod) | Could provide longitudinal data on both lean and fat mass since its accuracy is less likely to be affected by changes in fatness. | Does not provide regional data. | ||
| Deuterium dilution | Could estimate whole body lean mass | Results may be affected by the small differences in FFM hydration in obesity, but these have been quantified and could be adjusted for. Small differences in FFM hydration would introduce minor errors in the short term |
BIA, bioelectric impedance analysis; BMI, body mass index; DXA, dual energy x ray absorptiometry; FFM, fat‐free mass; FM, fat mass.
Conclusions: In practical terms, the techniques most useful in this patient are BMI and waist circumference for monitoring nutritional status and central fat distribution. If available, DXA could be used for assessing changes in limb lean mass, and densitometry or deuterium could also provide longitudinal data for both fat and lean mass.
Table 3 Illustration of the advantages and disadvantages of different techniques for measuring body composition in clinical practice. The patient is a 10 year old boy with chronic renal failure on peritoneal dialysis. Which measurements might be useful in terms of baseline assessment and monitoring of body composition? In this child, measurement of lean mass would be potentially useful as a guide for basing nutritional requirements or drug doses, but measurements may be complicated by oedema or variation in FFM hydration or both.
| Technique | Advantages | Disadvantages | ||
|---|---|---|---|---|
| Simple measurements | ||||
| Skinfold thickness | Useful index of regional fatness | Poorer accuracy and precision in obese/oedematous individuals | ||
| BMI | Useful as a simple baseline and longitudinal measurement of relative weight | Will not allow assessment of fat and lean masses and changes in fat which may be more relevant in terms of nutritional/drug requirements and metabolic risk | ||
| Waist circumference | Useful baseline and longitudinal measurement. Centile charts are available, and central fatness is of greater relevance to metabolic risk | Value may be limited in this child who is on peritoneal dialysis | ||
| Predictive measurements | ||||
| Skinfold thickness equations predicting FM and FFM | – | Poor precision and accuracy—any error will be to magnified by the use of prediction equations not derived from a comparable population | ||
| BIA | Could provide information on the direction of longitudinal changes in lean mass | Poor accuracy in absolute terms. Changes in body weight, the relative proportions of trunk and limbs, or FFM hydration will introduce further errors* | ||
| Two‐component techniques | ||||
| DXA | Could be used to measure regional (limb) lean mass which could provide information on which to base nutritional requirements or drug doses | Limited use for measuring baseline fat mass or longitudinal changes in fat mass, since measurements are known to be biased by body size (thickness). In this child, skinfold thicknesses may already provide information on regional fatness | ||
| Densitometry (BodPod) | Could provide longitudinal data on both lean and fat mass since its accuracy is less likely to be affected by changes in fatness | Variations in the hydration (and therefore density) of FFM in this child will invalidate assumptions of constant density | ||
| Deuterium dilution | Could estimate whole body lean mass if hydration known, and could potentially aid calculation of dialysis fluids | Results for both TBW and FFM may be affected by variation in hydration in this child |
*Multifrequency BIA could provide informative data on hydration in the future.
BIA, bioelectric impedance analysis; BMI, body mass index; DXA, dual energy x ray absorptiometry; FFM, fat‐free mass; FM, fat mass; TBW, total body water.
Conclusions: In practical terms, the techniques most useful in this patient are BMI ± skinfold thickness measurement for monitoring fat mass and distribution. If available, DXA could be used for assessing changes in (limb) lean mass, which might be more relevant for nutritional requirements and drug doses. This technique will be of greater use in practice once reference data are available.
The extended version of this paper is available on the journal website (http://www.archdischild.com/supplemental)
Copyright ©2006 BMJ Publishing Group & Royal College of Paediatrics and Child Health
Supplementary Material
Abbreviations
BIA - bioelectric impedance analysis
BMI - body mass index
DXA - dual energy x ray absorptiometry
FFM - fat‐free mass
FM - fat mass
TBW - total body water
WC - waist circumference
Footnotes
Competing interests: Dr Wells has received equipment gratis from Tanita UK for childhood body composition studies.
