Abstract
Aims
To describe the outcome of four years' nationwide neonatal screening for congenital toxoplasmosis in liveborn newborns.
Methods
Congenital toxoplasmosis was diagnosed if specific Toxoplasma gondii IgM antibodies were detected in eluate from the PKU Guthrie filter paper card from a child. Infants diagnosed with congenital toxoplasmosis were examined for intracranial and retinal lesions and treated for three months with sulphadiazine, pyrimethamine, and folinic acid continuously.
Results
Eluates from PKU‐cards from 262 912 newborns were analysed. The birth prevalence of congenital toxoplasma infection was 2.1 per 10 000 liveborns. Congenital toxoplasmosis was suspected in 96 infants and confirmed in 55. Forty seven children were examined for intracranial and retinal lesions soon after birth; 12 had clinical signs at this first examination. Of these, 5 had intracranial calcifications, 2 had retinochoroidal lesions, 4 had intracranial calcifications and retinochoroidal lesions, and 1 had hydrocephalus, intracranial calcifications, and retinochoroidal lesions. Ninety four eyes were examined soon after birth; there were central retinochoroidal lesions in 9. Two children had macular lesion of both eyes, five had macular lesions of one eye. At 1 year of age, 10/68 eyes had central lesions, and at 3 years of age, 5/32 had central lesions. Thus new retinochoroidal lesions developed in three eyes in the observation period.
Conclusions
Neonatal screening is feasible for diagnosing children with congenital toxoplasmosis at birth in low endemic areas. Retinochoroiditis with macular lesion was diagnosed in 9.6% of the eyes at birth and in 15.6% of the eyes examined at 3 years of age.
Keywords: toxoplasma gondii, congenital, neonatal screening, PKU‐card
The parasite Toxoplasma gondii is an obligate intracellular protozoan of animals and humans worldwide. Infection with T gondii during pregnancy may result in congenital infection of the fetus; the maternal‐fetal transmission rate rises from less than 2% at 4 gestational weeks to more than 80% at 36 weeks of gestational age.1 Children infected in early pregnancy are more likely to show signs of infection, but about 75% of children born with congenital toxoplasmosis have no clinical signs at birth.2,3 Clinical signs include retinochoroiditis, intracranial calcifications, and in severe cases, hydrocephalus. Over time the infection may reactivate causing retinochoroiditis, visual impairment, or even blindness in individuals without clinical signs initially. Reactivation may occur during childhood, through puberty, and into adult life.4
The main routes of infection after fetal life are by eating undercooked or raw meat containing T gondii cysts,5 or by ingestion of oocysts shed by cats into the environment. In theory it is possible to prevent the infection by health education, but this has never been shown to be effective in practice.6,7 Early diagnosis by prenatal or neonatal screening followed by treatment may reduce clinical signs and prevent secondary complications and relapses later in life; this is the rationale for the prenatal screening programmes in Austria, France, and Slovenia, and for the neonatal screening programmes in Denmark and New England (USA).2
Recent studies have shown that early treatment during pregnancy does not prevent transmission,8,9 and the effect on clinical signs in the infant is marginal.8,10,11 At the same time, invasive diagnostic procedures during pregnancy involving amniocentesis carry the risk of abortion; furthermore, the sensitivity of detecting T gondii by PCR in amniotic fluid is only about 70%.12 Neonatal screening has the advantage that diagnosis is easier because blood samples are easier to obtain. However, the small number of children infected early in pregnancy might be missed because of decline or disappearance of toxoplasma specific IgM and IgA antibodies at birth. It is probable that neonatal screening based on the detection of IgM antibodies at birth will identify infections from the second half of pregnancy.
Based on a pilot study conducted in 1992–96,3 the National Board of Health in Denmark decided to introduce a national neonatal screening programme. We report the results and outcome from the first four years of the screening programme.
Methods
Patients
The study population included all liveborn children in Denmark who participated in the national neonatal screening programme from its start on 1 January 1999 until 31 December 2002. It is estimated that >98% of newborns in Denmark participate in the screening programme since the number of PKU‐cards tested per year exceeds the number of children born per year; <2% of children are tested twice due to inadequate test result from the first test of the PKU‐card.
