Abstract
Treatment of head louse infection is primarily through topical insecticides. However, there is growing evidence of resistance. A representative population sample was tested using biochemical and molecular methods; it was shown that, in Wales, treatments containing pyrethroids are likely to be less effective in controlling head louse infection than those containing organophosphates.
Keywords: head lice, surveillance, insecticide resistance
Two classes of insecticides are commonly used for the treatment of head louse infections in the United Kingdom: organophosphates (malathion) and pyrethroids (permethrin and phenothrin). These formulated insecticides are available for purchase in community pharmacists, with pyrethroids the most widely used due to shorter contact time and less odour. A third class of insecticide, carbamates (carbaryl) is available in the UK on prescription only.
Insecticide resistance is often observed in insect populations where insecticides are heavily used, and resistance to pyrethroids and malathion has now been reported in UK head louse populations.1 Resistance to insecticides is conferred by a limited number of mechanisms in all species of insect analysed to date. These tend to involve either metabolic detoxification of the insecticide before it reaches its target site, or changes in sensitivity of the target site, so that it is no longer as susceptible to insecticide inhibition. In order to assist the Welsh Assembly Government in providing recommendations on the treatment and control of head louse infection in Wales, we carried out a cross‐sectional survey of insecticide resistance in head lice affecting primary school children.
Methods
A 3% random sample of schools was drawn in three of the five health authority areas in Wales from a list of 1644 local education authority run primary schools in January 2000 (total 288 387 pupils, mean 175 pupils per school). Pupils at the selected schools whose parents had given informed consent for participation were screened with detection combs on dry hair using a standard protocol.2 A case was defined by the presence of at least one living head louse. Parents of children infected with head lice were informed and all children were given a Welsh Assembly Government fact sheet on head lice control and treatment.
All lice collected during the screen were frozen at −70°C within six hours and transferred frozen to Liverpool School of Tropical Medicine (LSTM). At LSTM biochemical assays were carried out on individual head lice to assess the activity of glutathione S‐transferase, monooxygenases, and esterases. A PCR based diagnostic protocol was developed at LSTM to monitor the frequency of pyrethroid resistance in lice populations.
Results
Thirty one of the thirty two (97%) schools randomly selected agreed to participate. Schools recruited to the study were representative of those in the areas of Wales studied, in terms of both size (p = 0.27) and proportion of pupils eligible for free schools meals (p = 0.12), but in relation to Wales as a whole, were smaller (p = 0.02) and had fewer children eligible for free schools meals (p = 0.02). Of 4045 children enrolled in the 31 schools recruited, 69% (2793) were screened for head lice. Live head lice were detected in 231 of children screened (prevalence 8.3%; 95% CI 7.3–9.4%).
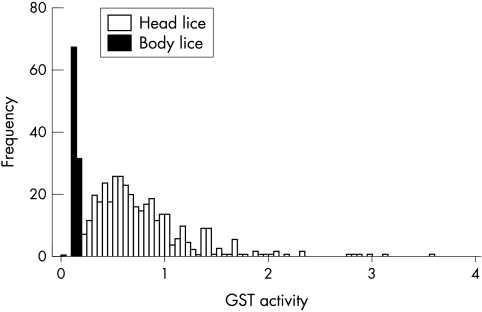
Biochemical assays were carried out on 393 head lice to assess the activity of glutathione S‐transferase, monooxygenases, and esterases. Glutathione S‐transferase (GST) activity, associated with primary DDT resistance and secondary pyrethroid resistance, ranged from 0.17 to 3.60 nmol/min/mg (mean 0.81, median 0.69) and was higher than that previously reported in a laboratory maintained susceptible population of body lice (p < 0.001) (fig 1).3 However, microsomal monooxygenase quantity (mean 0.00083 nmol/min/mg, median 0.00048, range 0.0000364–0.0074) and total esterase activity (mean 0.00019 nmol/min/mg, median 0.00014, range 0.00001–0.00154 with α‐naphthyl acetate as substrate; mean 0.00018 nmol/min/mg, median 0.00014, range 4.09e−7–0.00119 with β‐naphthyl acetate as substrate), both of which are associated with organophosphate resistance, were lower than in susceptible lice (p < 0.001).
Figure 1 Glutathione S‐transferase activity distribution in head lice collected from primary school children in Wales compared to an insecticide susceptible laboratory strain of body lice. Data taken from Hemingway et al.3
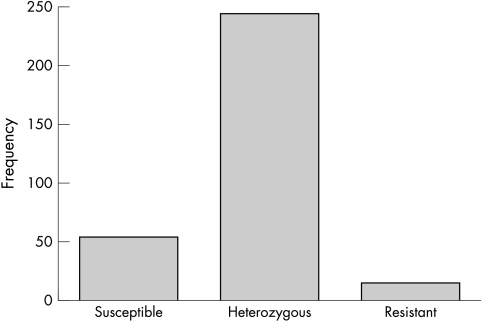
The frequency of pyrethroid resistance genes was measured in 316 lice. Fifty five of these lice (17.4%) were homozygous susceptible for pyrethroid and DDT target site sensitivity, 17 (5.4%) were homozygous resistant, and 244 (77.2%) were heterozygotes (fig 2).
