Abstract
Aim
To assess the prevalence of eosinophilic oesophagitis in a tertiary paediatric gastroenterology clinic population.
Methods
A retrospective audit of Western Australian children investigated for oesophageal disease by paediatric gastroenterologists in the years 1995, 1999 and 2004. Macroscopic appearance of the oesophagus at endoscopy, original histological findings and diagnosis were recorded for each child. Biopsy specimens were blindly re‐evaluated, with re‐coded histological diagnoses compared with original reports. Age, sex and socioeconomic status were identified for each child.
Results
The prevalence of eosinophilic oesophagitis in Western Australia increased over the decade 1995–2004, rising from 0.05 to 0.89 per 10 000 children, with a concomitant increase in the severity of oesophagitis as determined by inflammatory cell numbers and associated features of inflammation. Children diagnosed with eosinophilic oesophagitis had a median age of 78.9 months (6.58 years), with no associated predisposition by sex or socioeconomic status trend. Almost one third of cases were macroscopically normal at endoscopy. All children with an original diagnosis of eosinophilic oesophagitis had ⩾40 eosinophils per high‐power field.
Conclusion
Over the decade 1995–2004, a true increase was seen in the prevalence of eosinophilic oesophagitis, not accounted for by diagnostic shift. Histological samples should be taken at endoscopy to confirm or exclude the diagnosis of eosinophilic oesophagitis.
In 1982, Winter et al1 reported that the presence of intraepithelial eosinophils in the oesophagus was specific for oesophagitis secondary to acid reflux, with the eosinophil numbers and location correlating with disease severity. Subsequently, this became an accepted histological criterion for the diagnosis of gastro‐oesophageal reflux disease (GORD) in children, although the significance of eosinophilic infiltration remained unclear.1 Eosinophilic oesophagitis, characterised by a predominantly eosinophilic inflammatory infiltrate, was defined as a distinct clinicopathological entity in 1993,2 distinct from reflux oesophagitis and eosinophilic gastroenteropathies, and was increasingly recognised worldwide.2,3
Eosinophilic oesophagitis is a chronic condition localised to the oesophagus, often with intermittent symptoms.3,4,5 Symptoms are similar to those of GORD, making clinical differentiation difficult.6,7 Dysphagia and food bolus obstruction (with or without stricture formation) have been documented, more commonly in older children and adults, necessitating oesophogastroduodenoscopy, dilatation or disimpaction.3,8,9,10,11 In children, there may be associated features of atopy (asthma or eczema).5,7,12 Long‐term follow‐up studies are few in the literature, but no long‐term risk of malignancy has been reported.3,4
Biopsy of the oesophageal mucosa is necessary for the diagnosis of eosinophilic oesophagitis, as a proportion of mucosa specimens appear normal macroscopically.7 Histological criteria used for the diagnosis of eosinophilic oesophagitis include >20–24 intraepithelial eosinophils per high‐power field (HPF, ×40), which may be associated with epithelial or basal zone hyperplasia (>15%) and elongation of rete papillae (>2/3 normal).2
There seems to be a true increase in the prevalence of eosinophilic oesophagitis in the developed countries, which is not related to increasing awareness and diagnostic shift.7,11,13
The main aim of this study was to assess the prevalence of eosinophilic oesophagitis in a tertiary paediatric setting over 10 years. A secondary aim was to delineate the demographic features of Western Australian children diagnosed with eosinophilic oesophagitis. The main outcome measure was the prevalence of eosinophilic oesophagitis (per 10 000 children) for three representative years (1995, 1999 and 2004). Secondary measures included distribution by sex and age, correlation with socioeconomic status, and macroscopic and histological features.
Patients and methods
Study population and design
The study was conducted at the Princess Margaret Hospital (Perth, Western Australia, Australia), the state paediatric referral centre and the site of practice of all paediatric gastroenterologists in Western Australia. Biopsy specimens were assessed in the Anatomical Pathology Department at the Princess Margaret Hospital, encompassing almost 100% of all cases of oesophagitis confirmed by biopsy for 1995, 1999 and 2004. Ethical approval was obtained from the Women's and Children's Ethics Committee.