Diagnostic algorithm
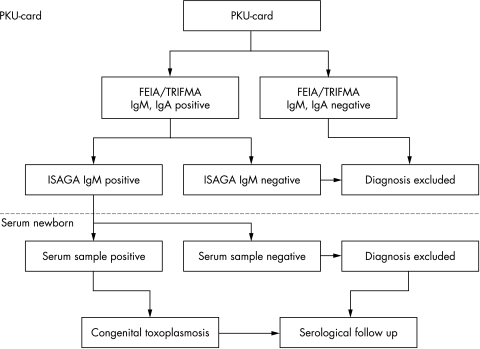
Eluates from the filter papers obtained 5–10 days after birth for the current analysis of phenylketonuria and hypothyroidism (Guthrie card, PKU‐card; Schleicher & Schuell no. 2992), were analysed at Statens Serum Institut, Copenhagen, Denmark. The diagnostic procedure was based on a two step detection of toxoplasma specific IgM and IgA antibodies eluted from a 3 mm blood spot punched from the card (fig 1).
Figure 1 Diagnostic algorithm for the neonatal screening programme for congenital toxoplasmosis.
Screening analysis (step one)
From 1 January 1999 to 31 January 2001, toxoplasma specific IgM antibodies were analysed using a μ‐capture enzyme immunoassay (fluorescence enzyme immunoassay, FEIA; Labsystems OY, Helsinki, Finland). From 1 February 2001, an in‐house assay based on automated time resolved immunofluorometric assay (TRIFMA), was introduced to take advantage of the AutoDELFIA system (Wallac, Turku, Finland). The assay was automated and integrated with the TSH analysis, and detected both toxoplasma specific IgM and IgA antibodies.13 The antibody assays were all based on eluate from a 3 mm blood spot from the PKU‐card in the first step as described previously.3
Analysis of screening positive samples (step two)
PKU‐cards with toxoplasma specific IgM antibodies above the cut‐off in the screening analysis were further analysed by a modified immunosorbent agglutination assay (ISAGA; BioMérieux, France) for toxoplasma specific IgM antibodies, using eluate from a second 3 mm blood spot. If the toxoplasma specific ISAGA IgM was negative, congenital toxoplasmosis was excluded. If the toxoplasma specific ISAGA IgM was positive, serum samples from the newborn and the mother were requested through the local paediatric department.
Analysis of serum samples
The paired mother and child serum samples were analysed for toxoplasma specific IgG and IgM antibodies by the Vidas system (BioMérieux, France) and for IgA by the Platelia system (PasteurDiagnostics, Paris), Sabin‐Feldman's dye test (in‐house), and ISAGA IgM (BioMérieux). In a few cases, a differentiated Western blot was needed to distinguish between infant and maternal toxoplasma specific IgG antibodies (kindly performed by Dr Jacqueline Franck, Marseille, France). Children with a positive ISAGA IgM on the PKU‐card, but negative when analysing a subsequent serum sample for toxoplasma specific IgM and IgA antibodies, were followed without treatment. When the child was 1 year of age, a new serum sample was tested for toxoplasma specific IgG antibodies to confirm that the child was not infected.
Children diagnosed with congenital toxoplasmosis at birth also had a new serum sample tested at 1 year of age for toxoplasma specific IgG antibodies for serological confirmation.
Guidelines on examinations and treatment
Examinations
The national Danish guidelines for follow up of children with congenital toxoplasmosis included a paediatric examination, ophthalmoscopy, and cerebral ultrasound examination at the time of diagnosis, shortly after birth, and a paediatric neurological evaluation at 3, 6, and 12 months of age, with a yearly follow up until 12 years of age. Examination of the retinae was performed at 1, 3, 6, 9, and 12 years of age, and individually when lesions had been observed.
Treatment
The recommended treatment was three months' continuous sulphadiazine (50–100 mg/kg/day), in two divided doses, and pyrimethamine (2 mg/kg on the first day, thereafter 1 mg/kg/day), supplemented with folinic acid (7.5 mg) administered twice a week.14 Prednisone was recommended in cases with active CNS involvement and/or active retinochoroiditis, never as monotherapy, but always administered together with anti‐parasitic drugs.15 Complete blood cell count with differential and platelet counts was performed weekly during treatment.
Follow up data
The children with congenital toxoplasmosis were followed longitudinally; data from the paediatric and ophthalmologic records of the children were collected after informed written consent had been obtained from the parents.
Ethics approval
The programme was recommended by the Danish National Board of Health, as an integrated part of the health service routinely offered to the population; as such the study did not need an ethics clearance. Parents were informed of the programme at the time of the PKU‐blood sample, and were free to refuse participation. Parents who refused were informed that it would have no impact on the future treatment and follow up of their child.
