Abstract
Aims
To review the effect of total splenectomy on lung function and nutrition in children with cystic fibrosis related liver disease (CFLD) and associated portal hypertension. The stated indications for surgery and the short and long term risks of the procedure were also documented.
Method
Over a 25 year period from January 1980 to June 2005, approximately 650 patients with cystic fibrosis (CF) were treated at the Royal Children's Hospital, Melbourne, Australia. Nine patients with CFLD who underwent a splenectomy during that time were identified and their medical records were reviewed.
Results
FEV1% predicted dropped by −16±11% in the two years pre‐splenectomy. This contrasts with the increase in FEV1% predicted of 2±16% in the two years post‐splenectomy (p = 0.05). The cumulative gain in WAZ score (ΔWAZ pre) over the two years prior to splenectomy of 0.045±0.69 was not significantly different from the cumulative gain in WAZ score (ΔWAZ post) for the two years after splenectomy of 0.15±0.36 (p = 0.65). The average age at splenectomy was 14.8 years (SD = 3 years). The average weight of an excised spleen was 983 g (SD = 414 g). There were no deaths associated with splenectomy. The median length of follow up post‐splenectomy was 6.0 years (range 0.7–15.8). There were no episodes of bacterial peritonitis or overwhelming sepsis.
Conclusions
Splenectomy may have a beneficial effect on lung function although this may not manifest itself until the second year post‐splenectomy. Splenectomy in patients with CFLD appears to be a safe procedure.
Keywords: cystic fibrosis, splenectomy, portal hypertension, nutrition, respiratory function tests
As the life expectancy of patients with cystic fibrosis (CF) continues to improve with improving pulmonary and nutritional care,1 associated liver disease is emerging as a challenging clinical entity in its own right. In approximately 5–15% of patients, the liver involvement is extensive resulting in cirrhosis.2 As portal hypertension advances, the associated massive splenomegaly can become the dominant clinical problem in this challenging subgroup of patients. It has been proposed that as the spleen enlarges it begins to impede diaphragmatic excursion, causing dyspnoea and a deterioration in lung function.3,4,5
The primary aim of this study was to review the effect of splenectomy on lung function and nutrition in children with cystic fibrosis related liver disease (CFLD) and associated portal hypertension. We also documented the indications for splenectomy, and its short and long term risks.
Patients and methods
The patient database of the Royal Children's Hospital was reviewed to identify patients with CF who had a splenectomy between January 1980 and June 2005. The medical notes of the identified patients were then reviewed using a pro‐forma template.
The diagnosis of CF was confirmed in all patients on the basis of clinical manifestations and abnormal sweat iontophoresis. The diagnosis of significant liver disease was based on clinical assessment, the finding of a persistently elevated ALT, and ultrasonographic findings consistent with portal hypertension. CFLD was diagnosed only after other causes of liver disease were ruled out, as per the CF Foundation Hepatobiliary Disease Consensus statement guidelines.2
All available spirometry data for two years before and two years after splenectomy were collected. The best FEV1 for each time period (see below) was taken for analysis, as was the most recent FEV1 recorded prior to splenectomy. The height and weight recorded at the time of FEV1 were used for comparison of anthropometric data.
Approval for this clinical audit was obtained from the ethics committees of the participating hospitals.
Statistics
Anthropometric data were converted to z‐score for weight‐for‐age (WAZ) and height‐for‐age (HAZ).6 Measured spirometry values were converted to percent predicted values using Zaplatel's equations.7 Comparison of FEV1, WAZ, and HAZ data for successive years was made using Student's t test. Linear regression analysis was used to allow longitudinal comparisons of lung function. Data were divided into four equal time periods: 2 years before, 1 year before, 1 year after, and 2 years after splenectomy.
Results
From our cohort of 650 patients we identified nine with clinically significant liver disease who underwent a splenectomy. Patient profiles are presented in table 1.