Histological analysis
We retrospectively identified 328 biopsy specimens from children who had undergone upper gastrointestinal endoscopy, using the Systematised Nomenclature of Pathology codes for oesophageal inflammation (T6200 M4000) and miscellaneous oesophageal conditions (T6200 M0002). Exclusion criteria for the study included structural abnormalities of the oesophagus (congenital and secondary), coeliac disease, inflammatory bowel disease and multiple biopsies on the same child (in the same year). No child had a diagnosis of eosinophilic gastrointestinal disorder.
Of the 328 biopsies, 32 were excluded because of structural abnormalities, inflammatory bowel disease or multiple biopsies (in the same year) in a child. Thus, 296 biopsy specimens or charts were included in the study.
The original histological diagnosis was obtained from the pathology database. Other data collected from reports included presence or absence of eosinophils, quantification of eosinophils (none, few, moderate or large), ulceration and biopsy site.
All biopsy specimens were re‐evaluated by a paediatric histopathologist (NMS) blinded to demographic and biopsy details, including original diagnosis. Re‐evaluation data included number of leucocytes and differential count, presence or absence of basal hyperplasia, elongation of rete papillae, formation of microabscesses and morphological diagnosis (oesophagitis with eosinophils, non‐eosinophilic oesophagitis, and minor changes that are resolving or normal). Eosinophils were graded from the site considered to be the worst affected on low‐power field.2,5 On the basis of eosinophil numbers, biopsy specimens were stratified into groups and correlated with demographic, macroscopic and original findings. Original and re‐evaluated specimens were classified as having eosinophilic oesophagitis if the pathologist identified >24 intraepithelial eosinophils/HPF (×40).13
Demographic details
Age of the child at the time of biopsy (months) and sex were obtained from the child's medical records. Socioeconomic status was assessed from postcode details of the family address, and converted using codes of the 2001 Socio‐Economic Index for Areas (SEIFA; Australian Bureau of Statistics; see http://www.abs.gov.au). Western Australian census data for 1996 and 2001 (Australian Bureau of Statistics tool for assessment of relative socioeconomic advantage) were used to calculate prevalences per 10 000 children (0–14 years of age).
Macroscopic details
Macroscopic oesophageal findings were obtained from endoscopy notes for 278 of the 296 patients. Endoscopies were carried out under general anaesthesia by accredited paediatric gastroenterologists or trainees under supervision.
Statistical analysis
Data were analysed using SPSS V.12.0 for Windows. Continuous outcome variables were compared using Student's t test or non‐parametric analysis (Mann–Whitney or Kruskal–Wallis tests) as appropriate. Associations between categorical variables were analysed using Pearson's χ2 test or Fisher's exact test. Results were adjusted using logistic or Poisson's regression analysis where appropriate (STATA V.8.20). Statistical significance was recorded at p<0.05 (two tailed).
Results
Demographics
Median (interquartile (IQR)) age at biopsy was 77.0 (18.0–141.0) months in 1995, 76.0 (27.5–148.5) months in 1999 and 85.5 (35.2–126.2) months in 2004, with an overall median age of 82.0 (28.2–139.0) months. Patient age at the time of biopsy was not different for any of the years assessed (Kruskal–Wallis χ2 = 1.072, df = 2, p = 0.585). Distribution by sex did not differ over the years (χ2 = 4.075, df = 2, p = 0.13). The male sex was over‐represented, with 66.2% biopsies carried out on boys (binomial p<0.001). Age at original biopsy did not differ with sex (median age for boys 80.0 months and for girls 96.5 months; Mann–Whitney z = −1.727, p = 0.084).
Original biopsy findings
The precise site of most (86.8%) oesophageal biopsy specimens was not specified, and multiple specimens were taken in 11.1% patients. Most biopsy findings were reported as non‐specific oesophagitis or minor changes (66.6% and 28.4%, respectively). Histological ulceration was uncommon (2.7% overall). No child was diagnosed with eosinophilic oesophagitis in 1995 or 1999. Only eight children (five boys and three girls; 2.7% overall) were originally classified as having eosinophilic oesophagitis in 2004, and none in the prior years.
Macroscopic (intraoperative) findings
The main macroscopic findings on endoscopy are listed in order of frequency, with multiple findings for most children (table 1). In all, 52.7% of children had ⩾1 abnormal finding. Of those cases reclassified as eosinophilic oesophagitis (>24 eosinophils on HPF, n = 54), 29.6% were macroscopically normal. White exudate was seen in 14 (25.9%), erythema in 14 (25.9%), nodularity in 7 (13.0%), linear erosions in 6 (11.1%) and tram‐tracking or furrows in 4 (7.4%) of the 54 children.
