Short abstract
Sleep may affect energy balance. Sleep may not be the only answer to the obesity pandemic, but its effect should be considered seriously, as even small changes in the energy balance are beneficial. Good sleep could be part of the obesity prevention approach.
We are currently facing a major obesity pandemic. Most alarming is the accelerated increase in overweight and obesity in children, with childhood obesity tracking into adulthood. Although there is a strong genetic contribution to obesity, the current pandemic has been driven by environmental factors. Unfortunately, interventions aiming to alter food selection (eg, less fat and sugar) and calorie intake (eg, smaller portions) and to increase physical activity have not been able to result in long‐term weight loss and maintenance. These approaches are confounded by the fact that only an insignificant daily energy surplus could result in obesity over time. Although changes in the basic balance between energy intake (food calories) and expenditure (physical activity) are obviously responsible for the current obesity pandemic, our understanding of the factors that alter this balance remains incomplete. Intriguingly, sleep may be a factor that alters both sides of the energy balance equation. The precise physiological functions of sleep are unknown, but the contribution of sleep to physical and psychological health, and its social and economic significance, is increasingly recognised.1 Sleep research has mainly concentrated on the cognitive consequences of sleep loss, on the basis of the belief that sleep is for the brain alone. Recently, however, there has been a shift in interest in the consequences of sleep loss for other organs and several physiological systems. Also, more laboratory studies on sleep are now concentrating on investigating the health and performance effects of chronic partial sleep restriction, which is truer of real life, rather than acute total sleep deprivation. On the basis of both population studies and laboratory studies on partial sleep restriction, there is increasing evidence that short sleep duration results in metabolic changes that may contribute to the development of obesity, insulin resistance, diabetes and cardiovascular disease.2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29
Several large population studies have identified an important dose–response relationship between short sleep duration, excess body weight and metabolic disturbances across all age groups and in several ethnic groups.2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,26,27,28,29 Table 1 lists the key findings of the main studies reporting associations between short sleep duration and excess body weight in children and adolescents. The relationship between short sleep duration and obesity seems to be more robust in children, in whom there is a linear dose–response relationship between shorter sleep and increased body weight. Interestingly, an analysis from the Avon Longitudinal Study of Parents And Children in the 1990s in the UK has identified that short sleep duration at an early age of 30 months predicts obesity at age 7 years.20 As it is believed that sleep is important for brain development and plasticity, this suggests that sleep loss at a young age may considerably alter the hypothalamic mechanisms that regulate appetite and energy expenditure.
Table 1 Summary of major studies reporting an association between short sleep duration and obesity.
| First author, publication year, country | Number studied, age group | Design | Key findings |
|---|---|---|---|
| Locard,17 1992, France | 1031, 5‐year‐olds | Cross‐sectional, case–control | Short sleep duration was associated with obesity (OR 4.9); this was independent of watching TV |
