Abstract
Rhodospirillum rubrum is a facultatively phototrophic bacterium that, under certain growth conditions, forms an intracytoplasmic chromatophore membrane (ICM) housing the photochemical apparatus. The puf operon of R. rubrum encodes protein subunits of the photochemical reaction center and the B880 light-harvesting antenna complex. Mutant strains of R. rubrum were constructed by interposon mutagenesis through which a kanamycin resistance gene cartridge was inserted into restriction sites and in place of restriction fragments of the puf region. Southern blot analysis demonstrated that the defective copies of puf sequences had replaced their normal chromosomal counterparts through homologous recombination. The phenotypes of the mutant strains were evaluated on the basis of puf gene expression, spectral analysis, pigment content of membranes, and electron-microscopic examination of thin sections of cells grown under semi-aerobic and dark anaerobic conditions. Alterations of the puf region affect phototrophic competence and the formation of the ICM. The latter result implies an obligatory role for puf gene products in ICM formation in R. rubrum. One mutant with a deletion in puf structural genes was complemented in trans to the wild-type phenotype. Other mutants could be restored to the wild-type phenotype only by recombination.
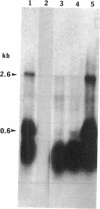
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Forrest M. E., Cohen S. N., Beatty J. T. Structural and functional analysis of transcriptional control of the Rhodobacter capsulatus puf operon. J Bacteriol. 1989 Jan;171(1):473–482. doi: 10.1128/jb.171.1.473-482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. P., Feher G. Crystallization of reaction center from Rhodopseudomonas sphaeroides: preliminary characterization. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4795–4799. doi: 10.1073/pnas.81.15.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: the cofactors. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5730–5734. doi: 10.1073/pnas.84.16.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: the protein subunits. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6162–6166. doi: 10.1073/pnas.84.17.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. P., Feher G., Yeates T. O., Rees D. C., Deisenhofer J., Michel H., Huber R. Structural homology of reaction centers from Rhodopseudomonas sphaeroides and Rhodopseudomonas viridis as determined by x-ray diffraction. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8589–8593. doi: 10.1073/pnas.83.22.8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C. E., Young D. A., Marrs B. L. Analysis of the Rhodobacter capsulatus puf operon. Location of the oxygen-regulated promoter region and the identification of an additional puf-encoded gene. J Biol Chem. 1988 Apr 5;263(10):4820–4827. [PubMed] [Google Scholar]
