Abstract
Background
Vitamin D deficiency is a chronic condition which contributes to general ill health and seems to be re‐emerging in our catchment area since funding of vitamin D supplementation by Primary Care Trusts ceased. This study aims to verify this situation and to assess the cost effectiveness of reintroducing vitamin D supplementation in the Burnley Health Care NHS Trust.
Methods
Vitamin D deficient patients presenting between January 1994 and May 2005 were identified and data retrospectively collected from their case notes. The cost of treatment and the theoretical cost of primary prevention for the Trust population were calculated using previous and current DoH guidelines.
Results
Fourteen patients were identified, of whom 86% presented in the last 5 years and 93% were of Asian origin. The incidence of vitamin D deficiency for our population is 1 in 923 children overall and 1 in 117 in children of Asian origin. The average cost of treatment for each such child is £2500, while the theoretical cost of prevention of vitamin D deficiency in the Asian population through primary prevention according to COMA guidance is £2400 per case.
Conclusions
Vitamin D deficiency is re‐emerging in our Trust. The overwhelming majority of our patients are of Asian origin. The cost of primary prevention for this high risk population compares favourably both medically and financially with treatment of established disease. We suggest that Primary Care Trusts provide funds for vitamin D supplementation of Asian children for at least the first 2 years of life.
Keywords: cost‐benefit, hypocalcaemia, rickets, treatment, vitamin D deficiency, vitamin D supplementation
Vitamin D deficiency in infants and children adversely affects calcium metabolism resulting in rickets, hypocalcaemic convulsions, dental problems, general ill health, and poor growth.1,2 The main source of vitamin D is solar UV radiation falling on the skin. However, there is no radiation of appropriate wavelength (290–310 nm) in Britain from the end of October to the end of March. Furthermore, increased skin pigmentation reduces the capacity of skin to synthesise vitamin D.3 A genetic predisposition to vitamin deficiency and altered vitamin D level has also been reported amongst Asian people.4 Over the past few years there has been growing concern about the resurgence of rickets in British Asian children and recent immigrants to the UK.5,6 This reflects the general decrease in the use of vitamins over the years: 12% of babies received vitamin supplementation in 1995 compared to 4% in 2000.7
Guidance from the Committee on Medical Aspects of Food and Nutritional Policy (COMA) states that the reference nutrient intake (RNI) of vitamin D should be 10 μg/day for pregnant and breastfeeding women, 8.5 μg/day for infants under 6 months of age, and 7 μg/day for children from 6 months to 3 years of age.8,9 COMA suggests that infants and young children should receive supplementary vitamin D for at least the first 2 years of life and this is the recommendation most UK based health professionals follow. In the USA, the Institute of Medicine of the National Academy of Sciences suggests a daily “adequate intake” of 5 μg from birth to 18 years of age.10
Current Department of Health (DoH) guidance states that, to avoid vitamin D deficiency, all pregnant and nursing mothers should take supplements containing 10 μg/day of vitamin D. Furthermore, all children under 5 years of age should take supplements containing 7 μg/day of vitamin D.11 Although the National Institute for Health and Clinical Excellence does not favour routine vitamin D supplementation for pregnant women because of the “absence of evidence of benefit”,12 the DoH does support this. The problem, at present, is the unavailability of a suitable preparation as multivitamin supplements are deemed unsafe because of their vitamin A content and the potential of the latter for teratogenicity.
Burnley Health Care NHS Trust in north west England provides a caring service to a population of approximately 242 000 within the boroughs of Burnley, Pendle, and Rossendale which have a large Asian community. Since funding for the provision of supplementary vitamins was discontinued, there is a feeling that the number of children seen in the paediatric department with clinical or biochemical signs of vitamin D deficiency has greatly increased. This study was therefore conducted in order to identify the number of patients presenting to the paediatric department in the period 1994–2005 with vitamin D deficiency, to establish whether these children had any vitamin supplementation prior to presentation, to identify the incidence of vitamin D deficiency in the Trust's population, and finally to determine whether re‐introduction of vitamin D supplementation would be beneficial.