Informed consent was obtained for publication of figure 2.
The extended version of this paper is available on the journal website (http://www.archdischild.com/supplemental)
References
- 1.Reilly J J, Methven E, McDowell Z C.et al Health consequences of obesity. Arch Dis Child 200388748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanner J M, Whitehouse R H. Revised standards for triceps and subscapular skinfolds in British children. Arch Dis Child 197550142–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole T J, Freeman J V, Preece M A. Body mass index reference curves for the UK, 1990. Arch Dis Child 19957325–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole T J, Bellizzi M C, Flegal K M.et al Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 20003201240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization ICD‐10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Geneva: WHO, 1992
- 6.Chan Y L, Leung S S F, Lam W W M.et al Body fat estimation in children by magnetic resonance imaging, bioelectrical impedance, skinfold and body mass index: a pilot study. J Paediatr Child Health 19983422–28. [DOI] [PubMed] [Google Scholar]
- 7.Pietrobelli A, Faith M S, Allison D B.et al Body mass index as a measure of adiposity among children and adolescents: a validation. J Pediatr 1998132204–210. [DOI] [PubMed] [Google Scholar]
- 8.Wells J C K. A Hattori chart analysis of body mass index in infancy and childhood. Int J Obesity 200024325–329. [DOI] [PubMed] [Google Scholar]
- 9.Wells J C K, Mok Q, Johnson A W. Nutritional status in children. Lancet 20013571293. [DOI] [PubMed] [Google Scholar]
- 10.Rimm A A, Hartz A J, Fischer M E. A weight shape index for assessing risk of disease in 44,820 women. J Clin Epidemiol 198841459–465. [DOI] [PubMed] [Google Scholar]
- 11.Walton C, Lees B, Crook D.et al Body fat distribution, rather than overall adiposity, influences serum lipids and lipoproteins in healthy men independently of age. Am J Med 199599459–464. [DOI] [PubMed] [Google Scholar]
- 12.Savva S C, Tornaritis M, Savva M E.et al Waist circumference and waist‐to‐height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obes 2000241453–1458. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy H D, Jarrett K V, Crawley H F. The development of waist circumference percentiles in British children aged 5.0–16.9 y. Eur J Clin Nutr 200155902–907. [DOI] [PubMed] [Google Scholar]
- 14.de Ridder C M, de Boer R W, Seidell J C.et al Body fat distribution in pubertal girls quantified by magnetic resonance imaging. Int J Obes 199216443–449. [PubMed] [Google Scholar]
- 15.Fox K, Peters D, Armstrong N.et al Abdominal fat deposition in 11‐year‐old children. Int J Obes 19931711–16. [PubMed] [Google Scholar]
- 16.Owens S, Litaker M, Allison J.et al Prediction of visceral adipose tissue from simple anthropometric measurements in youths with obesity. Obes Res 1999716–22. [DOI] [PubMed] [Google Scholar]
- 17.Brambilla P, Manzoni P, Sironi S.et al Peripheral and abdominal adiposity in childhood obesity. Int J Obes 199418795–800. [PubMed] [Google Scholar]
- 18.Brook C G D. Determination of body composition of children from skinfold measurements. Arch Dis Child 197146182–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston J L, Leong M S, Checkland E G.et al Body fat assessed from body density and estimated from skinfold thickness in normal children and children with cystic fibrosis. Am J Clin Nutr 1988481362–1366. [DOI] [PubMed] [Google Scholar]
- 20.Slaughter M H, Lohman T G, Boileau R A.et al Skinfold equations for estimation of body fatness in children and youth. Hum Biol 198860709–723. [PubMed] [Google Scholar]
- 21.Deurenberg P, Pieters J J L, Hautvast J G A. The assessment of the body fat percentage by skinfold thickness measurements in childhood and young adolescence. Br J Nutr 199063293–303. [DOI] [PubMed] [Google Scholar]
