Abstract
Persistent lower airway infection with inflammation is the major cause of morbidity and mortality in cystic fibrosis. This review examines the recent advances in the understanding of airway inflammation in cystic fibrosis, and focuses on the evidence that pulmonary inflammation is, under some circumstances, disassociated from infection, and the potential implications for therapeutic intervention.
Keywords: cystic fibrosis, infection, inflammation
The quality of life of children with cystic fibrosis (CF) has improved significantly over the last 20 years.1 However, the decline in lung function resulting from chronic inflammation remains a major problem.2 The underlying cause of reduced survival in CF is an inactive or inefficient functioning of the cystic fibrosis transmembrane conductance regulator (CFTR). CFTR dysfunction results in low levels of airway surface fluid volume, and impaired mucociliary function.1 Microorganisms such as Pseudomonas aeruginosa find a niche in this milieu by a variety of strategies including mucoid exopolysaccharides production and biofilm formation.1 Standard therapy is therefore directed at treating infection. However, recent data suggest that, under some circumstances, pulmonary inflammation in CF may be disassociated from bacterial infection. This opens up the possibility of targeting airway inflammation per se. To assess the potential for this approach, we review the evidence that: (1) lung inflammation causes lung damage in CF; (2) inflammation persists after clearance of bacteria by antimicrobial treatment; and (3) lung inflammation occurs before persistent microbial infection is established.
Pulmonary inflammation and lung damage
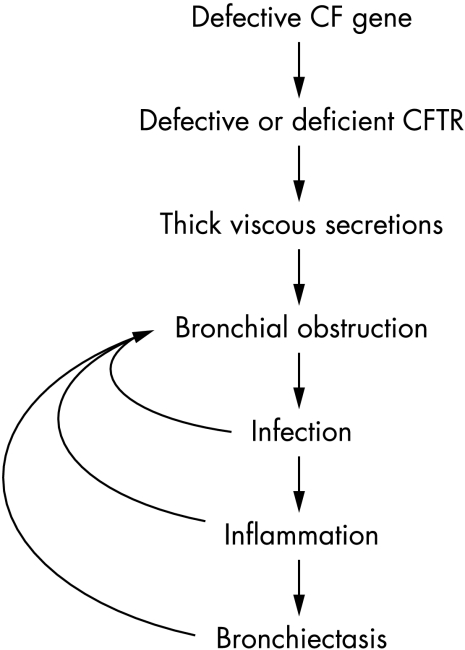
There is good evidence to support the paradigm that tissue damage in CF is due to mediators released by neutrophils that have transmigrated into the lung in response to chronic infection (fig 1). For example, Sagel et al3 reported that airway neutrophils are 10 times higher in CF children with infected sputum compared with healthy controls, and that the airway neutrophilia is associated with elevated levels of mediators that increase the migration of blood neutrophils into the lung (e.g. interleukin‐8; IL‐8), and damage lung tissue (neutrophil derived elastase).3 Antimicrobial therapy, by reducing the stimulus for neutrophil migration into the lung,4 thus attenuates lung tissue injury.
Figure 1 Traditional view of pathology of lung disease in CF. Inflammation is triggered by chronic bacterial infection.
Disassociation of inflammation from infection
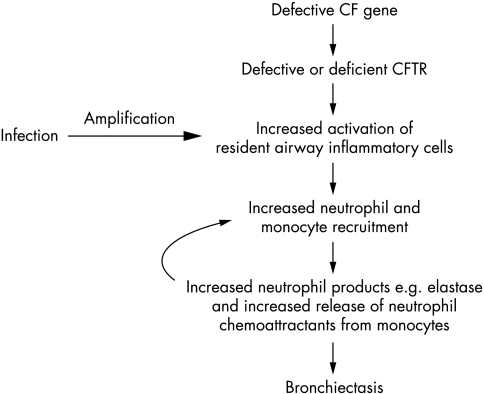
If bacterial infection is the only driver for inflammation in CF, complete clearance of bacteria should re‐establish a normal inflammatory milieu, and inflammation should not occur before the first bacterial infection. However, recent data suggest that there may be a disassociation between infection and inflammation within the CF airway. Muhlebach et al5 obtained bronchoalveolar lavage (BAL) fluid from CF and non‐CF children, with and without evidence of lower airway bacterial infection. Non‐infected and infected CF children had higher numbers of airway neutrophils compared to their controls, suggesting that the CF airway may be in a “pro‐inflammatory” state—i.e. primed for an excessive and prolonged inflammatory response to bacterial infection (fig 2). There is some indirect evidence that priming may occur before the first bacterial infection. First, Hubeau et al6 examined the lungs of 21 CF and 16 non‐CF fetuses, and found increased numbers of macrophages, a major source of neutrophil chemoattractants,1 infiltrating the CF airway before birth. Second, Pfeffer et al7 cultured blood monocytes from CF adults and controls in heterologous serum (to remove any influence of circulating stimuli), and found increased release of tumour necrosis factor alpha (TNF‐α) from CF monocytes. Third, Thomas et al8 reported that the macrophages derived from bone marrow cells in the CF mouse model exhibit four times higher levels of TNF‐α m‐RNA compared to cells from non‐CF wild‐type mice. In addition, airway tissue grafts from infection naïve CF fetal mice, when transplanted into mice with severe combined immunodeficiency, release increased levels of IL‐8,9 which over the long term, increases epithelial breakdown and luminal inflammation.9 Direct sampling studies of young infants also suggest that abnormal airway inflammation may occur before the bacterial colonisation is established. Using bronchoalveolar lavage (BAL), Khan et al10 found increased numbers of airway neutrophils in seven CF infants without any evidence of bacterial, viral, or fungal infection at the time of lavage. In a serial BAL study, Balough et al11 found that the majority of culture negative samples from children with CF contained high numbers of neutrophils, and Rosenfeld et al12 found evidence of neutrophilic inflammation in BAL samples from young children (<15 months age) with no clinical symptoms or detectable infection. An important limitation of these studies is that subject selection was based on a previous history of lower respiratory tract symptoms. Indeed, in a study which recruited 46 newly diagnosed CF infants, Armstrong et al13 reported that neutrophil counts in the BAL fluid in definitive infection naïve children were similar to the non‐CF controls. Furthermore, a follow up study found no significant differences in pro‐inflammatory mediators between infection naive CF infants and controls.14 However, these data are not the last word on whether priming occurs before the first infection, since 6/19 of the control group of Armstrong et al13 either had a confirmed infection in the BAL fluid, or developed respiratory tract symptoms within 48 hours of bronchoscopy. In summary, there is good evidence from animal and human lavage studies that inflammation in the CF airway is disproportionate to the levels of bacterial infection, but whether airway inflammation occurs before the first infection, remains unclear.