| Kagamimori,14 1999, Japan | 9668, 3‐year‐olds | Cross‐sectional, case–control Toyoma cohort study | Frequency of sleeping <10 h was greater in obese children (29.3%) v that in non‐obese children (13.7%) |
| Vioque,27 2000, Spain | 1772, ⩾15‐year‐olds | Cross‐sectional | Prevalence OR for obesity 0.43 (CI 0.27 to 0.67) for sleeping ⩾9 h v ⩽6 h; prevalence OR for obesity was 24% lower for each additional sleeping hour/day |
| von Kries,28 2002, Germany | 6862, 5–6‐year‐olds | Cross‐sectional | Short sleep duration was associated with overweight, obesity and increased body fat percentage; adjusted OR for obesity 0.52 (CI 0.34 to 0.78) for sleeping 10.5–11 h and 0.46 (CI 0.28 to 0.75) for sleeping 11.5 h |
| Sekine,21 2002, Japan | 8274, 6–7‐year‐olds | Cross‐sectional, based on Toyoma cohort study | MOR (adjusted for age, parental obesity, physical activity, watching TV, frequency of eating breakfast, snack frequency) v ⩾10 h was 1.49 (CI 1.08 to 2.14) for 9–10 h, 1.89 (CI 1.34 to 2.73) for 8–9 h and 2.87 (CI 1.61 to 5.05) for <8 h; ORs for boys were greater than for girls |
| Gupta,11 2002, USA | 383, 11–16‐year‐olds | Cross‐sectional Heartfelt study | Only study to use 1‐day actigraphy; short sleep associated with obesity and greater body fat; adjusted OR for obesity decreased by 20% for every hour of increased sleep |
| Agras,2 2004, USA | 150, 9½‐year‐olds | Prospective study of newborn infants from birth to 9.5‐year‐olds | Negative correlation between hours of sleep and overweight (−0.21); overweight children sleep less than 30 min on average than children with normal weight, the difference was mainly in daytime sleep; hours of sleep were negatively correlated with high activity levels |
| Gibson,9 2004, UK | 1294, 7–18‐year‐olds | Cross‐sectional, national diet and nutrition survey of young people | Obese children spent less time in bed (10–20 min 1st quintile of age‐adjusted BMI v 5th quintile), but this was statistically significant only in boys |
| Reilly,20 2005, UK | 8234, 7‐year‐olds | Children of the 90s (ALSPAC) Bristol | 25 factors examined at age 30 months that could predispose to obesity at age 7 years; 8 factors found to be significant, including short sleep duration; OR v 12.5 h of sleep was 1.04 (CI 0.76 to 1.42) for 11–11.9 h; 1.35 (CI 1.02 to 1.79) for 10.5–10.9 h; 1.45 (CI 1.10 to 1.89) for <10.5 h |
| Padez,18 2005, Portugal | 4511, 7–9‐year‐olds | Cross‐sectional | OR v 8 h sleep; overweight: OR 0.46 (CI 0.40 to 0.51) for 9–10 h, 0.44 (CI 0.38‐0.49) for ⩾11 h; obesity: OR 0.44 (CI 0.40 to 0.47) for 9–10 h, 0.39 (CI 0.35‐0.42) for ⩾11 h |
| Knutson,15 2005, USA | 4486, 15–18‐year‐olds | Cross‐sectional, national longitudinal study of adolescent health | Results significant for men only; linear regression: sleep duration significantly predicted BMI z score (β = −0.08, CI −0.12 to 0.03); logistic regression: sleep duration predicted risk of overweight (OR 0.90, CI 0.82 to 1.00) |
| Chaput,3 2006, Canada | 422, 5–10‐year‐olds | Cross‐sectional, “Quebec en Forme” project | MOR of obesity v 12–13 h sleep was 1.42 (CI 1.09 to 1.98) for 10.5–11.5 h sleep, 3.45 (CI 2.61 to 4.67) for 8‐10 h sleep; waist circumference negatively correlated with sleep duration (r = −0.24), but only significantly in boys |
| Chen,4 2006, Taiwan | 656, 13–18‐year‐olds | Cross‐sectional, case–control | High adequate sleep (defined as 6–8h sleep/night on >4 weekdays/week) was associated with non‐obesity, OR 1.74 (CI 1.3 to 2.4) |
ALSPAC, Avon Longitudinal Study of Parents And Children; BMI, body mass index (kg/m2); MOR, multivariate odds ratio, TV, television.
