Abstract
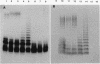
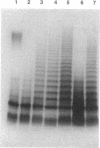
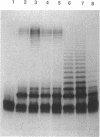
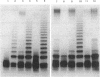
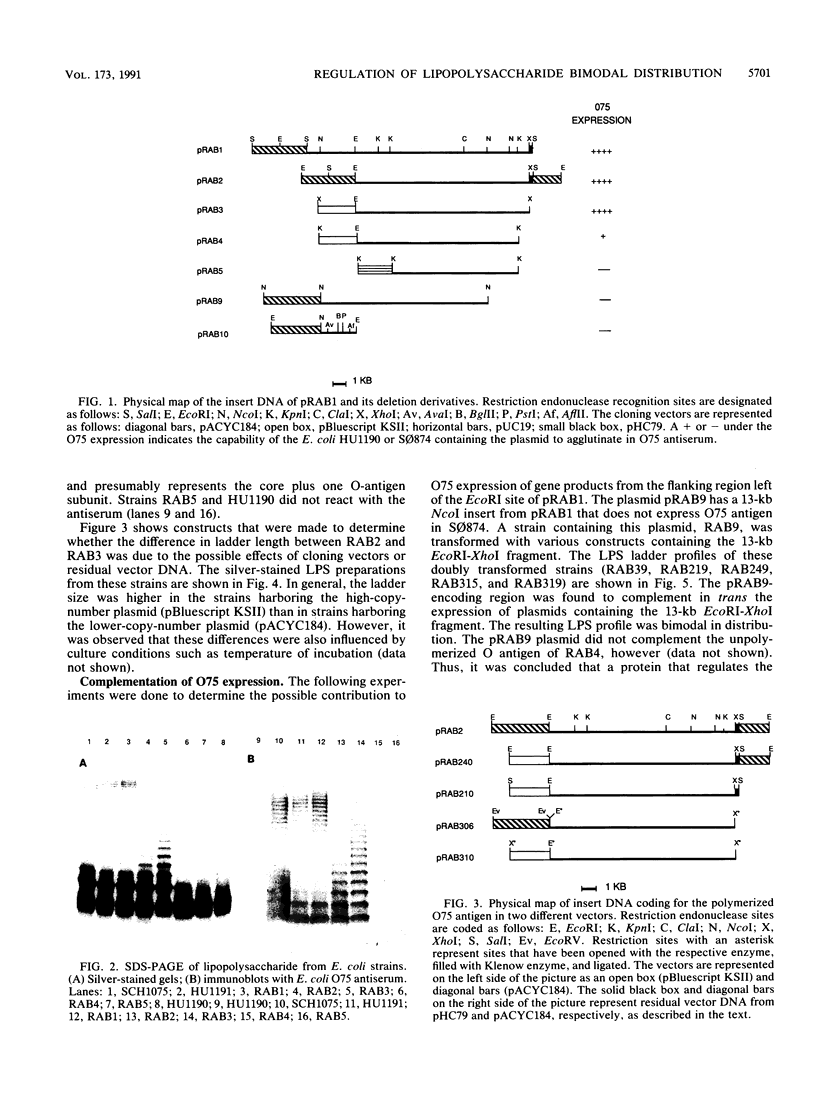
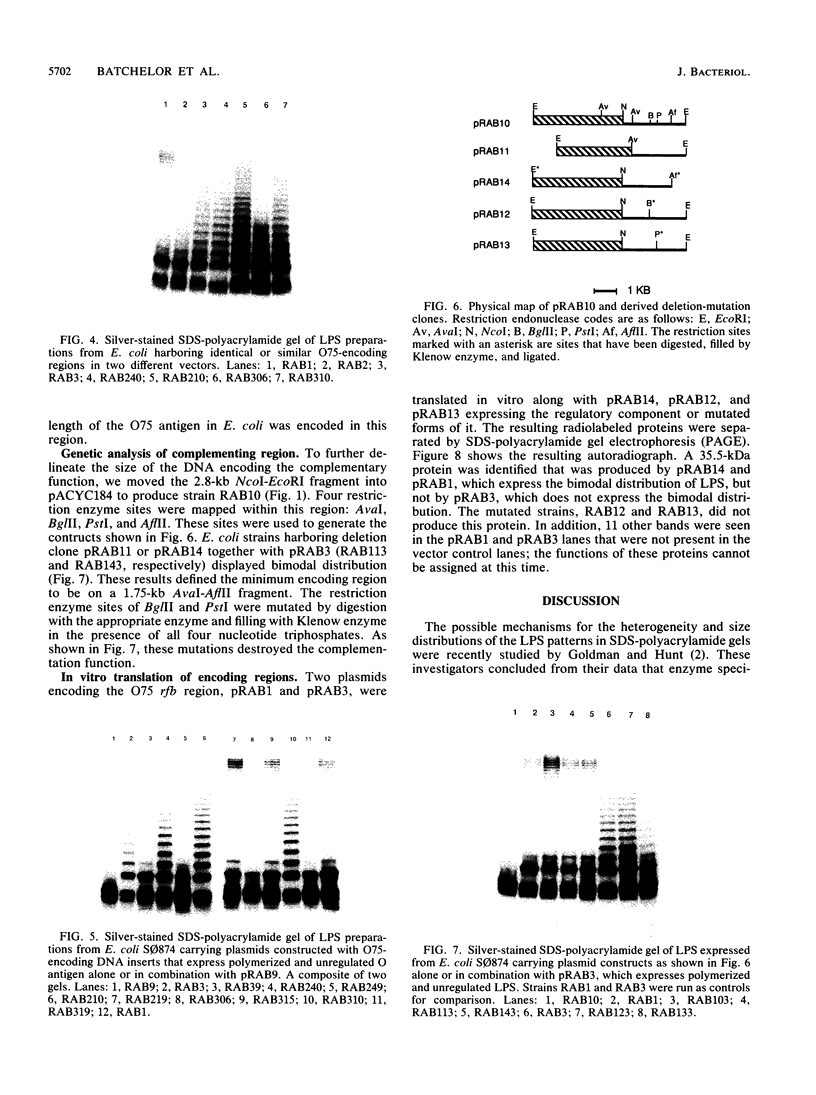
We report on the cloning and characterization of the rfb gene cluster of the O75 lipopolysaccharide from a urinary tract isolate of Escherichia coli. Deletion cloning defined the minimum region of DNA that expressed the O75 antigen in E. coli host strains to be on a 12.4-kb insert. However, the E. coli strain expressing this region did not produce a polymerized O chain as detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver staining. A slightly larger DNA clone of 13.4 kb produced a polymerized O chain in E. coli S phi 874 but was found to be abnormal in its distribution over the surface membrane. Normal wild-type E. coli, as with Salmonella spp., has a bimodal distribution of the lipopolysaccharide on the surface which is seen as an abundance of long and short O chains attached to the lipid A-core structure. We found in a region adjacent to the cloned rfb region, and on the opposite side from where the putative polymerase (rfc) is encoded, a novel protein of 35.5 kDa expressed from a 1.75-kb DNA fragment. This protein was shown to complement in trans the E. coli strains carrying plasmids that expressed abnormal, unregulated lipopolysaccharides. The expression of these complemented strains was bimodal in distribution. Mutation of the gene encoding this protein destroyed its ability to regulate O-chain distribution. We propose to call this regulator gene rol, for regulator of O length.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Hunt F. Mechanism of O-antigen distribution in lipopolysaccharide. J Bacteriol. 1990 Sep;172(9):5352–5359. doi: 10.1128/jb.172.9.5352-5359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Grossman N., Schmetz M. A., Foulds J., Klima E. N., Jimenez-Lucho V. E., Leive L. L., Joiner K. A., Jiminez V. Lipopolysaccharide size and distribution determine serum resistance in Salmonella montevideo. J Bacteriol. 1987 Feb;169(2):856–863. doi: 10.1128/jb.169.2.856-863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi G. E., Hull R. A., Krallmann-Wenzel U., Hull S. I. Molecular cloning and expression of the O4 polysaccharide gene cluster from Escherichia coli. Microb Pathog. 1989 Feb;6(2):123–132. doi: 10.1016/0882-4010(89)90015-6. [DOI] [PubMed] [Google Scholar]