Methods
Cases were identified by searching for “hypocalcaemic convulsions”, “hypocalcaemic tetany”, and “rickets” in the paediatric discharge books, the discharge letters of patients, and the relevant codes provided by the clinical coding department, from January 1994 to May 2005. The diagnosis of vitamin D deficiency was based on a combination of clinical findings such as bow legs, rickety rosary, tetany, convulsions due to hypocalcaemia, radiological evidence, biochemistry results such as raised alkaline phosphatase (ALP) with or without high parathyroid hormone levels, or low levels (<25 nmol/l) of 25‐hydroxycholecalciferol (25OHC), with improvement of symptoms and signs with vitamin D with or without calcium supplementation. Patients with medical conditions likely to predispose to vitamin D deficiency, such as cystic fibrosis or hepatic/renal abnormality, and those on anticonvulsants were excluded. Patient records were subsequently reviewed and information was collected using a specially designed form which included patient demographics, age and mode of presentation, earlier administration of vitamins, investigations and treatment, inpatient care, number of follow‐up appointments, and final outcome.
The average cost of treatment for each patient was then calculated from the total cost of investigations and hospital expenses based on figures provided by appropriate Trust departments and the total cost of medication based on British National Formulary values.13 The cost of medication was calculated on median duration of treatment (15 months), using median age (1 year) and estimated weight at this age (10 kg). The yearly cost of Abidec multivitamin drops was an average (£10.3) from the published range (£6.9–£13.7).14 Trust figures were used regarding the total number (2216) of new patients (outpatients and inpatients given follow‐up appointments, excluding babies admitted to our neonatal intensive care unit) seen by the paediatric department during 2004. Local data from the 2001 Census were used for the percentage of the population below the age of 15 who were Asian (12.63%) and the number of Asian children below the age of 1 (approximately 500). The theoretical cost of vitamin D supplementation was then calculated both for the Asian community and for the entire Trust population, based on COMA recommendations and current DoH guidelines.
As the study was retrospective in nature and only used data obtained for clinical purposes, ethical committee approval was not required.
Results
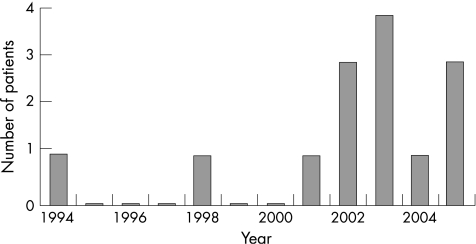
Seventeen patients were identified. However, three patients were excluded: two patients were diagnosed as suffering from osteopenia of prematurity, while the third had cystic fibrosis and was suffering from rickets secondary to intestinal malabsorption. Therefore, the data from 14 patients were analysed. The year of presentation for each case is shown in fig 1. Data on patient demographics, gender, age, and mode of presentation are shown in table 1. The mean number of patients with vitamin D deficiency seen over the 5 years was 2.4 patients per year. The incidence of vitamin D deficiency for the entire population was therefore approximately 1 in 923 (2.4/2216), while the incidence for the Asian population was 1 in 117 (2.4/(2216×12.63%)).
Figure 1 Chronology of presenting cases.
Table 1 Patient clinical information.
| Variable | Patients | |
|---|---|---|
| n | % | |
| Patient demographics | ||
| White | 1 | 7 |
| Asian | 13 | 93 |
| Gender | ||
| Males | 9 | 64 |
| Females | 5 | 36 |
| Age at presentation (years) | ||
| 0⩽0.5 | 5 | 36 |
| 0.5⩽1 | 4 | 29 |
| 1⩾1.5 | 1 | 7 |
| 1.5⩽2 | 1 | 7 |
| 2⩽2.5 | 1 | 7 |
| >2.5 | 2 | 14 |
| Mode of presentation | ||
| Rickets | 4 | 29 |
| Hypocalcaemic fits | 1 | 7 |
| Hypocalcaemic tetany | 1 | 7 |
| Incidental finding | 8 | 57 |
| Additional medical conditions | ||
| Iron deficiency anaemia | 10 | 71 |
Bone profiles (serum calcium, phosphate, and ALP) were investigated in 93% of patients. Hypocalcaemia was found in 36% of patients, while an equal percentage of our sample had hypophosphataemia. A raised ALP was found in 71% of patients. Serum 25OHC and parathyroid hormone levels were measured in 29% and 36% of patients, respectively and were abnormal in all cases. All patients had a full blood count and iron deficiency anaemia was found in 71%.
Eight (57%) of our patients were incidentally found to be vitamin D deficient during investigation of a different presenting complaint: two had eczema, two presented with lower respiratory tract infections, three were referred for investigation of failure to thrive, and one was admitted for intravenous rehydration following prolonged diarrhoea and vomiting.