Statistical analysis
The present study was primarily a descriptive study. Analysing distributions we used the median and centiles. The χ2 test was used to compare proportions, with p < 0.05 considered significant. Confidence intervals for qualitative data were estimated assuming sample means following normal distribution.
Results
During the four year period, 262 912 PKU‐cards were tested. Ninety six samples were found to be toxoplasma specific IgM positive by the ISAGA‐IgM. Paired mother‐child blood samples were then requested for confirmatory testing. Eventually 55 of the 96 children were diagnosed with congenital T gondii infection (table 1). The birth prevalence of congenital toxoplasmosis in the present study was 2.1 per 10 000 liveborn children. Two infants were subsequently found to have positive toxoplasma serologies; both were born to women with suspected or known toxoplasma infection during pregnancy. The two infants were not diagnosed; in one case, because of a negative PKU‐card IgM, and in the other case, because of a negative ISAGA‐IgM following a positive PKU‐card IgM result.
Table 1 Number of infants tested per year and the number who tested anti‐toxoplasma antibody positive at the screening test and at the confirmatory test.
| Year | 1999 | 2000 | 2001 | 2002 | Total |
|---|---|---|---|---|---|
| Number of infants tested | 66232 | 67081 | 65450 | 64149 | 262912 |
| Positive PKU‐card | 35 | 22 | 25 | 14 | 96 |
| Positive serum sample | 11 | 13 | 19 | 12 | 55 |
The PKU‐card blood samples were obtained at an average of 7 days postpartum. The median age of the child when congenital toxoplasmosis was confirmed was 29 days (10% centile: 20 days; 90% centile: 66 days). The median age of the child when treatment was initiated was 32 days (10% centile: 22 days; 90% centile: 106 days).
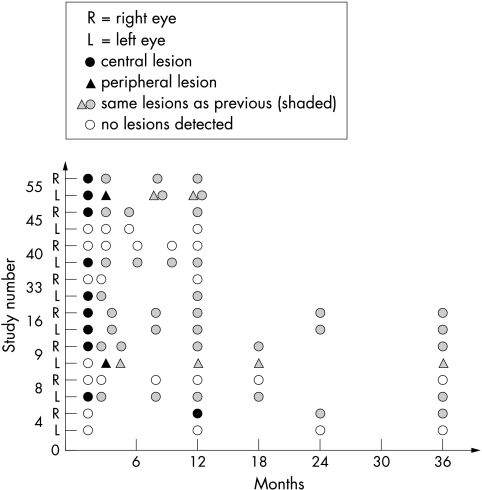
Parents of 49 of 55 newborns with congenital toxoplasma infection consented to provide data for the longitudinal study. One infant (number 17) received no treatment, and was excluded from the study. One infant (number 54) was treated according to protocol, but had eye examination only, and was also excluded from the study. This left a total of 47 study infants who had paediatric examination, ophthalmoscopy, and cerebral ultrasound examination performed shortly after birth. In this cohort based sample, 25.5% (12/47) had clinical signs of their congenital toxoplasmosis at birth. Of these 12 infants, five had intracranial calcifications only, four had intracranial calcifications and retinochoroiditis, one infant had intracranial calcifications, hydrocephalus, and bilateral retinochoroiditis, and two infants had retinochoroiditis only (data not shown). Thus 14.9% of infants (7/47) had retinal lesions when first evaluated; 23.5% (8/34) at 1 year of age, and 25% (4/16) at 3 years of age had retinal lesions. Of the 94 eyes examined at birth, 9.6% (9/94) showed clinical signs at the initial examination, all manifesting as central lesions. Two infants (numbers 16 and 55) had macular involvement of both eyes, whereas five infants had macular involvement of one eye (numbers 8, 9, 33, 40, and 45) (fig 2).
Figure 2 Children with eye lesions diagnosed shortly after birth or at later examinations.
Sixty eight of 94 eyes (72.3%) were examined when the children were 1 year of age. New retinochoroidal lesions were found in three eyes: a central lesion in a previously asymptomatic child (child number 4), a peripheral lesion in the fellow eye of a child who presented a central lesion in one eye at birth (child number 9), and a peripheral lesion in an eye with a formerly diagnosed central lesion (child number 55) (fig 2). At the end of this study, 22 of 47 children had reached 3 years of age at the latest cut‐off of the longitudinal study, and 16 of these children were examined (72.7%, 32/44). None of the children presented new retinochoroidal lesions after 1 year of age (fig 2).