Figure 2 Potential alternative mechanism for airway inflammation in CF.
Early inflammation and lung damage
Lung inflammation in the first years of life, whether resulting from intrinsic priming of lung cells, or in response to infection, may be clinically significant. High resolution computed tomography (HRCT) studies have shown dilated and thickened airways in the very early stages of CF,15 which could either be due to inflammation, or due to mucus plugging per se. A role for inflammation is suggested by the study of Dakin et al16 who measured lung function and pulmonary inflammation in 22 children (age range 6.7–44 months, mean 23.2 months), of whom 17 (77%) had chronic respiratory symptoms. The authors found that specific compliance and functional residual capacity were inversely correlated with proportion of neutrophils and concentration of IL‐8 in the BAL fluid.16 A similar inverse correlation between lung function and neutrophilic inflammation has been reported for stable asymptomatic young CF children using the low frequency forced oscillation technique.17 In contrast, Nixon et al18 showed no correlation between the concentration of airway neutrophils and IL‐8 and lung function in a group of young stable CF children, 81% of whom were diagnosed by neonatal screening. This lack of consistency between studies may be due to subject selection, concomitant antibiotic therapy, and differences in lung function measurement techniques. Although there are no definitive data to prove that early inflammation is a major factor in early lung function changes, it has not been completely excluded.
New insights from other respiratory diseases
Chronic obstructive airways disease (COPD) in adults shares some features with CF. In both conditions, there is a complex interaction between infection and inflammation, leading to irreversible lung damage. In both COPD and CF, resident alveolar macrophages, via the release of IL‐8 are considered to be a key cell in modulating the transmigration of circulating neutrophils into the lung. A recent study of adults with COPD found that a population of “small” airway macrophages also may be an important stimulus for neutrophil migration.19 “Small” airway macrophages are thought to derive from recently migrated monocytes, and exhibit an increased capacity to release pro‐inflammatory mediators.19 These cells are distinguished from resident alveolar macrophages by their small size and by increased expression of CD14, the receptor for lipopolysaccharide. Thus a putative sequence of events is that is that resident alveolar macrophages in CF are primed by infection (or by intrinsic abnormalities) to release chemoattractants for monocytes. Newly recruited monocytes are primed to release high levels of neutrophil chemoattractants, and thereby amplify neutrophil migration. Indeed, in a pilot study, we found increased concentrations of “recruitable” monocytes (CD14+CD16+) in the peripheral circulation of CF patients compared to controls.20
Implications for clinical practice and future research
To date, the focus for CF therapy has been treating infection, and thereby attenuating the stimulus for persistent neutrophilic inflammation. If, however, the association between active infection and neutrophilic inflammation is less clear cut, anti‐inflammatory drugs may be of use in infants without established bacterial infection, and blocking the mechanisms that recruit neutrophils into the airway may be clinically efficacious. Targeting other immune cells such as T cells is another option, since T lymphocytes cultured from whole blood of young asymptomatic CF patients show a higher production of the pro‐inflammatory cytokine IL‐2 following stimulation.21
Specific therapeutic options
Corticosteroids are powerful anti‐inflammatory drugs, and systemic steroids may slow down the decline of lung function in CF.22 Unfortunately, systemic steroids are associated with an increased incidence of adverse effects such as growth retardation, development of diabetes, and cataracts; inhaled steroids, though safer, are not as effective.23 A recent systematic review of high dose ibuprofen (a non‐steroidal anti‐inflammatory agent) found some beneficial effects on the decline in lung function with treatment of up to three months, predominantly in children less than 13 years of age. However, the review recommended that “further work needs to be undertaken to confirm these findings”, and there are major concerns about side effects such as gastrointestinal bleeding and renal failure.24 It has been speculated that recombinant DNase may attenuate neutrophil mediated lung injury,25 but a randomised control trial (36 months of treatment with DNase), found no reduction in BAL neutrophil counts in children with mild CF lung disease.25 Therapies with dual anti‐infective and anti‐inflammatory activity, such as azithromycin are currently being evaluated, with some evidence to suggest that a six month course improves lung function.26 The improvement in lung function with long term azithromycin therapy is clinically small,26 and side effects such as diarrhoea may limit efficacy.