Initial investigations and treatments with their cost are shown in table 2. Half of the patients required inpatient care ranging from 1 to 10 days with a median duration of 2 days. A total of 26 inpatient days were spent in hospital and the cost for these is shown in table 2. The length of follow up ranged from 5 months to 10 years with a median duration of 15 months. A total of 76 follow‐up appointments were made, ranging from 1 to 20 per patient with a median of 4. Additionally, 12 dietician reviews were conducted ranging from 0 to 4 per patient with a median of 1. The cost of follow up and subsequent investigations is also shown in table 2. Overall, the cost of treatment for the 14 patients was £35 075.12, which is £2505.37 per patient. The problem was resolved in 43% of the patients, while it was still ongoing in the remaining 57%.
Table 2 Cost of treatment for vitamin D deficiency.
| Type of intervention | Cost (£) | Total cost (£) |
|---|---|---|
| Initial investigations | ||
| X rays: 13×£32 | 416.00 | |
| Bone profiles: 13×£11 | 143.00 | |
| Urea and electrolytes: 11×£11 | 121.00 | |
| Full blood count: 14×£11 | 154.00 | |
| Parathyroid hormone levels: 5×£23 | 115.00 | |
| Vitamin D levels: 4×£23 | 92.00 | |
| Total | 1041.00 | 1041.00 |
| Treatment | ||
| 1‐Alphacalcidol: 114 bottles×£24.18 | 2756.52 | |
| Calcium supplements: 92 bottles×£3.39 | 311.88 | |
| Phosphate supplements: 92 | 302.68 | |
| packets×£3.29 | ||
| Total | 3371.08 | 3371.08 |
| Inpatient care | ||
| 26×£342.59 | 8907.34 | |
| Total | 8907.34 | 8907.34 |
| Follow up | ||
| First follow‐up appointments: 14×£326 | 4564.00 | |
| Subsequent follow‐up appointments: | 11 373.90 | |
| 62×£183.45 | ||
| First dietician reviews: 8×£326 | 2608.00 | |
| Subsequent dietician reviews: | 733.80 | |
| 4×£183.45 | ||
| Total | 19 279.70 | 19 279.70 |
| Subsequent investigations | ||
| Bone profiles : 108×£11 | 1188.00 | |
| Urea and electrolytes: 49×£11 | 539.00 | |
| Full blood count: 43×£11 | 473.00 | |
| Parathyroid hormone levels: 7×£23 | 161.00 | |
| Vitamin D levels: 5×£23 | 115.00 | |
| Total | 2476.00 | 2476.00 |
| Total cost for 14 patients | 35 075.12 | |
| Total cost per patient | 2505.37 |
Data about prior use of vitamin supplements was derived from patients' notes and by direct communication with affected families. None of these families had used vitamin supplementation in the past. The cost for supplementing one child with vitamin D according to COMA and current DoH recommendations is shown in table 3. With an incidence of vitamin D deficiency of 1 in 923 children in our population, £19 013.8 or £47 534.5 would have to be spent to prevent just one case of vitamin D deficiency according to COMA and current DoH guidelines, respectively. For the Trust's Asian population where the incidence of vitamin D deficiency is 1 in 117, the respective costs are £2410.2 and £6025.5.
Table 3 Cost of primary prevention/vitamin D supplementation in children.
| Primary prevention (COMA) | Primary prevention (DoH) |
|---|---|
| Abidec for 2 years: £20.6/patient | Abidec for 5 years: £51.5/patient |
| Cost to prevent 1 case of rickets in Trust population (1:923): | |
| 923×£20.6 = £19 013.8 | 923×£51.5 = £47 534.5 |
| Cost to prevent 1 case of rickets in Asian population (1:117): | |
| 117×£20.6 = £2410.2 | 117×£51.5 = £6025.5 |
| Entire Trust population primary prevention: | |
| 4000×£20.6 = £82 400/year | 4000×£51.5 = £206 000/year |
| Trust population treatment cost: 4000/923×£2505.37 = £10 857.5/year | |
| Total Asian population primary prevention: | |
| 500×£20.6 = £10 300/year | 500×£51.5 = £25 750/year |
| Asian population treatment cost: 500/117×£2505.37 = £10 706.7/year | |
There are about 4000 births per year in our Trust, ∼500 of which are of Asian origin.