Table 1 Frequency of macroscopic findings on upper gastrointestinal endoscopy per year of biopsy.
| Macroscopic finding | 1995 | 1999 | 2004 | Total |
|---|---|---|---|---|
| Normal | 35 | 42 | 62 | 140 |
| Increased erythema | 26 | 31 | 38 | 95 |
| Increased nodularity | 1 | 3 | 16 | 20 |
| White plaque or exudate | 2 | 0 | 17 | 19 |
| Erosions | 4 | 5 | 7 | 16 |
| Ulceration | 3 | 0 | 0 | 3 |
| Tram‐tracking/furrows | 0 | 0 | 5 | 5 |
| Other* | 1 | 5 | 1 | 7 |
*Other includes Barrett's syndrome (n = 1), stricture (n = 1), thickened distal oesophagus (n = 3), tortuous distally (n = 1) and non‐specific irregular appearance (n = 1).
Histological re‐evaluation of biopsy specimens
Table 2 shows leucocyte counts in the worst affected regions, with neutrophil count not varying with year (Kruskal–Wallis χ2 = 0.068, df = 2, p = 0.966). Mean lymphocyte and eosinophil counts increased significantly over the decade (Kruskal–Wallis χ2 = 99.086, df = 2, p<0.001; Kruskal–Wallis χ2 = 29.267, df = 2, p<0.001, respectively).
Table 2 Leucocyte count per high‐power field per year.
| Leucocyte/HPF | 1995 | 1999 | 2004 | Overall |
|---|---|---|---|---|
| Lymphocytes | 1.25 (3.01) | 7.49 (11.41) | 14.11 (14.43) | 9.16 (12.86) |
| Eosinophils | 3.36 (8.67) | 9.94 (16.92) | 22.41 (34.60) | 14.34 (27.13) |
| Neutrophils | 0.17 (1.11) | 0.28 (1.31) | 0.38 (2.23) | 0.30 (1.78) |
HPF, high‐power field. Values are mean (SD).
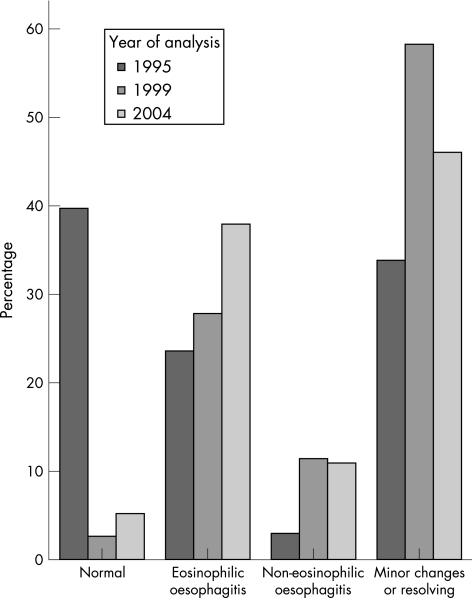
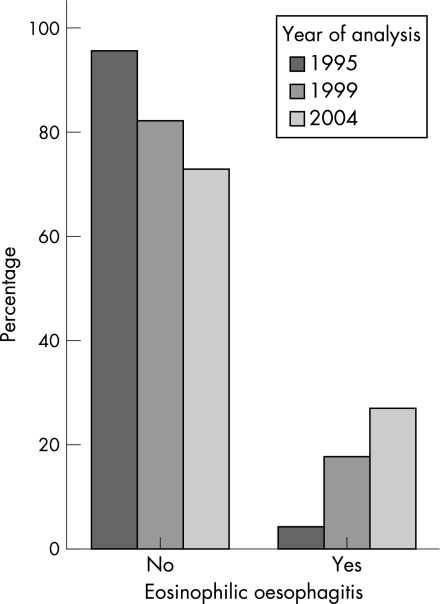
A morphological diagnosis was made on the re‐evaluated biopsy specimens (fig 1), and they were classified into non‐eosinophilic oesophagitis (n = 213, mean (standard deviation (SD)) eosinophil count 2.8 (4.86)) and eosinophilic oesophagitis in case of >24 eosinophils on HPF (n = 54, mean (SD) eosinophil count 63.8 (27.8); fig 2). Patients with eosinophilic oesophagitis had a significant increase in the median number of eosinophils by year: 1995, 37; 1999, 41; and 2004, 80 (Kruskal–Wallis χ2 = 13.22, df = 2, p<0.001). Eosinophil count was not influenced by sex (Mann–Whitney z = −1.38, p = 0.168).