Ultrasound examination of cerebrum revealed that 10 of the 47 infants (numbers 3, 4, 6, 8, 16, 19, 29, 40, 45, and 55) had intracerebral calcifications, of which one infant (number 16) also had hydrocephalus.
Severe neurological signs, gross intracranial calcification, and hydrocephalus were found in one patient (infant number 16). Paediatric neurological examination did not reveal any abnormality in the remaining study children.
Discussion
The diagnostic algorithm for the present national neonatal screening programme was designed as a two step analysis of blood eluted from the PKU‐card: in case the PKU‐card was toxoplasma specific IgM positive it was followed by a wide range of tests on serum samples from both the mother and the child. The first analysis on the PKU‐card in the screening programme was designed to have a high sensitivity and a low specificity, to avoid false negative results, and the second step to have a high specificity.
The 1992–96 pilot study showed that 84% of children with congenital toxoplasmosis at birth had toxoplasma specific IgM and/or IgA antibodies which could be eluted from their PKU‐card.3 Children with congenital toxoplasmosis, but without detectable toxoplasma specific IgM or IgA antibodies at birth therefore will not be found by neonatal screening based on detection of toxoplasma specific IgM and/or IgA antibodies. Other studies have found that only about 50% of children with congenital toxoplasmosis have toxoplasma specific IgM antibodies at birth.7,16,17,18 However, in these studies the mothers received treatment during pregnancy, and it is conceivable that treatment reduces the duration of the IgM response.7 In the 1992–96 pilot study, 16% of infants (4/25) showed a toxoplasma specific IgG seroconversion and negative IgM in the PKU‐card eluate.3
The neonatal screening programme for congenital toxoplasmosis was performed under the assumption that neonatal treatment reduces the risk of reactivation of toxoplasmosis. After serological confirmation we gave continuous treatment with pyrimethamine and sulphadiazine for three months, excluding spiramycin. This is a modification of the WHO recommendations which further includes systemic medication with spiramycin.14,19
Recommendations on duration of treatment vary in different centres (from three months to two years), as do the drugs of choice.17 We selected the protocol of three months' therapy based on the recommendations of the WHO working group, since there are at present no studies that have shown improved outcome with extended treatment regimens in this patient category.
The potential myelosuppression of the therapy was monitored by weekly blood examination. Compliance of the therapy was monitored by weekly follow up and contact with the family by the paediatrician when indicated.
Typical retinochoroidal scars indicative of ocular toxoplasmosis were found in 14.9% of the newborns in this study, which is comparable to earlier findings.3 It is well documented that retinochoroiditis may recur later in life in previously unaffected eyes despite early treatment.20 The reactivation process of retinal T gondii cysts is not well understood and in contrast to the cysts in the brain, reactivations in the retina generally occur in immunocompetent individuals.
Signs of recurrent disease (newly discovered retinochoroidal scars) were found in three eyes during the relatively short observation period. Two of these lesions were located in the retinal periphery with no impact on visual acuity and may have been overlooked at earlier examination. Two of 47 affected children had bilateral visual impairment due to ocular toxoplasmosis. The decline in percentage of children followed from 100% in the newborn period to approximately 72% at 1 and 3 years of age, might introduce a follow up bias since the children lost for follow up were all without clinical signs at the initial examination.
Ten infants had intracerebral calcifications according to the ultrasonographic examination. We are aware that ultrasound may underestimate the number of intracerebral calcifications. Computerised tomography may be a more sensitive method to show intracerebral calcifications, but due to the radiation load that this method implies,21 we abstained from using this method as a routine. Magnetic resonance imaging could be an alternative method, but this is much more time and resource consuming, and for calcifications it is not a specific examination.
Early evidence of severe neurological impairment was observed in one child. This, however, does not exclude more subtle deficits eventually to be disclosed, for example minor learning difficulties at school age.