- Belasco J. G., Beatty J. T., Adams C. W., von Gabain A., Cohen S. N. Differential expression of photosynthesis genes in R. capsulata results from segmental differences in stability within the polycistronic rxcA transcript. Cell. 1985 Jan;40(1):171–181. doi: 10.1016/0092-8674(85)90320-4. [DOI] [PubMed] [Google Scholar]
- Biel A. J., Marrs B. L. Transcriptional regulation of several genes for bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulata in response to oxygen. J Bacteriol. 1983 Nov;156(2):686–694. doi: 10.1128/jb.156.2.686-694.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger G., Bérard J., Corriveau P., Gingras G. The structural genes coding for the L and M subunits of Rhodospirillum rubrum photoreaction center. J Biol Chem. 1988 Jun 5;263(16):7632–7638. [PubMed] [Google Scholar]
- Bélanger G., Gingras G. Structure and expression of the puf operon messenger RNA in rhodospirillum rubrum. J Biol Chem. 1988 Jun 5;263(16):7639–7645. [PubMed] [Google Scholar]
- Bérard J., Bélanger G., Corriveau P., Gingras G. Molecular cloning and sequence of the B880 holochrome gene from Rhodospirillum rubrum. J Biol Chem. 1986 Jan 5;261(1):82–87. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins M. L., Niederman R. A. Membranes of Rhodospirillum rubrum: isolation and physicochemical properties of membranes from aerobically grown cells. J Bacteriol. 1976 Jun;126(3):1316–1325. doi: 10.1128/jb.126.3.1316-1325.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. L., Niederman R. A. Membranes of Rhodospirillum rubrum: physicochemical properties of chromatophore fractions isolated from osmotically and mechanically disrupted cells. J Bacteriol. 1976 Jun;126(3):1326–1338. doi: 10.1128/jb.126.3.1326-1338.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook S. M., Treml S. B., Collins M. L. Immunocytochemical ultrastructural analysis of chromatophore membrane formation in Rhodospirillum rubrum. J Bacteriol. 1986 Jul;167(1):89–95. doi: 10.1128/jb.167.1.89-95.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J., Donohue T. J., Kaplan S. Construction, characterization, and complementation of a Puf- mutant of Rhodobacter sphaeroides. J Bacteriol. 1988 Jan;170(1):320–329. doi: 10.1128/jb.170.1.320-329.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHoff B. S., Lee J. K., Donohue T. J., Gumport R. I., Kaplan S. In vivo analysis of puf operon expression in Rhodobacter sphaeroides after deletion of a putative intercistronic transcription terminator. J Bacteriol. 