Figure 1 Morphological diagnosis of re‐assessed biopsy specimens by year of original analysis (expressed as percentage of biopsies/year).
Figure 2 Diagnosis of eosinophilic oesophagitis (>25 eosinophils/high‐power field) by year of original analysis.
We found a significant increase in the number of eosinophilic oesophagitis diagnoses over the decade (χ2 15.449, df 2, p<0.001), with a corresponding increase in the prevalence of eosinophilic oesophagitis per 10 000 children: 1995, 0.05; 1999, 0.31; and 2004, 0.89 (table 3). Using Poisson regression analysis, the incidence of eosinophilic oesophagitis in 1999 was found to be six times as great as that in 1995 (IRR 5.93, p = 0.02, 95% confidence interval (CI) 1.33 to 26.51), with the incidence in 2004 rising to 17 times that in 1995 (IRR 17.31, p<0.001, 95% CI 4.16 to 71.96). We found no significant difference between the group with non‐eosinophilic oesophagitis and that with eosinophilic oesophagitis by sex (χ2 = 1.04, df = 1, p = 0.308), mean age at biopsy (89.3 v 78.9 months; Mann–Whitney z = −0.891, p = 0.373) or socioeconomic status by mean SEIFA score (1011.76 v 1015.5; t = −0.415, df = 270, p = 0.679).
Table 3 Increasing diagnosis of eosinophilic oesophagitis by year of original analysis.
| Variable | 1995 | 1999 | 2004 | Overall | p Value |
|---|---|---|---|---|---|
| Original report diagnosis (%) | n = 71 | n = 85 | n = 140 | n = 296 | <0.001 |
| 0 (0) | 0 (0) | 8 (5.71) | 8 (2.70) | ||
| Reclassified EO diagnosis (%; >24 eosinophils/HPF) | n = 69 | n = 79 | n = 137 | n = 285 | <0.001 |
| 3 (4.35) | 14 (17.72) | 37 (27.01) | 54 (18.95) | ||
| Prevalence of diagnosed EO/10 000 children | 0.051 | 0.305 | 0.891 |
EO, eosinophilic oesophagitis; HPF, high‐power field. Values are mean (SD).
In biopsy specimens reclassified as having eosinophilic oesophagitis, basal hyperplasia >15% (χ2 = 21.285, df = 1, p<0.001), elongation of rete papillae >2/3 normal (χ2 = 48.595, df = 1, p<0.001) and microabscess formation (χ2 = 90.131, df = 1, p<0.001) were all increased. Significant interyear variation was also shown for basal hyperplasia, elongation of rete papillae and formation of microabscesses using logistic regression (table 4). We found no significant association between eosinophilic oesophagitis and basal spongiosis, ulceration or atrophy (Kruskal–Wallis χ2 = 1.925, p = 0.165).
Table 4 Percentage of children with re‐coded diagnosis of eosinophilic oesophagitis and histological features per year of analysis.
| Variable | 1995 | 1999 | 2004 | p Value |
|---|---|---|---|---|
| EO and basal hyperplasia | 1.41 | 16.47 | 26.43 | <0.001 |
| EO and elongation of rete papillae | 2.82 | 15.29 | 24.29 | <0.001 |
| EO and microabscess formation | 2.82 | 2.35 | 14.29 | 0.001 |
EO, eosinophilic oesophagitis.
Biopsy specimens were stratified depending on eosinophil counts/HPF (0, 1–7, 8–24, >25) in keeping with the published literature.14,15 In 2004, 27% of biopsy specimens had >24 eosinophils/HPF. Of those reclassified as having eosinophilic oesophagitis, 42 (77.8%) children had ⩾40 eosinophils/HPF, which included all children (n = 8) with an original diagnosis of eosinophilic oesophagitis. Of these, 30 (71.4%) children were boys, but sex was not significant (χ2 = 0.59, p = 0.808).