Figure 2 Molecular detection of pyrethroid/DDT resistance in 316 lice collected from children.
Discussion
This paucity of data on the epidemiology of head lice in the UK has hampered the development of a sound evidence based approach to control and treatment.
In the past, studies of insecticide resistance in head lice have been hampered by the lack of success in maintaining colonies of lice in the laboratory, and the difficulties in measuring effectiveness directly by observing the effect of insecticides on small numbers of live lice in vitro. Molecular and biochemical techniques, developed for measuring insecticide resistance in mosquitoes, have now been applied to head lice. These techniques have the advantage of accommodating larger samples and can be used on lice stored frozen after collection, although these techniques have not previously been applied to a systematically collected representative sample. This is the first large scale systematic survey of head lice prevalence and insecticide resistance status in a representative population sample of primary school children.
Metabolic resistance in individual insects can be detected through increased quantities of enzyme, most commonly glutathione transferases, monooxygenases, or esterases. By these methods we found evidence of resistance to DDT and pyrethroids, but not organophosphates. Pyrethroids act primarily on the nervous system where the target site is the voltage sensitive sodium channel. Target site resistance, termed “knock‐down resistance” (kdr), can be identified by the presence of a mutation in a region of the sodium channel. Each louse contains two copies (alleles) of the sodium channel gene and can be scored as homozygous resistant (two copies of the resistant allele), homozygous susceptible (two susceptible alleles), or heterozygous (one copy each of resistant and susceptible alleles). A normal wild type insecticide susceptible population of insects would contain less than one in a million individuals carrying a resistance associated mutation.
The over‐representation of heterozygotes in this population suggests that the population is currently under active selection pressure. As relatively few lice were homozygous for the resistant allele there may be a substantial fitness cost associated with this genotype in the absence of insecticide selection. As homozygous resistant or heterozygous lice are regarded as resistant to pyrethroids, this means that four out of five head lice affecting Welsh primary school children are resistant to pyrethroids.
The 8% point prevalence observed in this study is probably typical for primary children the United Kingdom. Whether the findings on resistance can be applied to other parts of the United Kingdom will depend largely on local patterns of insecticide usage. It is important to note that in this study resistance was measured to the active ingredients and not the formulated product. Formulations may vary slightly by country. In the United States, for example, the formulation of malathion based insecticides differs to that in the United Kingdom, containing isopropyl alcohol, a chemical which is reported to have pediculicidal properties.4
In conclusion, we would recommend that, in Wales, products containing organophosphates, rather than pyrethroids, be used as first line treatment for head louse infection in Wales. Since carrying out this study, a new product, 4% dimeticone lotion, has become licensed for use against head louse infection in the UK, and has been shown to be as effective and safe as phenothrin.5 Where rates of resistance to pyrethroids are high, such as appears to be the case in Wales, this silicone based insecticide may be a suitable alternative. No data are yet available on the relative effectiveness of dimeticone and malathion in the management of head louse infection.
Acknowledgements
This paper is dedicated to the late Dr Fran Collins. Dr Bill Smith conceived the project. The project would not have been possible without the participation of School Nursing staff and the cooperation and support of all head teachers and staff at the schools visited. Richard Lewis and Claire Nash provided administrative support. Ethical approval to carry out the work was provided by the All Wales MREC and LRECs in the areas participating. The study was part funded by the Wales Office of R&D for Health and Social Care.
Footnotes
Competing interests: none
References
- 1.Downs A M R, Stafford K A, Hunt L P.et al Widespread insecticide resistance in head lice to the over‐the‐counter pediculocides in England, and the emergence of carbaryl resistance. B J Dermatol 200214688–93. [DOI] [PubMed] [Google Scholar]
- 2.Roberts R J, Casey D, Morgan D A.et al Comparison of wet combing with malathion for treatment of head lice in the UK: pragmatic randomized controlled trial. Lancet 2000356540–544. [DOI] [PubMed] [Google Scholar]
- 3.Hemingway J, Miller J, Mumcuoglu K Y. Pyrethroid resistance mechanisms in the head louse Pediculus capitis from Israel: implications for control. Med Vet Entomol 19991389–96. [DOI] [PubMed] [Google Scholar]
- 4.Burkhart C N, Burkhart C G. Head lice revisited: in vitro standardized tests and differences in malathion formulations. Arch Dermatol 2004140488–489. [DOI] [PubMed] [Google Scholar]
- 5.Burgess I F, Brown C M, Lee P N. Treatment of head louse infestation with 4% dimeticone lotion: randomised controlled equivalence trial. BMJ 20053301423–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]