A great deal is known about the components of the inflammatory cascade that lead to neutrophil transmigration into the lung, and the options for selectively blocking individual components of this cascade are expanding rapidly. For example, a human monoclonal IgG2 antibody directed against human IL‐8 (ABX‐IL‐8) has shown promise in adults with chronic obstructive airways disease.27 Genistein, an isoflavanoid tryrosine kinase inhibitor, when combined with 4‐phenylbutyrate, increases the activity of CFTR and inhibits IL‐8 production. This combination is currently in phase II trials in adults with CF.28 Alpha‐1 antitrypsin neutralises neutrophil derived elastase, a histotoxic mediator released from neutrophils; it is safe to deliver to humans by nebulisation, but efficacy has not been established.29 The recent disaster in the trial of a fully humanised monoclonal antibody designed to bind to CD28,30 shows that manipulation of the immune system should be done with great caution. Anti‐inflammatory treatments could, for example, attenuate the useful effects of inflammation. A recent phase II clinical trial of BIIL 284 BS, a leukotriene B4 receptor antagonist in CF, was stopped prematurely because of an increase in pulmonary infective exacerbations in the treatment group.31 More studies are therefore needed to determine the relationship between inflammation and infection in CF, in order to inform researchers of the potential adverse (and beneficial) effects of novel anti‐inflammatory therapies.
In summary, while appropriate nutritional supplementation and aggressive management of airway infection remains the cornerstone of CF treatment, a renewed therapeutic focus on the initiation and regulation of airways inflammation is justified by data showing the persistence of inflammation in the absence of infection in the early stages of the disease. However, improvements and standardisation in the monitoring of inflammation and assessment of lung function in young children are first needed before anti‐inflammatory therapies can be rationally targeted.
Footnotes
Competing interests: none
References
- 1.Chmiel J, Davis P. State of the Art: Why do the lungs of patients with cystic fibrosis become infected and why can't they clear the infection? Respir Res 200348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weller P. Implications of early inflammation and infection in cystic fibrosis: a review of new and potential interventions. Pediatr Pulmonol 199724143–5 discussion 15961. [DOI] [PubMed] [Google Scholar]
- 3.Sagel S, Kapsner R, Osberg I.et al Airway inflammation in children with cystic fibrosis and healthy children assessed by sputum induction. Am J Respir Crit Care Med 2001164(8 pt 1)1425–1431. [DOI] [PubMed] [Google Scholar]
- 4.Ordoñez C, Henig N R, Mayer‐Hamblett N.et al Inflammatory and microbiologic markers in induced sputum after intravenous antibiotics in cystic fibrosis. Am J Respir Crit Care Med 20031681471–1475. [DOI] [PubMed] [Google Scholar]
- 5.Muhlebach M, Stewart P W, Leigh M W.et al Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am J Respir Crit Care Med 1999160186–191. [DOI] [PubMed] [Google Scholar]
- 6.Hubeau C, Puchelle E, Gaillard D. Distinct pattern of immune cell population in the lung of human fetuses with cystic fibrosis. J Allergy Clin Immunol 2001108524–529. [DOI] [PubMed] [Google Scholar]
- 7.Pfeffer K, Huecksteadt T, Hoidal J. Expression and regulation of tumor necrosis factor in macrophages from cystic fibrosis patients. Am J Respir Cell Mol Biol 19939511–519. [DOI] [PubMed] [Google Scholar]
- 8.Thomas G, Costelloe E A, Lunn D P.et al G551D cystic fibrosis mice exhibit abnormal regulation of inflammation in lungs and macrophages. J Immunol 20001643870–3877. [DOI] [PubMed] [Google Scholar]
- 9.Tirouvanziam R, de Bentzmann S, Hubeau C.et al Inflammation and infection in naive human cystic fibrosis airway grafts. Am J Respir Cell Mol Biol 200023121–127. [DOI] [PubMed] [Google Scholar]