Discussion
This study has shown a significant increase in prevalence of eosinophilic oesophagitis during the past decade in Western Australian children, from 0.05 to 0.89 diagnosed cases per 10 000 children, an 18‐fold increase (table 3). Although this may be partially accounted for by diagnostic shift and increasing awareness of the significance of mucosal eosinophilia, there is also an increase in severity of inflammation, with increasing eosinophil count and associated features of inflammation in oesophageal biopsy specimens over the study period. We believe that there has been a real increase in the prevalence of eosinophilic oesophagitis. A parallel increase is probably occurring elsewhere, as evidenced by the increasing number of literature reports regarding this disorder: a Medline search yielded 1 article in the 1970s, 8 in the 1980s, 128 in the 1990s and 132 in the 5 years to the end of 2005.
Our findings of a rising prevalence is in keeping with the recent report from the US by Noel et al,13 documenting a childhood prevalence of 0.991 increasing to 3.106/10 000 population over the period 2000–2004, with an annual incidence of 1.28/10 000. This group documented a male predominance and familial clustering, and distribution according to population distribution, with no urban–rural gradient.13 The distribution in Western Australia is in keeping with this, and in addition we have not shown any association with socioeconomic gradient. Studies on an adult Swiss population reported similar prevalence and incidence estimates.16
At issue for the study is whether different populations were assessed in 1995 and 2004 at issue for this study. The denominator, the number of children in Western Australia, is relatively stable over this period, and clinical practice and the practitioners remain the same. Few young children will undergo gastrointestinal endoscopy in other settings in Western Australia. The decreasing proportion of children with a normal biopsy indicates that there has been some shift in patient selection. It remains possible that the clinicians are more selective and are choosing for biopsy those children who are more likely to have eosinophilic oesophagitis; however, this cannot be established and we think there may be a real increase in the prevalence of eosinophilic oesophagitis. Clearly, there is an increasing awareness of the disorder, as seen by the low rate of specific diagnosis at the time of original assessment (2.7%) in comparison with that at the time of review during this study (18.9%).
The rising prevalence of eosinophilic oesophagitis mirrors the rise in atopic disease, and there seems to be an overlapping spectrum of GORD, allergy and eosinophilic oesophagitis.6,11,14 A family or personal history of atopy is associated with as many as 50% of cases.11,17 It is postulated that immune dysregulation, in particular Th2‐mediated mechanisms, may have a role in disease pathogenesis.8,18,19 The rise in prevalence of atopy is a complex phenomenon in which multiple factors have been implicated. These factors include decreased exposure to infections in early life, sibship size, high body weight in early childhood and changes in gut microflora.20
Other studies have not identified an increase in the severity of inflammation documented here. Numbers of eosinophils and lymphocytes (but not neutrophils) increased, as did the proportion of biopsy specimens showing structural changes in basal hyperplasia and rete papillary elongation. Clinical experience, however, is that paediatric gastroenterologists are seeing increasing numbers of children with severe disease such as food bolus impaction, which may be associated with more severe or chronic inflammation. Atopic disease is increasing in general and food allergy in particular, with poorly documented suggestions of increasing severity of disease.21
Dietary antigens are the most frequently identified triggers in the pathogenesis of eosinophilic oesophagitis. Identification of positive skin prick or patch test reactions to specific food allergens and their subsequent dietary elimination can lead to improvement in oesophageal mucosal biopsy specimens in up to 77% of patients.22,23 Evidence from animal studies suggests that sensitisation may lead to eosinophilic oesophagitis via inhaled antigens and via damaged skin.19,24
Biopsy of the oesophageal mucosa is necessary for the diagnosis of eosinophilic oesophagitis, an issue highlighted by the number of cases with an endoscopically normal macroscopic appearance. A quarter of this cohort had white exudate formation, with no evidence of fungal infection. With chronicity, increased structural change occurs in addition to intraepithelial eosinophilia. The mucosal surface becomes more irregular, erythematous and wrinkled.8,10 In more severe cases, endoscopy may show linear fissuring or furrows, nodularity, white exudate, oesophageal rings or trachealisation, erosions or ulceration, and stricture formation.3,7,8,25,26 Straumann et al26 showed that white exudates are seen in as many as 50% of adults with eosinophilic oesophagitis, correlating histologically with increased intraepithelial eosinophilia and clinically with dysphagia.