1988 Oct;170(10):4681–4692. doi: 10.1128/jb.170.10.4681-4692.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Schmidhauser T., Yakobson E., Lu P., Liang X. W., Finlay D. R., Guiney D., Helinski D. R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985 Mar;13(2):149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farchaus J. W., Oesterhelt D. A Rhodobacter sphaeroides puf L, M and X deletion mutant and its complementation in trans with a 5.3 kb puf operon shuttle fragment. EMBO J. 1989 Jan;8(1):47–54. doi: 10.1002/j.1460-2075.1989.tb03347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice W. P., Saari L. L., Lowery R. G., Ludden P. W., Roberts G. P. Genes coding for the reversible ADP-ribosylation system of dinitrogenase reductase from Rhodospirillum rubrum. Mol Gen Genet. 1989 Aug;218(2):340–347. doi: 10.1007/BF00331287. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- HOLT S. C., MARR A. G. LOCATION OF CHLOROPHYLL IN RHODOSPIRILLUM RUBRUM. J Bacteriol. 1965 May;89:1402–1412. doi: 10.1128/jb.89.5.1402-1412.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch P. R., Beringer J. E. A physical map of pPH1JI and pJB4JI. Plasmid. 1984 Sep;12(2):139–141. doi: 10.1016/0147-619x(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Kiley P. J., Donohue T. J., Havelka W. A., Kaplan S. DNA sequence and in vitro expression of the B875 light-harvesting polypeptides of Rhodobacter sphaeroides. J Bacteriol. 1987 Feb;169(2):742–750. doi: 10.1128/jb.169.2.742-750.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley P. J., Kaplan S. Molecular genetics of photosynthetic membrane biosynthesis in Rhodobacter sphaeroides. Microbiol Rev. 1988 Mar;52(1):50–69. doi: 10.1128/mr.52.1.50-69.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug G., Cohen S. N. Combined actions of multiple hairpin loop structures and sites of rate-limiting endonucleolytic cleavage determine differential degradation rates of individual segments within polycistronic puf operon mRNA. J Bacteriol. 1990 Sep;172(9):5140–5146. doi: 10.1128/jb.172.9.5140-5146.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mueller P. R., Collins M. L. Identification of two distinct lactate dehydrogenases in Rhodospirillum rubrum. J Bacteriol. 1983 Mar;153(3):1562–1566. doi: 10.1128/jb.153.3.1562-1566.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Myers C. R., Collins M. L. Cell-cycle-specific fluctuation in cytoplasmic membrane composition in aerobically grown Rhodospirillum rubrum. J Bacteriol. 1987 Dec;169(12):5445–5451. doi: 10.1128/jb.169.12.5445-5451.