Table 1 Patient profiles.
| Gender | Genotype | Age liver palpable (yr) | Age spleen palpable (yr) | Age at splenectomy (yr) | Splenic weight (g) | |
|---|---|---|---|---|---|---|
| Patient 1 | F | DF508/DF508 | 1.7 | 6.4 | 13.7 | 740 |
| Patient 2 | F | DF508/DF508 | 6.8 | 6.8 | 9.4 | 287 |
| Patient 3 | M | DF508/621+1 | 8.6 | 10.2 | 16.4 | 1421 |
| Patient 4 | F | DF508/DF508 | 10 | 11.6 | 13.5 | 850 |
| Patient 5 | M | DF508/DF508 | NI | 16.7 | 19.3 | 1670 |
| Patient 6 | M | NI | NI | NI | 13.5 | 642 |
| Patient 7 | F | DF508/DF508 | 10.8 | 10.8 | 14.2 | 1035 |
| Patient 8 | M | DF508/? | 11.8 | 11.8 | 18.7 | 1098 |
| Patient 9 | M | G551D/G542X | 6.5 | 7.1 | 14.4 | 1104 |
| Mean | 8 | 10.2 | 14.8 | 983 | ||
| SD | 3.4 | 3.4 | 3.0 | 414.1 |
NI, no information available; ?, unidentified mutation; SD, standard deviation.
All patients were pancreatic insufficient and were chronically colonised with Pseudomonas aeruginosa. All nine patients had a total splenectomy, two were performed laparoscopically (patients 2 and 7), and none had a porto‐systemic shunt created.
Indications
The stated indications for splenectomy extracted from the clinical files are presented in table 2.
Table 2 Stated indications for splenectomy.
| Indication | |
|---|---|
| 1 | Low platelet count (5) |
| 2 | Risk of splenic rupture (4) |
| 3 | Hypersplenism (4) |
| 4 | Portal hypertension (2) |
| 5 | Haemoptysis (1) |
| 6 | Risk of infection (1) |
| 7 | Risk of bleeding (1) |
| 8 | Impaired lung function (1) |
| 9 | Abdominal pain and discomfort (1) |
| 10 | Recurrent splenic infarcts (1) |
Number of occurrences in parentheses.
There was a median of 2 indications per patient (range 1–4). The three most common indications were low platelet count, risk of splenic rupture, and hypersplenism.
Early postoperative complications
Two patients developed atelectasis during their postoperative recovery, which resolved at the one month follow up clinic. There were no deaths associated with splenectomy.
Lung function
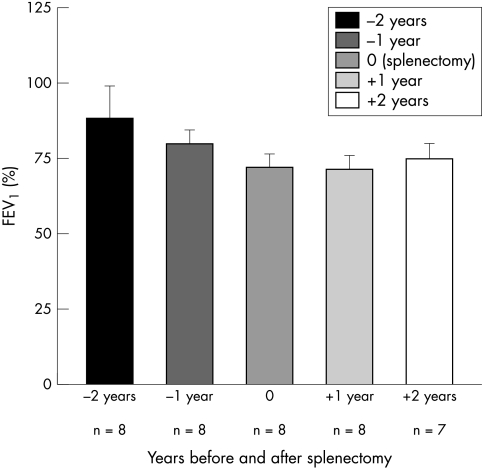
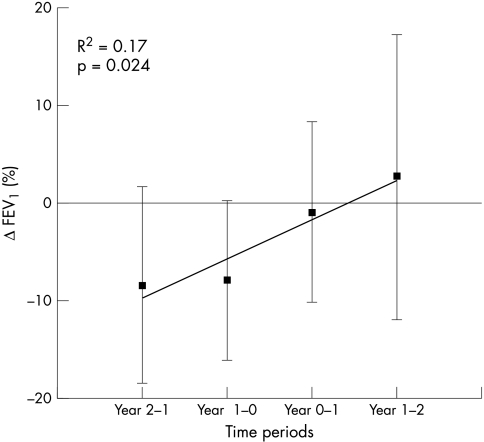
FEV1% predicted data were available for eight patients (see fig 1). The mean FEV1% predicted two years prior to splenectomy was 89±11%. The cumulative loss in FEV1% predicted (ΔFEV1% pre) over the two years prior to splenectomy of −16±11% was significantly different from the cumulative gain in FEV1% predicted (ΔFEV1% post) of 2±16% in the two years post‐splenectomy (p = 0.05). Linear regression analysis showed a progressive improvement in the rate of decline in lung function over the first three years and improvement in the fourth year (fig 2).