There are no undisputed histological criteria for diagnosis, some studies using 24 intraepithelial eosinophils/HPF, the value used in this study,13,27 whereas other groups have used 20 eosinophils/HPF.2,14 When a lower value of >20 eosinophils/HPF was used, only one extra child was identified in our population. Interestingly, 77.8% of our cohort with eosinophilic oesophagitis had >40 eosinophils/HPF. Other reported features useful in diagnosis are epithelial or basal zone hyperplasia and elongation of rete papillae,1,2,17,18 originally associated with GORD and significantly increased in our group with eosinophilic oesophagitis.
A limitation of our study is the fact that biopsy location was not specified in most cases; however, recent studies have shown that the number of eosinophils (as opposed to location in the oesophagus) is the key diagnostic feature.7,28 In the past, the presence of eosinophils was supposed to be the hallmark of GORD; however, diagnosis is now informally classified according to the eosinophil count visualised on HPF.1,11,15 This rough histological grouping may guide clinical diagnosis and potential treatment: 0 eosinophils/HPF, normal finding; 1–7 eosinophils/HPF, suggestive of GORD; >25+ eosinophils/HPF, suggestive of eosinophilic oesophagitis; and an intermediate group with 8–24 eosinophils/HPF, suggestive of uncertain aetiology that may represent an overlap between GORD and allergy.15 Most of this cohort had eosinophil counts that placed them in the normal or GORD group, which is in keeping with the literature. As this study did not correlate diagnosis with treatment or atopy status, further correlations cannot be drawn from our results.
Conclusion
The prevalence of eosinophilic oesophagitis is increasing very rapidly in Western Australian children, in keeping with a general increase in atopic disorders in Western countries. The increase in prevalence is accompanied by an increase in disease severity. The reasons for these increases are unclear, but are likely to be complex. It is important to diagnose eosinophilic oesophagitis on the basis of histological features, because of a normal macroscopic appearance in a larger proportion of patients. Further studies are necessary to define more concise diagnostic criteria and delineate the underlying pathogenesis, so that appropriate strategies for the recognition and management of eosinophilic oesophagitis may be devised.
What is known on this topic
Eosinophilic oesophagitis seems to be increasing in prevalence, mirroring the rise in atopic disease in developed countries.
A count of >20–24 intraepithelial eosinophils per high‐power field is suggestive of eosinophilic oesophagitis, although no definitive diagnostic criterion exists.
Macroscopic change increases with chronicity and includes features of furrowing, white exudates and stricture formation, but appearance can be normal early in the disease.
What this study adds
Over the decade, a true increase was seen in the prevalence of eosinophilic oesophagitis in Western Australian children.
Histological samples are required to confirm or exclude the diagnosis of eosinophilic oesophagitis.
Acknowledgements
We thank Elaine Pascoe, biostatistician, and the staff of the Departments of Gastroenterology and Anatomical Pathology, Princess Margaret Hospital, for their advice and assistance.
Abbreviations
GORD - gastro‐oesophageal reflux disease
HPF - high‐power field
SEIFA - Socio‐Economic Index for Areas
Footnotes
Competing interests: None declared.