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers C. R., Collins M. L. Cell-cycle-specific oscillation in the composition of chromatophore membrane in Rhodospirillum rubrum. J Bacteriol. 1986 Jun;166(3):818–823. doi: 10.1128/jb.166.3.818-823.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Abdulaev N. G., Shmuckler B. E., Zargarov A. A., Kutuzov M. A., Telezhinskaya I. N., Levina N. B., Zolotarev A. S. Photosynthetic reaction centre of Chloroflexus aurantiacus. Primary structure of M-subunit. FEBS Lett. 1988 May 23;232(2):364–368. doi: 10.1016/0014-5793(88)80770-1. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Abdulaev N. G., Zolotarev A. S., Shmukler B. E., Zargarov A. A., Kutuzov M. A., Telezhinskaya I. N., Levina N. B. Photosynthetic reaction centre of Chloroflexus aurantiacus. I. Primary structure of L-subunit. FEBS Lett. 1988 Apr 11;231(1):237–242. doi: 10.1016/0014-5793(88)80739-7. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schultz J. E., Weaver P. F. Fermentation and anaerobic respiration by Rhodospirillum rubrum and Rhodopseudomonas capsulata. J Bacteriol. 1982 Jan;149(1):181–190. doi: 10.1128/jb.149.1.181-190.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockett R. E., Donohue T. J., Varga A. R., Kaplan S. Control of photosynthetic membrane assembly in Rhodobacter sphaeroides mediated by puhA and flanking sequences. J Bacteriol. 1989 Jan;171(1):436–446. doi: 10.1128/jb.171.1.436-446.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Taylor D. P., Cohen S. N., Clark W. G., Marrs B. L. Alignment of genetic and restriction maps of the photosynthesis region of the Rhodopseudomonas capsulata chromosome by a conjugation-mediated marker rescue technique. J Bacteriol. 1983 May;154(2):580–590. doi: 10.1128/jb.154.2.580-590.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadeboncoeur C., Noël H., Poirier L., Cloutier Y., Gingras G. Photoreaction center of photosynthetic bacteria. 1. Further chemical characterization of the photoreaction center from Rhodospirillum rubrum. Biochemistry. 1979 Oct 2;18(20):4301–4308. doi: 10.1021/bi00587a007. [DOI] [PubMed] [Google Scholar]
- Van der Rest M., Gingras G. The pigment complement of the photosynthetic reaction center isolated from Rhodospirillum rubrum. J Biol Chem. 1974 Oct 25;249(20):6446–6453. [PubMed] [Google Scholar]
- Wellington C. L., Taggart A. K., Beatty J. T. Functional significance of overlapping transcripts of crtEF, bchCA, and puf photosynthesis gene operons in Rhodobacter capsulatus. J Bacteriol. 1991 May;173(9):2954–2961. doi: 10.1128/jb.173.9.2954-2961.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiessner C., Dunger I., Michel H. Structure and transcription of the genes encoding the B1015 light-harvesting complex beta and alpha subunits and the photosynthetic reaction center L, M, and cytochrome c subunits from Rhodopseudomonas viridis. J Bacteriol. 