Although supportive data from prospective studies are emerging, most of the studies showing an association between short sleep and obesity have been cross‐sectional and do not prove causality or reverse causality. It may therefore be argued that the association between short sleep duration and changes in body weight is spurious or is a surrogate marker for another factor that impinges on body weight regulation. However, in addition to the extensive epidemiological data suggesting a link between short sleep duration and obesity, recent evidence has begun to suggest a mechanistic link involving metabolic hormones and sympathetovagal balance. In a large adult population sample (Wisconsin Sleep Cohort Study), short sleep duration was shown to be associated with obesity.26 Sleep duration determined by polysomnography and by self‐reported habitual sleep time was associated with changes in metabolic hormones that affect appetite and energy expenditure. Circulating leptin and ghrelin levels, two opposing hormones in appetite regulation, were measured.26 Leptin is released by adipocytes to signal the extent of fat stores to the hypothalamus. Low leptin levels signal an energy deficit and have a much stronger effect than high leptin levels, which is usually observed in obesity, where there is leptin resistance.30 Ghrelin is released mainly by the stomach.31 Its levels are highest before meals and diminish with food intake, suggesting that it signals hunger. Exogenous ghrelin increases food intake. Children with Prader–Willi syndrome, which is associated with voracious appetite, have high circulating ghrelin levels. In the Wisconsin Sleep Cohort Study cohort, short sleep duration was associated with low leptin levels, with a predicted 15.5% lower level for a habitual sleep of 5 h v 8 h, and high ghrelin levels, with a predicted 14.9% higher level for nocturnal (polysomnographic) sleep of 5 h v 8 h.26 These relationships were robust to correction for multiple possible confounding factors including age, sex, body mass index (weight (kg)/height2 (m2)), self‐reported exercise and sleep‐disordered breathing. These hormone changes are usually observed in reaction to food restriction and weight loss, and are typically associated with increased appetite.32 Recently, data from human laboratory studies using the partial sleep restriction paradigm have suggested that sleep restriction is associated with similar changes in leptin and ghrelin levels, changes in other metabolic hormones (eg, cortisol), increased appetite and an increased desire for energy‐dense food.23,24,25,33 This laboratory work suggests that as little as 2–3 nights of sleep restriction in young adults can have profound effects on metabolic hormones.
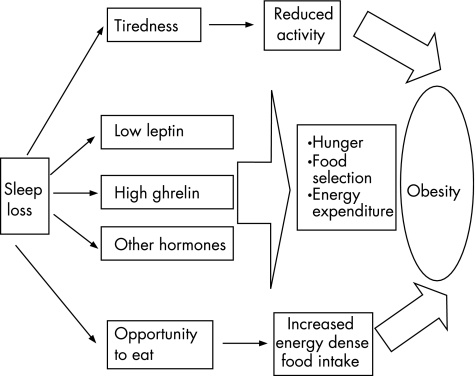
The combination of a large population and laboratory studies points to a novel physiological interaction between sleep and metabolism. Many questions, however, remain to be answered. Most studies investigating the effect of sleep on metabolism have concentrated on adults; hence, little is known about adolescents and younger children who may be most vulnerable to the consequences of sleep loss. Figure 1 shows the potential mechanisms through which short sleep duration may result in obesity. As this interaction is complex, multiple inter‐related factors possibly operate downstream of sleep duration and these combine to result in the observed phenotype. Additionally, obesity and its associated physiological changes can affect sleep adversely, resulting in the development of a vicious cycle. Sleep duration may alter the balance between energy intake and energy expenditure by affecting both sides of the equation. Sleep deprivation results in fatigue and excessive daytime sleepiness. Could this fatigue contribute to reduced daytime physical activity, which many believe is a major contributor to the current obesity pandemic? One study found that about 40% of 12–16‐year‐olds in their survey reported waking up tired34; this could have a serious adverse effect on daily physical activity. Additionally, physical activity has a beneficial effect on sleep, suggesting negative synergy between poor sleep and low physical activity. Sleep loss results in changes in levels of several hormones including leptin, ghrelin, insulin, cortisol, interleukin 6 and growth hormone. Many other potential hormones may also be associated, including peptide tyrosine tyrosine, adiponectin, resistin, visfatin and tumour necrosis factor α. Hormonal changes could contribute to selection of calorie‐dense food, excessive food intake, changes in energy expenditure and insulin resistance. The precise mechanism through which the brain signals the changes in hormone release with sleep deprivation is unknown, but one possibility is increased sympathetic nervous system activity.23,33
Figure 1 The potential mechanisms through which short sleep duration could result in obesity. Short sleep duration can affect both energy intake and energy expenditure. It results in tiredness that may hamper physical activity, and alters metabolic hormones to increase appetite and affect food selection. Additionally, extra time awake provides increased opportunity for food intake. Other potential mechanisms include effects of sleep on basal metabolic rate, thermic effect of food and non‐exercise activity thermogenesis.