Discussion
In the paediatric population of Burnley Health Care NHS Trust, of the 14 cases of vitamin D deficiency diagnosed over the last 11 years, 12 were in the last 5 years. The incidence of vitamin D deficiency in our population is 1 in 923. The great majority (13/14 or 93%) of the children affected were of Asian origin. The incidence of the condition in the Asian population is therefore 1 in 117 and identifies them as a high risk group, in agreement with previous reports.15 This increased incidence is probably secondary to a variety of factors such as environment, skin pigmentation, and genetic predisposition.3,4
Our results are also in agreement with recent reports highlighting the resurgence of rickets in the British Asian population.5,6,16 The 5 year period during which 86% of our cases presented coincides with the discontinuation of funding for vitamin D supplementation in eligible infants. In addition, none of the children affected were found to have received any form of vitamin supplementation prior to the development of rickets. Lack of vitamin D supplementation therefore appears to be the main cause for the increase in the incidence of vitamin D deficiency during this period.
Diagnosis of vitamin D deficiency is based on a low serum concentration of 25OHC (<25 nmol/l) and radiographic long bone changes.14,17,18 However, rickets does not always manifest itself radiologically, especially in the very young and in adolescents.19 Initial investigations should include serum calcium, phosphate, and ALP.20 In our study these investigations were conducted in 93% of cases with 36% having hypocalcaemia and an equal percentage manifesting hypophosphataemia. A raised ALP was found in 71% of patients. Serum 25OHC and parathyroid hormone levels were measured in only 29% and 36% of patients and in all cases were abnormal. All of our patients had a full blood count and in 71% iron deficiency anaemia was found, confirming an already known link with vitamin D deficiency.21,22 The high percentage of patients younger than 6 months in our population sample (36%) is possibly secondary to the high prevalence of hypocalcaemia in the very young as suggested by Ladhani et al.19 This is turn is probably a reflection of more severe vitamin D deficiency in mothers since the withdrawal of supplementation.
Most of our cases (57%) were picked up incidentally: two had eczema, two presented with lower respiratory tract infections, three were referred for investigation of failure to thrive, and one was admitted for intravenous rehydration following prolonged diarrhoea and vomiting. These findings should raise awareness among clinicians when high risk patients are seen and highlight the need to conduct all appropriate investigations from the outset.
Clinical trials suggest that 10 μg/day vitamin D supplementation is sufficient to prevent vitamin D deficiency.23,24 Guidelines for vitamin D supplementation in both the UK and USA seem therefore to be very reasonable.8,9,10 However, a dilemma appears to arise regarding the costs involved in applying these guidelines. Since the incidence in the general population has in recent years been low, possibly reflecting prior successful public health initiatives, local health authorities appear to question the appropriateness of allocating resources for primary prevention of vitamin D deficiency. More recently, the DoH has changed its guidance and now advises vitamin D supplementation in children for the first 5 years of life, thus potentially further increasing the costs involved.11
The DoH runs a Welfare Food Scheme under which pregnant or breastfeeding women and children under 5 years of age are given free vitamin supplements if they come from families fulfilling certain criteria25; the “Healthy Start” initiative of DoH includes a programme to promote vitamin D supplementation. However, despite the theoretical availability of recommended vitamins as either drops or tablets, neither is currently manufactured in the UK. Alternatives for nursing infants and children include Abidec and Dalivit multivitamin drops. As these preparations contain vitamin A they should not be given to pregnant women.
The cost of Abidec supplementation for primary prevention of vitamin D deficiency was used for our calculations. Based on the incidence of vitamin D deficiency and implementation of the current DoH recommendation, £206 000 would be required every year (in contrast to the £10 857 which is now spent on treatment) for primary prevention of vitamin D deficiency in the Burnley Health Care NHS Trust population of 242 000. The cost of prevention if the previous COMA recommendations were to be followed would drop to £82 400. In both cases the expenses for primary prevention outweigh those for treatment by several thousand pounds per year. Focusing on the high risk Asian population, either £25 750 or £10 300 would be required every year for primary prevention depending on whether the DoH or COMA recommendations were followed. It appears that implementation of the COMA recommendations for the Asian population would be cost effective. When one considers that most of our patients were picked up incidentally, thus indicating that there are many more patients with clinically silent vitamin D deficiency, and the fact that some patients were either treated in primary care or other neighbouring hospitals, this becomes an economically attractive proposition.