1990 Jun;172(6):2877–2887. doi: 10.1128/jb.172.6.2877-2887.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Feher G., Simon M. I. Primary structure of the L subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7303–7307. doi: 10.1073/pnas.81.23.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Ogden R. C., Simon M. I., Feher G. Primary structure of the M subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6505–6509. doi: 10.1073/pnas.80.21.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yeates T. O., Komiya H., Rees D. C., Allen J. P., Feher G. Structure of the reaction center from Rhodobacter sphaeroides R-26: membrane-protein interactions. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6438–6442. doi: 10.1073/pnas.84.18.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. A., Bauer C. E., Williams J. C., Marrs B. L. Genetic evidence for superoperonal organization of genes for photosynthetic pigments and pigment-binding proteins in Rhodobacter capsulatus. Mol Gen Genet. 1989 Jul;218(1):1–12. doi: 10.1007/BF00330558. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Alberti M., Begusch H., Bylina E. J., Hearst J. E. Reaction center and light-harvesting I genes from Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1984 Jan;81(1):189–192. doi: 10.1073/pnas.81.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Bylina E. J., Alberti M., Begusch H., Hearst J. E. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction-center, B870 antenna, and flanking polypeptides from R. capsulata. Cell. 1984 Jul;37(3):949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Ismail S., Bylina E. J. Chromosomal deletion and plasmid complementation of the photosynthetic reaction center and light-harvesting genes from Rhodopseudomonas capsulata. Gene. 1985;38(1-3):19–30. doi: 10.1016/0378-1119(85)90199-4. [DOI] [PubMed] [Google Scholar]
- Zhu Y. S., Kaplan S. Effects of light, oxygen, and substrates on steady-state levels of mRNA coding for ribulose-1,5-bisphosphate carboxylase and light-harvesting and reaction center polypeptides in Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Jun;162(3):925–932. doi: 10.1128/jb.162.3.925-932.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
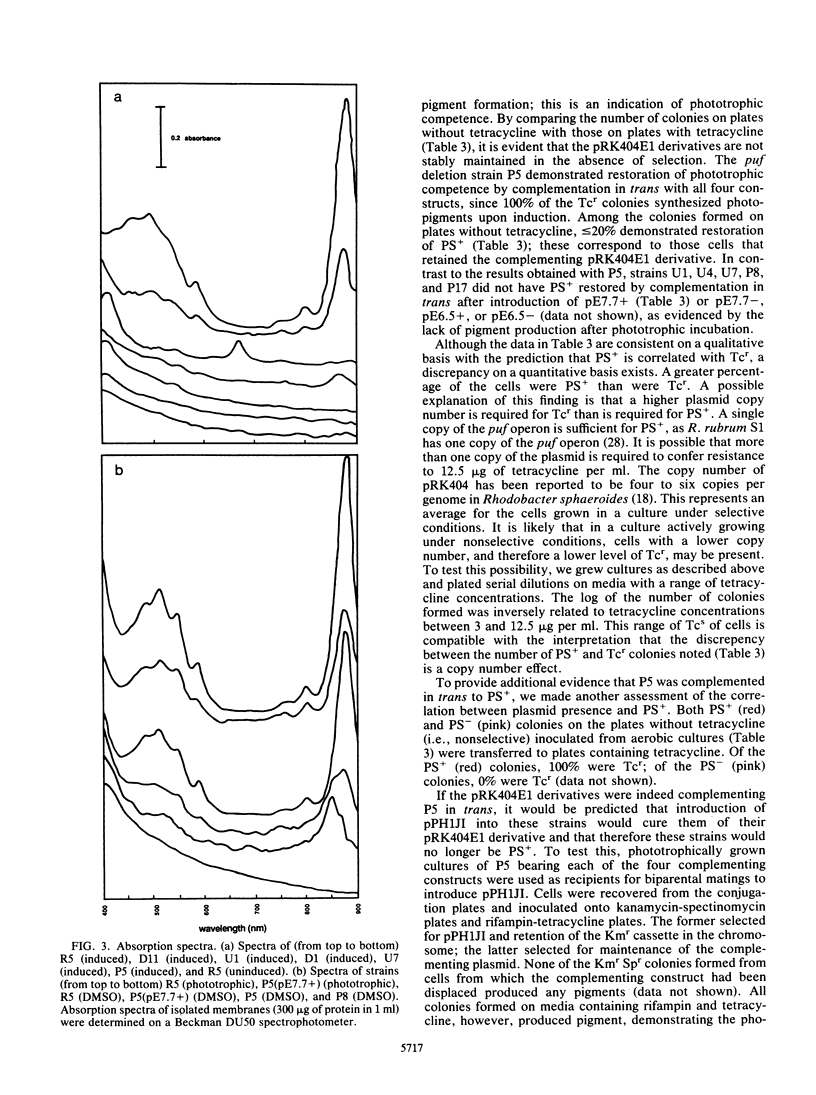
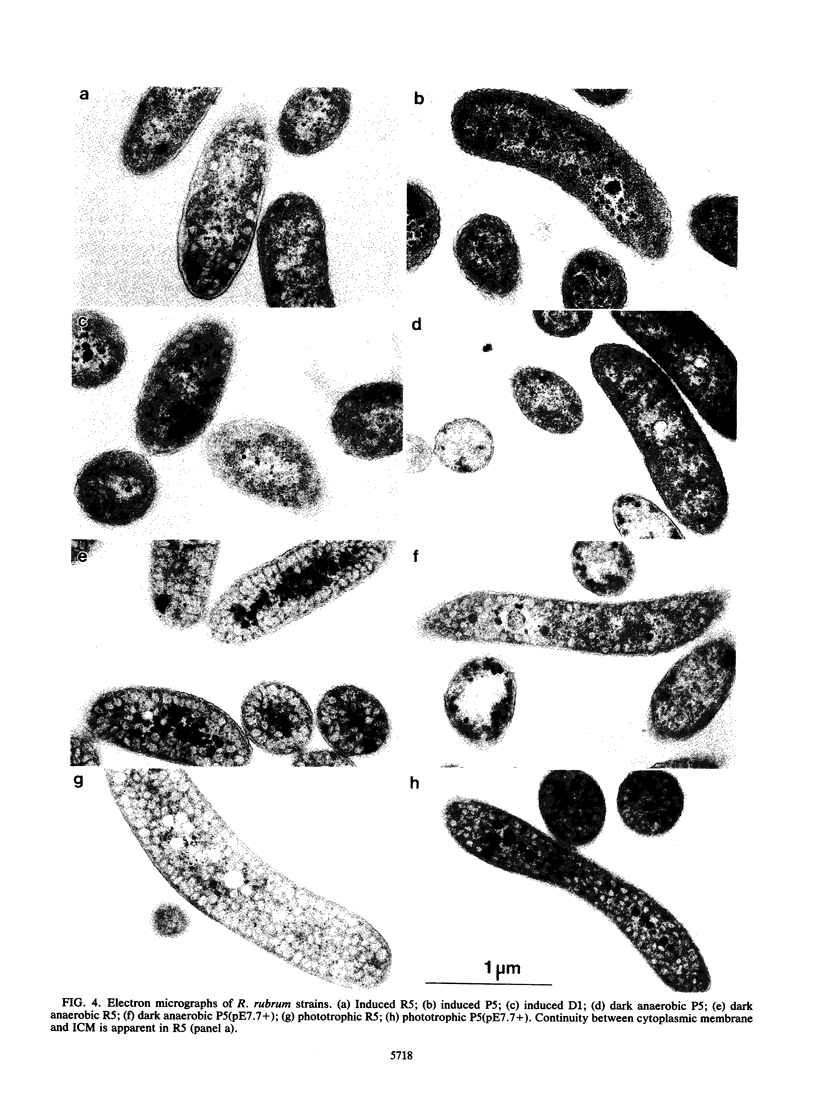
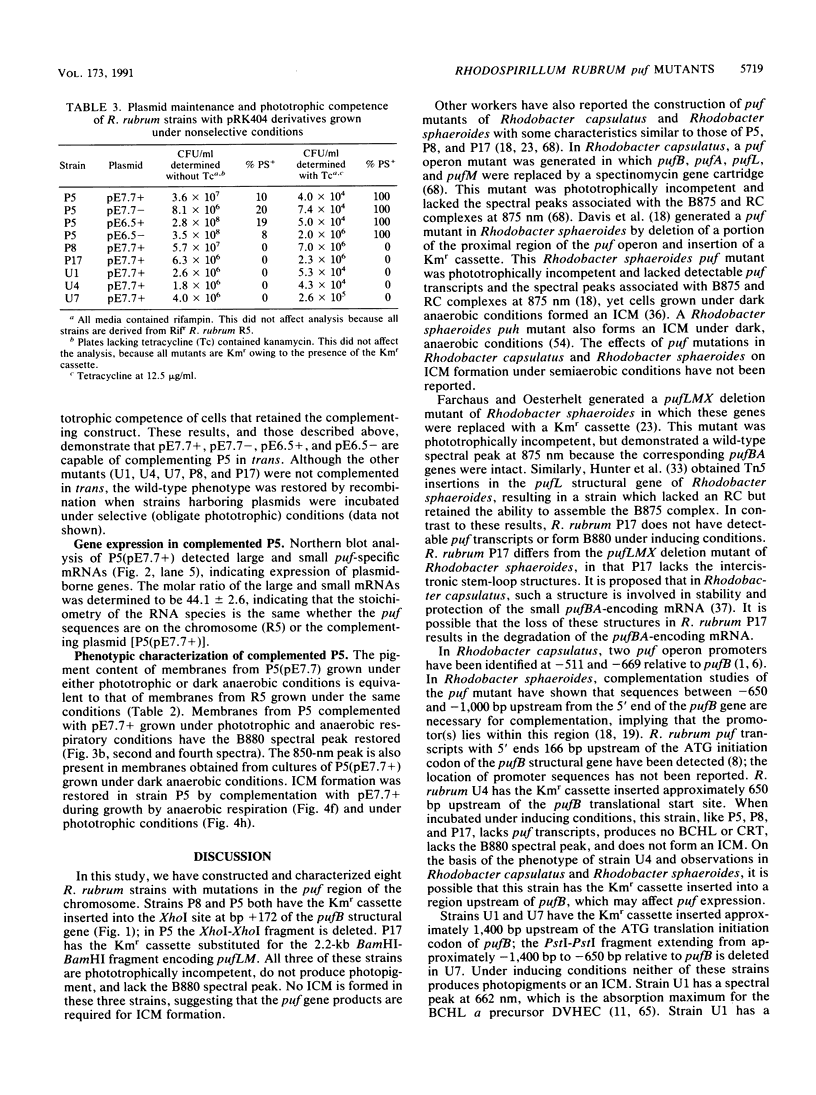
- Zhu Y. S., Kiley P. J., Donohue T. J., Kaplan S. Origin of the mRNA stoichiometry of the puf operon in Rhodobacter sphaeroides. J Biol Chem. 1986 Aug 5;261(22):10366–10374. [PubMed] [Google Scholar]