Little comprehensive data are available regarding sleep duration over time, but the data available suggest that sleep duration has decreased over the years.33,35 Sleep duration would have declined particularly at the same time as the rise in obesity.35 Studies on children and adolescents suggest that these groups are likely to be sleep deprived. Adolescence is a critical developmental period where sleep deprivation commonly occurs, as there are changes in the biological timing of sleep such that adolescents go to sleep later, which reduces the amount of sleep they get because of the requirements of going to school in the early morning.36 Total sleep need also seems to be greater in adolescents, thus compounding the sleep deprivation. Sleep problems are commonly reported in adolescence (ranging from 5% to 38%), and have considerable morbidity. Sleep deprivation in children and adolescents is believed to be driven by increased television viewing, computer games, and use of the internet and mobile phones, all of which have become progressively available to this age group and are increasingly found in their bedrooms. Television viewing is a factor that is usually found to be associated with obesity. Although this association has been attributed to sedentary behaviour, a recent report from the UK suggests that most television viewing by children is carried out at or near bedtime,37 and television viewing has been reported to adversely affect sleep.38
There is now enough evidence that sleep could have an effect on energy balance. The precise mechanisms for this are currently under investigation. Sleep is probably not the only answer to the obesity pandemic, but its effect should be taken seriously, as even small changes in energy balance are beneficial. It may prove difficult to unequivocally prove a causal relationship between short sleep duration and obesity, as we are dealing with highly complex physiological systems and animal models are inadequate. Some have argued that a trial of weight loss in obese people with sleep extension may provide the necessary proof.29,35 This trial, however, cannot be placebo controlled or blinded, and once a person has become obese, it may be too late. Although sleep extension is certainly possible, few groups have studied the effect of this over a long period. Furthermore, it may be difficult to precisely determine the optimal sleep duration. Nevertheless, recommendations can be made based on sleep duration data from large population samples.39 While we are waiting for additional supportive data, an obesity prevention approach in children and adolescents that promotes a healthy diet, physical activity and adequate sleep could be adopted. Good sleep could be promoted by removal of gadget distractions from bedrooms and restricting their use, observance of strict bedtimes and other sleep hygiene measures (see boxes). Ensuring adequate sleep in children and adolescents may not only help fighting against obesity but also could have other added health and educational benefits—for example, improvements in academic performance.40
Box 1: Sleep hygiene measures
Ensure a regular bedtime routine
Ensure strict bed and wake times
Ensure a quiet, dark and relaxing bedroom environment that is neither too hot nor too cold
Ensure a comfortable bed that is used only for sleeping and not for other activities (eg, reading, watching television or listening to music)
Undertake physical activity but not within a few hours of bedtime
Remove televisions, computers and gadgets from the bedroom
Avoid large meals near bedtime
Box 2: Additional measures for adolescents
Avoid caffeinated drinks after lunchtime
Avoid nicotine, alcohol and drugs
Avoid activities that may be arousing around bedtime (eg, heavy study, computer games, texting on mobile phone or arguing)
Avoid bright light in the evening
Ensure exposure to bright light on awakening in the morning
Allow sleeping in during weekends, but no more than 2–3 h beyond the usual wake time (as this disrupts the circadian clock)
Avoid staying up all night (eg, to study)
Acknowledgements
I thank Julian Shield, Peter Fleming, Andy Ness, John Henderson and the BRIO group (www.bris.ac.uk/brio) working at the University of Bristol for valuable discussions.