Of the infants with congenital toxoplasmosis, 25.5% had clinical signs at birth, which is comparable to the 15% found in the 1992–96 pilot study.3 This is also comparable to results from centres performing prenatal screening: one study reported that 16.6% of newborns with congenital toxoplasmosis had clinical abnormalities at birth,10 and in another study from another centre, 13% of children with serologically diagnosed congenital toxoplasmosis were reported to have clinical abnormalities.8
The study of toxoplasma infection in Danish pregnant women (1992–96), comparing IgG seroconversion during pregnancy with neonatal diagnosis based on detection of toxoplasma specific IgM and IgA antibodies at birth showed that 2.8 per 1000 seronegative women seroconverted during pregnancy. Though attaining a lower figure, the birth prevalence of children with congenital toxoplasmosis of 2.1 per 10 000 liveborn newborns in this study, did not significantly differ from the 4.2 per 10 000 liveborn newborns diagnosed in the study cohort 1992–96 (χ2 = 2.39; p = 0.12).3
For the future, we would expect a declining number of both infected women during pregnancy, and newborns with congenital toxoplasmosis in Denmark, because the literature suggests a decline in the prevalence of T gondii infections in other European countries, Sweden, and the Netherlands.22,23,24 Further, the national screening programme has generated public awareness of the infection in Denmark and awareness of measures to prevent infection, which may also reduce the risk of infections.
The results of the present study are similar to the results from the New England Newborn Screening Programme which found that 1 per 10 000 liveborns had congenital toxoplasmosis in an area with half the seroprevalence of Denmark when compared to Denmark.2 The maternal‐fetal transmission rate in the previous study was 19.4%,3 which is low when compared to transmission rates of 40–50% reported in previous studies.25
What is already known on this topic
Neonatal screening for congenital toxoplasmosis is feasible, but data are limited
The majority of data on clinical outcome of this congenital disease comes from centres which perform prenatal screening
What this study adds
A national neonatal screening programme for congenital toxoplasmosis as opposed to a prenatal screening programme is a possible solution in a low endemic area such as Denmark, and can be included in a programme screening for several congenital infections
Continued surveillance of sensitivity and specificity of the serological test assays is essential. Data from follow up of these children are comparable to data from children diagnosed through prenatal screening
A neonatal screening programme for congenital toxoplasma infection is feasible, and a recent study from Brazil showed that neonatal screening for congenital toxoplasmosis can be included in a programme of screening for several congenital infections.26 The rationale for performing a neonatal screening programme is that treatment during the first year of life is effective in preventing the untoward occurrence or relapse of retinochoroiditis. Such safe data are still missing. However, a recent study on the clinical evolution of ocular lesions reported an overall good ocular prognosis when infection was identified and treated early.20
Clearly, additional data are needed to determine the most effective approach to prevent ocular and cerebral lesions caused by congenital toxoplasmosis.
Acknowledgements
We are grateful for the support of the parents of the children with congenital toxoplasmosis who consented to provide data for this study. We are indebted to the staff at the Departments of Paediatrics and Departments of Ophthalmology and Ophthalmologists in private practice in Denmark who performed the clinical examinations, supervised the treatment, and provided access to the clinical data. Last but not least, we thank the staff at Laboratory of Parasitology, Statens Serum Institut, Copenhagen, Denmark.
Abbreviations
FEIA - fluorescence enzyme immunoassay
Ig - immunoglobulin
ISAGA - immunosorbent agglutination assay
PKU - phenylketonuria
TRIFA - time resolved immunofluorometric assay
TSH - thyroid stimulating hormone
Footnotes
Competing interests: none declared
References
- 1.Dunn D, Wallon M, Peyron F.et al Mother to child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet 19993531829–1833. [DOI] [PubMed] [Google Scholar]
- 2.Guerina N G, Hsu H W, Meissner H.et al Neonatal serologic screening and early treatment for congenital Toxoplasma gondii infection. The New England Regional Toxoplasma Working Group. N Engl J Med 19943301858–1863. [DOI] [PubMed] [Google Scholar]