References
- 1.Winter H S, Madara J L, Stafford R J.et al Intraepithelial eosinophils: a new diagnostic criterion for reflux esophagitis. Gastroenterology 198283818–823. [PubMed] [Google Scholar]
- 2.Attwood S E, Smyrk T C, Demeester T R.et al Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 199338109–116. [DOI] [PubMed] [Google Scholar]
- 3.Straumann A, Spichtin H P, Grize L.et al Natural history of primary eosinophilic esophagitis: a follow‐up of 30 adult patients for up to 11.5 years. Gastroenterology 20031251660–1669. [DOI] [PubMed] [Google Scholar]
- 4.Esposito S, Marinello D, Paracchini R.et al Long‐term follow‐up of symptoms and peripheral eosinophil counts in seven children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 200438452–456. [DOI] [PubMed] [Google Scholar]
- 5.Kelly K J, Lazenby A J, Rowe P C.et al Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid‐based formula. Gastroenterology 19951091503–1512. [DOI] [PubMed] [Google Scholar]
- 6.Rothenberg M E. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol 200411311–28. [DOI] [PubMed] [Google Scholar]
- 7.Liacouras C A, Spergel J M, Ruchelli E.et al Eosinophilic esophagitis: a 10‐year experience in 381 children. Clin Gastroenterol Hepatol 200531198–1206. [DOI] [PubMed] [Google Scholar]
- 8.Cheung K M, Oliver M R, Cameron D J.et al Esophageal eosinophilia in children with dysphagia. J Pediatr Gastroenterol Nutr 200337498–503. [DOI] [PubMed] [Google Scholar]
- 9.Sant'Anna A M, Rolland S, Fournet J C.et al Eosinophilic esophagitis in children: symptoms, histology and ph probe results. J Pediatr Gastroenterol Nutr 200439373–377. [DOI] [PubMed] [Google Scholar]
- 10.Walsh S V, Antonioli D A, Goldman H.et al Allergic esophagitis in children: a clinicopathological entity. Am J Surg Pathol 199923390–396. [DOI] [PubMed] [Google Scholar]
- 11.Liacouras C A, Ruchelli E. Eosinophilic esophagitis. Curr Opin Pediatr 200416560–566. [DOI] [PubMed] [Google Scholar]
- 12.Liacouras C A, Wenner W J, Brown K.et al Primary eosinophilic esophagitis in children: successful treatment with oral corticosteroids. J Pediatr Gastroenterol Nutr 199826380–385. [DOI] [PubMed] [Google Scholar]
- 13.Noel R J, Putnam P E, Rothenberg M E. Eosinophilic esophagitis. N Engl J Med 2004351940–941. [DOI] [PubMed] [Google Scholar]
- 14.Rothenberg M E, Mishra A, Collins M H.et al Pathogenesis and clinical features of eosinophilic esophagitis. J Allergy Clin Immunol 2001108891–894. [DOI] [PubMed] [Google Scholar]
- 15.Ruchelli E, Wenner W, Voytek T.et al Severity of esophageal eosinophilia predicts response to conventional gastroesophageal reflux therapy. Pediatr Dev Pathol 1999215–18. [DOI] [PubMed] [Google Scholar]
- 16.Straumann A, Simon H U. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol 2005115418–419. [DOI] [PubMed] [Google Scholar]
- 17.Remedios M, Campbell C, Jones D M.et al Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc 2006633–12. [DOI] [PubMed] [Google Scholar]
- 18.Cury E K, Schraibman V, Faintuch S. Eosinophilic infiltration of the esophagus: gastroesophageal reflux versus eosinophilic esophagitis in children—discussion on daily practice. J Pediatr Surg 200439e4–e7. [DOI] [PubMed] [Google Scholar]
- 19.Mishra A, Hogan S P, Brandt E B.et al An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest 200110783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Mutius E. Infection: friend or foe in the development of atopy and asthma? The epidemiological evidence. Eur Respir J 200118872–881. [DOI] [PubMed] [Google Scholar]
- 21.Burks W. Peanut allergy: a growing phenomenon. J Clin Invest 2003111950–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spergel J M, Andrews T, Brown‐Whitehorn T F.et al Treatment of eosinophilic esophagitis with specific food elimination diet directed by a combination of skin prick and patch tests. Ann Allergy Asthma Immunol 200595336–343. [DOI] [PubMed] [Google Scholar]
- 23.Liacouras C A. Eosinophilic esophagitis in children and adults. J Pediatr Gastroenterol Nutr 200337(Suppl 1)S23–S28. [DOI] [PubMed] [Google Scholar]
- 24.Akei H S, Mishra A, Blanchard C.et al Epicutaneous antigen exposure primes for experimental eosinophilic esophagitis in mice. Gastroenterology 2005129985–994. [DOI] [PubMed] [Google Scholar]
- 25.Sundaram S, Sunku B, Nelson S P.et al Adherent white plaques: an endoscopic finding in eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 200438208–212. [DOI] [PubMed] [Google Scholar]
- 26.Straumann A, Spichtin H P, Bucher K A.et al Eosinophilic esophagitis: red on microscopy, white on endoscopy. Digestion 200470109–116. [DOI] [PubMed] [Google Scholar]
- 27.Attwood S E, Lewis C J, Bronder C S.et al Eosinophilic oesophagitis: a novel treatment using Montelukast. Gut 200352181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markowitz J E, Liacouras C A. Eosinophilic esophagitis. Gastroenterol Clin North Am 200332949–966. [DOI] [PubMed] [Google Scholar]