Footnotes
Competing interests: None.
References
- 1.Wilson J F. Is sleep the new vital sign? Ann Intern Med 2005142877–880. [DOI] [PubMed] [Google Scholar]
- 2.Agras W S, Hammer L D, McNicholas F.et al Risk factors for childhood overweight: a prospective study from birth to 9.5 years. J Pediatr 200414520–25. [DOI] [PubMed] [Google Scholar]
- 3.Chaput J P, Brunet M, Tremblay A. Relationship between short sleeping hours and childhood overweight/obesity: results from the ‘Quebec en Forme' Project. Int J Obes (London) 2006301080–1085. [DOI] [PubMed] [Google Scholar]
- 4.Chen M Y, Wang E K, Jeng Y J. Adequate sleep among adolescents is positively associated with health status and health‐related behaviors. BMC Public Health 2006659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cournot M, Ruidavets J B, Marquie J C.et al Environmental factors associated with body mass index in a population of Southern France. Eur J Cardiovasc Prev Rehabil 200411291–297. [DOI] [PubMed] [Google Scholar]
- 6.Fallone G, Seifer R, Acebo C.et al How well do school‐aged children comply with imposed sleep schedules at home? Sleep 200225739–745. [DOI] [PubMed] [Google Scholar]
- 7.Gangwisch J E, Heymsfield S B, Boden‐Albala B.et al Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension 200647833–839. [DOI] [PubMed] [Google Scholar]
- 8.Gangwisch J E, Malaspina D, Boden‐Albala B.et al Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep 2005281289–1296. [DOI] [PubMed] [Google Scholar]
- 9.Gibson S, Lambert J, Neate D. Associations between weight status, physical activity, and consumption of biscuits, cakes and confectionery among young people in Britain. Nutr Bull 200429301–309. [Google Scholar]
- 10.Gottlieb D J, Punjabi N M, Newman A B.et al Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med 2005165863–867. [DOI] [PubMed] [Google Scholar]
- 11.Gupta N K, Mueller W H, Chan W.et al Is obesity associated with poor sleep quality in adolescents? Am J Hum Biol 200214762–768. [DOI] [PubMed] [Google Scholar]
- 12.Hasler G, Buysse D J, Klaghofer R.et al The association between short sleep duration and obesity in young adults: a 13‐year prospective study. Sleep 200427661–666. [DOI] [PubMed] [Google Scholar]
- 13.Heslop P, Smith G D, Metcalfe C.et al Sleep duration and mortality: the effect of short or long sleep duration on cardiovascular and all‐cause mortality in working men and women. Sleep Med 20023305–314. [DOI] [PubMed] [Google Scholar]
- 14.Kagamimori S, Yamagami T, Sokejima S.et al The relationship between lifestyle, social characteristics and obesity in 3‐year‐old Japanese children. Child Care Health Dev 199925235–247. [DOI] [PubMed] [Google Scholar]
- 15.Knutson K L. Sex differences in the association between sleep and body mass index in adolescents. J Pediatr 2005147830–834. [DOI] [PubMed] [Google Scholar]
- 16.Kripke D F, Garfinkel L, Wingard D L.et al Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry 200259131–136. [DOI] [PubMed] [Google Scholar]
- 17.Locard E, Mamelle N, Billette A.et al Risk factors of obesity in a five year old population. Parental versus environmental factors. Int J Obes Relat Metab Disord 199216721–729. [PubMed] [Google Scholar]
- 18.Padez C, Mourao I, Moreira P.et al Prevalence and risk factors for overweight and obesity in Portuguese children. Acta Paediatr 2005941550–1557. [DOI] [PubMed] [Google Scholar]
- 19.Patel S R, Ayas N T, Malhotra M R.et al A prospective study of sleep duration and mortality risk in women. Sleep 200427440–444. [DOI] [PubMed] [Google Scholar]