- Heuzenroeder M. W., Beger D. W., Thomas C. J., Manning P. A. Molecular cloning and expression in Escherichia coli K-12 of the O101 rfb region from E. coli B41 (O101:K99/F41) and the genetic relationship to other O101 rfb loci. Mol Microbiol. 1989 Mar;3(3):295–302. doi: 10.1111/j.1365-2958.1989.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R., Bieler S., Falkow S., Hull S. Chromosomal map position of genes encoding P adhesins in uropathogenic Escherichia coli. Infect Immun. 1986 Feb;51(2):693–695. doi: 10.1128/iai.51.2.693-695.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann B., Reske K., Jann K. Heterogeneity of lipopolysaccharides. Analysis of polysaccharide chain lengths by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Eur J Biochem. 1975 Dec 1;60(1):239–246. doi: 10.1111/j.1432-1033.1975.tb20996.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lomberg H., Hellström M., Jodal U., Leffler H., Lincoln K., Svanborg Edén C. Virulence-associated traits in Escherichia coli causing first and recurrent episodes of urinary tract infection in children with or without vesicoureteral reflux. J Infect Dis. 1984 Oct;150(4):561–569. doi: 10.1093/infdis/150.4.561. [DOI] [PubMed] [Google Scholar]
- Marolda C. L., Welsh J., Dafoe L., Valvano M. A. Genetic analysis of the O7-polysaccharide biosynthesis region from the Escherichia coli O7:K1 strain VW187. J Bacteriol. 1990 Jul;172(7):3590–3599. doi: 10.1128/jb.172.7.3590-3599.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath B. C., Osborn M. J. Localization of the terminal steps of O-antigen synthesis in Salmonella typhimurium. J Bacteriol. 1991 Jan;173(2):649–654. doi: 10.1128/jb.173.2.649-654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschel L. H., Larsen L. J. The sensitivity of smooth and rough gram-negative bacteria to the immune bactericidal reaction. Proc Soc Exp Biol Med. 1970 Jan;133(1):345–348. doi: 10.3181/00379727-133-34472. [DOI] [PubMed] [Google Scholar]