Figure 1 Lung function data (FEV1%, mean±SD).
Figure 2 Change in delta FEV1% over the four year study period.
Anthropometric data
The cumulative gain in WAZ score (ΔWAZ pre) over the two years prior to splenectomy of 0.045±0.69 was not significantly different from the cumulative gain in WAZ score (ΔWAZ post) for the two years after splenectomy of 0.15±0.36 (p = 0.65). Similarly there was no significant change in the HAZ score over the four year period (p = 0.96).
Long term follow up
The median length of follow up after splenectomy was 6.0 years (range 0.7–15.8). The patient‐years were combined to give a total time exposed to asplenia of 53.8 years, during which there were no episodes of bacterial peritonitis or overwhelming sepsis.
Six (75%) patients were vaccinated pre‐splenectomy against encapsulated organisms, with insufficient information on the remaining three. Six (75%) patients received penicillin prophylaxis. Two were not on prophylaxis, with insufficient information on the third child.
Two patients died during the follow up period. Patient 4 died 10 years post‐splenectomy from decompensated liver disease after lung transplant. Patient 9 died 1.3 years post‐splenectomy in a motor vehicle accident.
Discussion
The aim of this study was to assess the effect of splenectomy on lung function and anthropometric measurements in children with significant CFLD complicated by portal hypertension. We showed an accelerated decline in lung function prior to splenectomy which was arrested after the spleen was removed. There was no significant change in anthropometric data.
Previous publications report a stabilisation or improvement in lung function after removal of the spleen, but the extent and timing of this improvement have not been described.3,4,5,8
The mean FEV1% predicted in the two years prior to surgery declined by 8% per annum; approximately four times the 2% rate for the CF population taken as a whole.1 This accelerated decline halted post‐splenectomy. Noble‐Jamieson et al described a similar pattern in a group of 11 patents with CFLD who underwent liver transplant.9 This may be due to relief of the mass effect of the spleen splinting the diaphragm.3,5,9 Our patients were not considered for liver transplant as they did not have severe variceal disease and their synthetic liver function remained adequate.2 All our patients were chronically colonised with pseudomonas, which is a plausible alternative explanation for their more rapid decline in FEV1 pre‐splenectomy, but does not explain the improvement post‐splenectomy. We acknowledge there is a selection bias in the design of this study as we do not have a control group who did not undergo splenectomy, and so we are unaware of what the natural clinical course would be if treated conservatively. However, the general trend in the CF population is a regular deterioration in lung function, so it is somewhat surprising that the FEV1 in our cohort plateaued post‐splenectomy.
Our findings are consistent with previous publications which show no single absolute indication for splenectomy. Instead a combination of factors tips the risk:benefit ratio in favour of splenectomy.2,4,5,10 Indeed there is a significant degree of overlap between many of the indications in table 1, and one could challenge the validity of some. For example, it has been shown previously that a low platelet count with portal hypertension is not associated with increased risk of bleeding from oesophageal varices, or other causes.11,12,13
What is already known on this topic
Abdominal surgery can have a detrimental effect on lung function in patients with cystic fibrosis
Splenectomy may play a role in the treatment of patients with cystic fibrosis related liver disease (CFLD) with associated portal hypertension; splenectomy is associated with an increased risk of overwhelming bacterial infection
What this study adds
The accelerated rate of decline in lung function in the two years pre‐splenectomy appears to be arrested post‐splenectomy
There are multiple indications for performing splenectomy in patients with CFLD; splenectomy performed for CFLD can be safe both in terms of initial postoperative recovery and long term risk of overwhelming bacterial infection.