Alphacalcidol was one of the common medications used for treatment of vitamin D deficiency. This is a frequent mistake made by paediatricians. Treatment of vitamin D deficiency should be with ergocalciferol or cholecalciferol and not with alphacalcidol which is not licensed for primary vitamin D deficiency.4,13,14 There are no published randomised controlled trials comparing vitamin D with alphacalcidol. Furthermore, there is a difference in the cost of the two types of treatment with alphacalcidol being significantly more expensive.
What is already known on this topic
Vitamin D deficiency in infants and children adversely affects calcium metabolism resulting in rickets, hypocalcaemic convulsions, dental problems, general ill health, and poor growth.
There is growing concern about the resurgence of rickets in British Asian children and recent immigrants to the UK.
What this study adds
There has been a dramatic increase in the incidence of rickets especially in British Asian children since the cessation of vitamin D supplementation.
It makes economic and health sense to supplement all Asian children with 7 μg/day of vitamin D for at least the first 2 years of their lives.
Apart from its financial aspects, vitamin D deficiency is a chronic condition causing poor general health in the individual. It has been linked to an increased risk for multiple sclerosis, some cancers, cardiovascular disease, diabetes, osteoporosis, and mental illness.26,27,28,29 Additionally, the condition imposes a significant psychological and social stress on both the family and the growing child.
In light of the above, we therefore suggest that supplementation with vitamin D of all babies of Asian origin for the first 2 years of life might be the economic answer to a growing problem. Furthermore, campaigns targeting the high risk groups on vitamin D supplementation would be of great value as shown in the past.30,31,32 However, one should not forget that vitamin D deficiency also occurs in white populations as is nicely illustrated in our sample where one patient was white. The incidence of vitamin D deficiency in white populations is very low but does exist, particularly among recent arrivals from Eastern Europe.
We acknowledge certain limitations of our study such as the fact that it is a retrospective study. In addition, our population characteristics may be special in terms of low socioeconomic status, thus perhaps rendering generalisation of our results and recommendations problematic.
In conclusion, this study has detected 14 paediatric cases of vitamin D deficiency over the period 1994–2005 and has confirmed the rising incidence of vitamin D deficiency in the paediatric British Asian community with an incidence of 1 in 117 compared to 1 in 923 in the general population. Taking into account the fact that none of the children had received initial vitamin supplementation, the higher cost of treating vitamin D deficient Asian children compared to primary prevention, and the morbidity associated with the condition, we propose that local health authorities provide funds to supplement all Asian children with 7 μg/day of vitamin D for at least the first 2 years of their lives.
Acknowledgements
The authors wish to acknowledge Dr I Elbeshlawi for his assistance with data collection and all the paediatricians in Burnley General Hospital for allowing us to include their patients in our study.
Abbreviations
ALP - alkaline phosphatase
COMA - Committee on Medical Aspects of Food and Nutritional Policy
DoH - Department of Health
25OHC - 25‐hydroxycholecalciferol
RNI - reference nutrient intake
Footnotes
Competing interests: None declared.
References
- 1.Hochberg Z, Bereket A, Davenport M.et al Consensus development for the supplementation of vitamin D in childhood and adolescence. Horm Res 20025839–51. [DOI] [PubMed] [Google Scholar]
- 2.Muhe L, Lulseged S, Mason K E.et al Case‐control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet 19973491801–1804. [DOI] [PubMed] [Google Scholar]
- 3.Clemens T L, Adams J S, Henderson S L.et al Increased skin pigmentation reduces the capacity of skin to synthesise vitamin D3. Lancet 1982174–76. [DOI] [PubMed] [Google Scholar]
- 4.Shaw N J, Pal B R. Vitamin D deficiency in UK Asian families: activating a new concern. Arch Dis Child 200286147–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashraf S, Mughal M Z. The prevalence of rickets among non‐Caucasian children. Arch Dis Child 200287263–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mughal M Z, Salama H, Greenway T.et al Florid rickets associated with prolonged breast feeding without vitamin D supplementation. BMJ 199931839–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamlyn B, Brooker S, Oleinikova K.et alInfant feeding 2000. London: Stationary Office, 2002