- 3.Lebech M, Andersen O, Christensen N C.et al Feasibility of neonatal screening for toxoplasma infection in the absence of prenatal treatment. Lancet 19993531834–1837. [DOI] [PubMed] [Google Scholar]
- 4.Koppe J G, Loewer‐Sieger D H, de Roever‐Bonnet H. Results of a 20‐year follow‐up of congenital toxoplasmosis. Lancet 1986i254–256. [DOI] [PubMed] [Google Scholar]
- 5.Cook A J, Gilbert R E, Buffolano W.et al Sources of toxoplasma infection in pregnant women: European multicentre case‐control study. European Research Network on Congenital Toxoplasmosis. BMJ 2000321142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambroise‐Thomas P, Petersen E. eds. Congenital toxoplasmosis: scientific background, clinical management and control. France: Springer‐Verlag, 2000
- 7.Foulon W, Naessens A, Ho‐Yen D. Prevention of congenital toxoplasmosis. J Perinat Med 200028337–345. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert R, Dunn D, Wallon M.et al Ecological comparison of the risk of mother to child transmission and clinical manifestations of congenital toxoplasmosis according to prenatal treatment period. Epidemiol Infect 2001127113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert R, Gras L, and the European Multicentre Study on Congenital Toxoplasmosis Effect of timing and type of treatment on the risk of mother to child transmission of Toxoplasma gondii. Br J Obstet Gyneaecol 2003110112–120. [DOI] [PubMed] [Google Scholar]
- 10.Villena I, Aubert D, Leroux B.et al Pyrimethamine‐sulfadoxine treatment of congenital toxoplasmosis: follow‐up of 78 cases between 1980 and 1997. Reims Toxoplasmosis Group. Scand J Infect Dis 199830295–300. [DOI] [PubMed] [Google Scholar]
- 11.Gras L, Wallon M, Pollak A.et al European Multicenter Study on Congenital Toxoplasmosis. Association between prenatal treatment and clinical manifestations of congenital toxoplasmosis in infancy: a cohort study in 13 European centres. Acta Paediatr 2005941721–1731. [DOI] [PubMed] [Google Scholar]
- 12.Romand S, Wallon M, Franck J.et al Prenatal diagnosis using polymerase chain reaction on amniotic fluid for congenital toxoplasmosis. Obstet Gynecol 200197296–300. [DOI] [PubMed] [Google Scholar]
- 13.Sørensen T, Spenter J, Jaliashvili I.et al An automated time‐resolved immunofluometric assay for detection of Toxoplasma gondii specific IgM and IgA antibodies in filterpaper samples from newborns. Clin Chem 2002481981–1986. [PubMed] [Google Scholar]
- 14.WHO Report of the WHO Consultation on Public Health Aspects of Toxoplasmosis. Hannover, Federal Republic of Germany 6–8 October 1987. WHO/CDS/VPH/88. 74. Geneva: WHO, 1988
- 15.Bosch‐Driessen E H, Rothova A. Sense and nonsense of corticosteroid administration in the treatment of ocular toxoplasmosis. Br J Ophthalmol 199882858–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foulon W, Naessens A, Volckaert M.et al Congenital toxoplasmosis: a prospective survey in Brussels. Br J Obstet Gynaecol 198491419–423. [DOI] [PubMed] [Google Scholar]
- 17.Foulon M, Villena I, Stray‐Pedersen B.et al Treatment of toxoplasmosis during pregnancy: a multicenter study of impact on fetal transmission and children's sequelae at 1 year. Am J Obstet Gynecol 1999180410–415. [DOI] [PubMed] [Google Scholar]
- 18.Pratlong F, Boulot P, Issert E.et al Fetal diagnosis of toxoplasmosis in 190 women infected during pregnancy. Prenat Diag 199414191–198. [DOI] [PubMed] [Google Scholar]
- 19.Wallon M, Liou C, Garner P.et al Congenital toxoplasmosis: systematic review of evidence of efficacy of treatment in pregnancy. BMJ 19993181511–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallon M, Kodjikian L, Binquet C.et al Long‐term ocular prognosis in 327 children with congenital toxoplasmosis. Pediatrics 20041131567–1572. [DOI] [PubMed] [Google Scholar]
- 21.Brenner D J, Elliston C. D, Hall EJ, et al. Estimated risks of radiation induced fatal cancer from pediatric CT. AJR 2001176289–296. [DOI] [PubMed] [Google Scholar]
- 22.Forsgren M, Gille E, Ljungstrom I.et al Toxoplasma antibodies in pregnant women in Sweden in 1969, 1979 and 1987. Lancet 19913371413–1414. [DOI] [PubMed] [Google Scholar]
- 23.Evengård B, Peterson K, Engman M L.et al Low incidence of toxoplasma infection during pregnancy and in newborns in Sweden. Epidemiol Infect 2001127121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kortbeek L M, Melker de H E, Veldhuijzen I K.et al Population‐based Toxoplasma seroprevalence study in the Netherlands. Epidemiol Infect 2004132839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desmonts G, Couvreur J. Congenital toxoplasmosis: a prospective study of 378 pregnancies. N Engl J Med 19742901110–1116. [DOI] [PubMed] [Google Scholar]
- 26.Neto E C, Rubin R, Schulte J.et al Newborn screening for congenital infectious diseases. Emerg Infect Dis 2004101069–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]