- 22.Reilly J J, Wilson J, Durnin J V G A. Determination of body composition from skinfold thickness: a validation study. Arch Dis Child 199573305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells J C K, Fuller N J, Dewit O.et al Four‐component model of body composition in children: density and hydration of fat‐free mass and comparison with simpler models. Am J Clin Nutr 199969904–912. [DOI] [PubMed] [Google Scholar]
- 24.Wabitsch M, Braun U, Heinze E.et al Body composition in 5–18‐y‐old obese children and adolescents before and after weight reduction as assessed by deuterium dilution and bioelectrical impedance analysis. Am J Clin Nutr 1996641–6. [DOI] [PubMed] [Google Scholar]
- 25.Puiman P J, Francis P, Buntain H.et al Total body water in children with cystic fibrosis using bioelectrical impedance. J Cystic Fibrosis 20043243–247. [DOI] [PubMed] [Google Scholar]
- 26.Arpadi S M, Wang J, Cuff P A.et al Application of bioimpedance analysis for estimating body composition in prepubertal children infected with human immunodeficiency virus type 1. J Pediatr 1996129755–757. [DOI] [PubMed] [Google Scholar]
- 27.Parker L, Reilly J J, Slater C.et al Validity of six field and laboratory methods for measurement of body composition in 10–14 year old boys. Obes Res 200311852–858. [DOI] [PubMed] [Google Scholar]
- 28.Williams J E, Wells J C K, Wilson C M.et al Evaluation of Lunar Prodigy dual‐energy X‐ray absorptiometry for assessing body composition in healthy individuals and patients by comparison with the criterion four‐component model. Am J Clin Nutr. (in press). [DOI] [PubMed]
- 29.Siri W E. Body composition from fluid spaces and density: analysis of methods. In: Brozek J, Henschel A, editors. Techniques for measuring body composition. Washington DC: National Academy of Sciences NRC, 1961223–244.
- 30.Fomon S J, Haschke F, Ziegler E E.et al Body composition of reference children from birth to age 10 years. Am J Clin Nutr 1982351169–1175. [DOI] [PubMed] [Google Scholar]
- 31.Dewit O, Fuller N J, Fewtrell M S.et al Whole‐body air‐displacement plethysmography compared to hydrodensitometry for body composition analysis. Arch Dis Child 200082159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nunez C, Kovera A J, Pietrobelli A.et al Body composition in children and adults by air displacement plethysmography. Eur J Clin Nutr 199953382–387. [DOI] [PubMed] [Google Scholar]
- 33.Urlando A, Dempster P, Aitkens S. A new air displacement plethysmograph for the measurement of body composition in infants. Pediatr Res 200353486–492. [DOI] [PubMed] [Google Scholar]
- 34.Ma G, Yao M, Liu Y.et al Validation of a new paediatric air‐displacement plethysmograph for assessing body composition in infants. Am J Clin Nutr 200479653–660. [DOI] [PubMed] [Google Scholar]
- 35.Davies P S W, Wells J C K. Calculation of total body water in infancy. Eur J Clin Nutr 199448490–495. [PubMed] [Google Scholar]
- 36.Jennings G, Bluck L, Wright A.et al The use of infrared spectrophotometry for measuring body water spaces. Clin Chem 1999451077–1081. [PubMed] [Google Scholar]
- 37.Wells J C K, Fuller N J, Wright A.et al Evaluation of air‐displacement plethysmography in children aged 5–7 years using a three‐component model of body composition. Br J Nutr 200390699–707. [DOI] [PubMed] [Google Scholar]
- 38.Butte N F, Hopkinson J M, Wong W W.et al Body composition during the first 2 years of life: an updated reference. Pediatr Res 200047578–585. [DOI] [PubMed] [Google Scholar]
- 39.Brambilla P, Bedogni G, Moreno L A.et al Crossvalidation of anthropometry against magnetic resonance imaging for the assessment of visceral and subcutaneous adipose tissue in children. Int J Obes 20063023–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.