- 8.Department of Health Department of Health Report on Health and Social Subjects. 41 Dietary reference values for food, energy and nutrients for the United Kingdom. Report of the Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy. London: HMSO, 1991 [PubMed]
- 9.Department of Health Department of Health Report on Health and Social Subjects. 49 Nutrition and bone health with particular reference to calcium and vitamin D. Report of the Subgroup on Bone Health, Working Group on the Nutritional Status of the Population of the Committee on Medical Aspects of Food Policy. London: HMSO, 1998 [PubMed]
- 10.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes Food and Nutrition Board Institute of Medicine Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academy Press, 1997
- 11.Chief Medical Officer Meeting the need for vitamin D. CMO Update 2005426 (available at http://www.dh.gov.uk/assetRoot/04/11/56/64/04115664.pdf, accessed 28 July 2006) [Google Scholar]
- 12.National Institute for Clinical Excellence Antenatal care. Routine care for the healthy pregnant woman. London, NICE, 2003 (available at http://www.nice.org.uk/pdf/CG6_ANC_NICEguideline.pdf, accessed 28 July 2006)
- 13.Paediatric Formulary Committee BNF for children. London: BMJ Publishing Group, Royal Pharmaceutical Society of Great Britain, and RCPCH Publications, 2005
- 14.Iheanacho I. Primary vitamin D deficiency in children. Drug Ther Bull 20064412–16. [DOI] [PubMed] [Google Scholar]
- 15.Pal B R, Marshall T, James C.et al Distribution analysis of vitamin D highlights differences in population subgroups: preliminary observations from a pilot study in UK adults. J Endocrinol 2003179119–129. [DOI] [PubMed] [Google Scholar]
- 16.Allgrove J. Is nutritional rickets returning? Arch Dis Child 200489699–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holick M F. Vitamin D: the underappreciated D‐lightful hormone that is important for skeletal and cellular health. Curr Opin Endocrinol Diabetes 2002987–98. [Google Scholar]
- 18.Greer F R. Issues in establishing vitamin D recommendations for infants and children. Am J Clin Nutr 2004801759S–62S. [DOI] [PubMed] [Google Scholar]
- 19.Ladhani S, Srinivasan L, Buchanan C.et al Presentation of vitamin D deficiency. Arch Dis Child 200489781–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wharton B, Bishop N. Rickets. Lancet 20033621389–1400. [DOI] [PubMed] [Google Scholar]
- 21.Grindulis H, Scott P H, Belton N R.et al Combined deficiency of iron and vitamin D in Asian toddlers. Arch Dis Child 198661843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawson M, Thomas M. Vitamin D concentrations in Asian children aged 2 years living in England: population survey. BMJ 199931828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ala‐Houhala M, Koskinen T, Terho A.et al Maternal compared with infant vitamin D supplementation. Arch Dis Child 1986611159–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothberg A D, Pettifor J M, Cohen D F.et al Maternal‐infant vitamin D relationships during breast‐feeding. J Pediatr 1982101500–503. [DOI] [PubMed] [Google Scholar]
- 25.Department of Health Welfare food scheme. Free milk and vitamins: a guide for families. London, DoH, 2004 (available at http://www.dh.gov.uk/assetRoot/04/07/37/38/04073738.pdf, accessed 27 June 2006)
- 26.Mungen K L, Zhang S M, O'Reilly F.et al Vitamin D intake and incidence of multiple sclerosis. Neurology 200462(1)60–65. [DOI] [PubMed] [Google Scholar]
- 27.Guyton K Z, Kensler T W, Posner G H. Vitamin D and vitamin D analogs as cancer chemopreventive agents. Nutr Rev 200361(7)227–238. [DOI] [PubMed] [Google Scholar]
- 28.Holick M F. Vitamin D: importance in the prevention of cancers, type I diabetes, heart disease and osteoporosis. Am J Clin Nutr 200479(3)362–371. [DOI] [PubMed] [Google Scholar]
- 29.Schneider B, Weber B, Frensch A.et al Vitamin D in schizophrenia, major depression and alcoholism. J Neural Transm 2000107(7)839–842. [DOI] [PubMed] [Google Scholar]
- 30.Dunnigan M G, McIntosh W B, Sutherland G R.et al Policy for prevention of Asian rickets in Britain: a preliminary assessment of the Glasgow rickets campaign. BMJ 1981282357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goel K M, Sweet E M, Campbell S.et al Reduced prevalence of rickets in Asian children in Glasgow. Lancet 19812405–407. [DOI] [PubMed] [Google Scholar]
- 32.Dunnigan M G, Glekin B M, Henderson J B.et al Prevention of rickets in Asian children: assessment of the Glasgow campaign. BMJ 1985291239–242. [DOI] [PMC free article] [PubMed] [Google Scholar]