- 20.Reilly J J, Armstrong J, Dorosty A R.et al Early life risk factors for obesity in childhood: cohort study. BMJ 20053301357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sekine M, Yamagami T, Handa K.et al A dose‐response relationship between short sleeping hours and childhood obesity: results of the Toyama Birth Cohort Study. Child Care Health Dev 200228163–170. [DOI] [PubMed] [Google Scholar]
- 22.Shigeta H, Shigeta M, Nakazawa A.et al Lifestyle, obesity, and insulin resistance. Diabetes Care 200124608. [DOI] [PubMed] [Google Scholar]
- 23.Spiegel K, Leproult R, L'Hermite‐Baleriaux M.et al Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 2004895762–5771. [DOI] [PubMed] [Google Scholar]
- 24.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 19993541435–1439. [DOI] [PubMed] [Google Scholar]
- 25.Spiegel K, Tasali E, Penev P.et al Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004141846–850. [DOI] [PubMed] [Google Scholar]
- 26.Taheri S, Lin L, Austin D.et al Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med 20041e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vioque J, Torres A, Quiles J. Time spent watching television, sleep duration and obesity in adults living in Valencia, Spain. Int J Obes Relat Metab Disord 2000241683–1688. [DOI] [PubMed] [Google Scholar]
- 28.von Kries R, Toschke A M, Wurmser H.et al Reduced risk for overweight and obesity in 5‐ and 6‐y‐old children by duration of sleep—a cross‐sectional study. Int J Obes Relat Metab Disord 200226710–716. [DOI] [PubMed] [Google Scholar]
- 29.Vorona R D, Winn M P, Babineau T W.et al Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med 200516525–30. [DOI] [PubMed] [Google Scholar]
- 30.Leibel R L. The role of leptin in the control of body weight. Nutr Rev. 2002;60(Pt 2): S15–19, Discussion in Nutr Rev200260(Pt 2)S68–S87. [DOI] [PubMed] [Google Scholar]
- 31.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev 200585495–522. [DOI] [PubMed] [Google Scholar]
- 32.Hansen T K, Dall R, Hosoda H.et al Weight loss increases circulating levels of ghrelin in human obesity. Clin Endocrinol (Oxford) 200256203–206. [DOI] [PubMed] [Google Scholar]
- 33.Spiegel K, Knutson K, Leproult R.et al Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol 2005992008–2019. [DOI] [PubMed] [Google Scholar]
- 34.Knutson K L. The association between pubertal status and sleep duration and quality among a nationally representative sample of U. S. adolescents. Am J Hum Biol 200517418–424. [DOI] [PubMed] [Google Scholar]
- 35.Cizza G, Skarulis M, Mignot E. A link between short sleep and obesity: building the evidence for causation. Sleep 2005281217–1220. [DOI] [PubMed] [Google Scholar]
- 36.Carskadon M A, Acebo C, Jenni O G. Regulation of adolescent sleep: implications for behavior. Ann N Y Acad Sci 20041021276–291. [DOI] [PubMed] [Google Scholar]
- 37.Ofcom Child obesity‐food advertising in context. 2004. http://www.ofcom.org.uk/research/tv/reports/food_ads/ (accessed 10 Aug 2006)
- 38.Owens J, Maxim R, McGuinn M.et al Television‐viewing habits and sleep disturbance in school children. Pediatrics 1999104e27. [DOI] [PubMed] [Google Scholar]
- 39.Taheri S, Fleming P, Leary S.et al Team preliminary data regarding childhood sleep from the Avon Longitudinal Study of Parents And Children (ALSPAC). Sleep 200629(Suppl)O225–A26. [Google Scholar]
- 40.Wolfson A R, Carskadon M A. Understanding adolescents' sleep patterns and school performance: a critical appraisal. Sleep Med Rev 20037491–506. [DOI] [PubMed] [Google Scholar]