The risk of overwhelming bacterial infection in children post‐splenectomy has been known for over 50 years.14,15,16 In 53.8 cumulative patient‐years there were no episodes of overwhelming sepsis in our case series. This may be partially explained by the frequent use of antibiotics in patients with CF. Despite this, significant bacterial infection remains a risk for many years after removal of the spleen and ongoing vigilance seems prudent.15
In conclusion, splenectomy in patients with CFLD appears to be a safe procedure when performed in a centre with extensive experience caring for such patients. There is no absolute indication for splenectomy; instead a combination of factors tips the balance in favour of removing the spleen. We suggest that an accelerated decline in lung function in patients with massive splenomegaly could be considered as an additional indication for splenectomy. Although difficult, a large multicentre prospective trial would be needed to assess the true effect of splenectomy on symptomatology, nutrition, and lung function.
Abbreviations
CF - cystic fibrosis
CFLD - cystic fibrosis related liver disease
FEV1 - forced expiratory volume in 1 second
HAZ - z‐score for height‐for‐age
WAZ - z‐score for weight‐for‐age
Footnotes
Competing interests: none
References
- 1.Cystic Fibrosis Foundation Patient Registry 2003 Annual Report. Bethesda, MD 2004
- 2.Sokol R J, Durie P R. Recommendations for management of liver and biliary tract disease in cystic fibrosis. Cystic Fibrosis Foundation Hepatobiliary Disease Consensus Group. J Pediatr Gastroenterol Nutr 199928S1–13. [DOI] [PubMed] [Google Scholar]
- 3.Zach M S, Thalhammer G H, Eber E. Partial splenectomy in CF patients with hypersplenism. Arch Dis Child 200388649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westwood A T, Millar A J, Ireland J D.et al Splenectomy in cystic fibrosis patients. Arch Dis Child 2004891078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thalhammer G H, Eber E, Uranus S.et al Partial splenectomy in cystic fibrosis patients with hypersplenism. Arch Dis Child 200388143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dibley M J, Goldsby J B, Staehling N W.et al Development of normalized curves for the international growth reference: historical and technical considerations. Am J Clin Nutr 198746736–748. [DOI] [PubMed] [Google Scholar]
- 7.Zapletal A, Motoyama E K, Van de Woestijne K P.et al Maximum expiratory flow‐volume curves and airway conductance in children and adolescents. J Appl Physiol 196926308–316. [DOI] [PubMed] [Google Scholar]
- 8.Louis D, Chazalette J P. Cystic fibrosis and portal hypertension interest of partial splenectomy. Eur J Pediatr Surg 1993322–24. [DOI] [PubMed] [Google Scholar]
- 9.Noble‐Jamieson G, Barnes N, Jamieson N.et al Liver transplantation for hepatic cirrhosis in cystic fibrosis. J R Soc Med 19968931–37. [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly D A. Commentary. Arch Dis Child 200388145–146. [Google Scholar]
- 11.Peck‐Radosavljevic M. Hypersplenism. Eur J Gastroenterol Hepatol 200113317–323. [DOI] [PubMed] [Google Scholar]
- 12.Basili S, Ferro D, Leo R.et al Bleeding time does not predict gastrointestinal bleeding in patients with cirrhosis. The CALC Group. Coagulation Abnormalities in Liver Cirrhosis. J Hepatol 199624574–580. [DOI] [PubMed] [Google Scholar]
- 13.Goulis J, Armonis A, Patch D.et al Bacterial infection is independently associated with failure to control bleeding in cirrhotic patients with gastrointestinal hemorrhage. Hepatology 1998271207–1212. [DOI] [PubMed] [Google Scholar]
- 14.Diamond L K. Splenectomy in childhood and the hazard of overwhelming infection. Pediatrics 196943886–889. [PubMed] [Google Scholar]
- 15.Eraklis A J, Kevy S V, Diamond L K.et al Hazard of overwhelming infection after splenectomy in childhood. N Engl J Med 19672761225–1229. [DOI] [PubMed] [Google Scholar]
- 16.Goldstone J. Splenectomy for massive splenomegaly. Am J Surg 1978135385–388. [DOI] [PubMed] [Google Scholar]