- Neuhard J., Thomassen E. Altered deoxyribonucleotide pools in P2 eductants of Escherichia coli K-12 due to deletion of the dcd gene. J Bacteriol. 1976 May;126(2):999–1001. doi: 10.1128/jb.126.2.999-1001.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnalue N. A., Newton S., Stocker B. A. Lysogenization of Salmonella choleraesuis by phage 14 increases average length of O-antigen chains, serum resistance and intraperitoneal mouse virulence. Microb Pathog. 1990 Jun;8(6):393–402. doi: 10.1016/0882-4010(90)90026-m. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Porat R., Johns M. A., McCabe W. R. Selective pressures and lipopolysaccharide subunits as determinants of resistance of clinical isolates of gram-negative bacilli to human serum. Infect Immun. 1987 Feb;55(2):320–328. doi: 10.1128/iai.55.2.320-328.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So M., Dallas W. S., Falkow S. Characterization of an Escherichia coli plasmid encoding for synthesis of heat-labile toxin: molecular cloning of the toxin determinant. Infect Immun. 1978 Aug;21(2):405–411. doi: 10.1128/iai.21.2.405-411.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr P. I., Neill M. A., Clausen C. R., Newland J. W., Neill R. J., Moseley S. L. Genotypic variation in pathogenic Escherichia coli O157:H7 isolated from patients in Washington, 1984-1987. J Infect Dis. 1989 Feb;159(2):344–347. doi: 10.1093/infdis/159.2.344. [DOI] [PubMed] [Google Scholar]
- Tomás J. M., Ciurana B., Benedí V. J., Juarez A. Role of lipopolysaccharide and complement in susceptibility of Escherichia coli and Salmonella typhimurium to non-immune serum. J Gen Microbiol. 1988 Apr;134(4):1009–1016. doi: 10.1099/00221287-134-4-1009. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Valvano M. A., Crosa J. H. Molecular cloning and expression in Escherichia coli K-12 of chromosomal genes determining the O7 lipopolysaccharide antigen of a human invasive strain of E. coli O7:K1. Infect Immun. 1989 Mar;57(3):937–943. doi: 10.1128/iai.57.3.937-943.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]