- 10.Khan T, Wagener J S, Bost T.et al Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med 19951511075–1082. [DOI] [PubMed] [Google Scholar]
- 11.Balough K, McCubbin M, Weinberger M.et al The relationship between infection and inflammation in the early stages of lung disease from cystic fibrosis. Pediatr Pulmonol 19952063–70. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfeld M, Gibson R L, McNamara S.et al Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr Pulmonol 200132356–366. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong D, Grimwood K, Carzino R.et al Lower respiratory infection and inflammation in infants with newly diagnosed cystic fibrosis. BMJ 19953101571–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstrong D, Hook S M, Jamsen K M.et al Lower airway inflammation in infants with cystic fibrosis detected by newborn screening. Pediatr Pulmonol 200540500–510. [DOI] [PubMed] [Google Scholar]
- 15.Long F, Williams R, Castile R. Structural airway abnormalities in infants and young children with cystic fibrosis. J Pediatr 2004144154–161. [DOI] [PubMed] [Google Scholar]
- 16.Dakin C, Numa A H, Wang H.et al Inflammation, infection, and pulmonary function in infants and young children with cystic fibrosis. Am J Respir Crit Care Med 2002165904–910. [DOI] [PubMed] [Google Scholar]
- 17.Brennan S, Hall G L, Horak F.et al Correlation of forced oscillation technique in preschool children with cystic fibrosis with pulmonary inflammation. Thorax 200560159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nixon G, Armstrong D S, Carzino R.et al Early airway infection, inflammation, and lung function in cystic fibrosis. Arch Dis Child 200287306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frankenberger M, Menzel M, Betz R.et al Characterization of a population of small macrophages in induced sputum of patients with chronic obstructive pulmonary disease and healthy volunteers. Clin Exp Immunol 2004138507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao S, Wright A K A, Ziegler‐Heitbrock L.et al CD14+CD16+ blood monocytes in cystic fibrosis. Thorax 200560(suppl 2)ii93 [Google Scholar]
- 21.Hubeau C, Le Naour R, Abély M.et al Dysregulation of IL‐2 and IL‐8 production in circulating T lymphocytes from young cystic fibrosis patients. Clin Exp Immunol 2004135528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng K, Ashby D, Smyth R. Oral steroids for cystic fibrosis. Cochrane Database Syst Rev 2000CD000407. [DOI] [PubMed]
- 23.Balfour‐Lynn I, Walters S, Dezateux C. Inhaled corticosteroids for cystic fibrosis. Cochrane Database Syst Rev 2000CD001915. [DOI] [PubMed]
- 24.Lands L C, Dezateux C, Crighton A. Oral non‐steroidal anti‐inflammatory drug therapy for cystic fibrosis. Cochrane Database Syst Rev 1999CD001505. [DOI] [PubMed]
- 25.Paul K, Rietschel E, Ballmann M.et al Effect of treatment with dornase alpha on airway inflammation in patients with cystic fibrosis. Am J Respir Crit Care Med 2004169719–725. [DOI] [PubMed] [Google Scholar]
- 26.Southern K W, Barker P M, Solis A. Macrolide antibiotics for cystic fibrosis. Cochrane Syst Rev 2004. CD002203 [DOI] [PubMed]
- 27.Di Stefano A, Capelli A, Donner C. Role of interleukin‐8 in the pathogenesis and treatment of COPD. Chest 2004126676–678. [DOI] [PubMed] [Google Scholar]
- 28.Koehler D, Downey G P, Sweezey N B.et al Lung inflammation as a therapeutic target in cystic fibrosis. Am J Respir Cell Mol Biol 200431377–381. [DOI] [PubMed] [Google Scholar]
- 29.Martin S L, Downey D, Bilton D.et al Safety and efficacy of recombinant alpha1‐antitrypsin therapy in cystic fibrosis. Pediatr Pulmonol 200641177–183. [DOI] [PubMed] [Google Scholar]
- 30.MHRA Investigations in to adverse incidents during clinical trials of TGN1412. A report on the MHRA inquiry. www.mhra.gov.uk
- 31.Konstan M W, Doring G, Lands L C. Results of a phase II clinical trial of BIIL 284 BS (an LTB4 receptor antagonist) for the treatment of CF lung disease. Pediatr Pulmonol 200540(suppl 28)125–126. [Google Scholar]