Short abstract
Coordination of targeted toxicological studies is needed
Keywords: adverse effects, endocrine disrupting chemicals, environment, puberty, reproductive disorders
It is widely acknowledged that our environment is becoming increasingly contaminated with man‐made chemicals. Mammals, as well as lower organisms, are vulnerable to exposure to these agents through a variety of different sources and routes and there are concerns that they may be having a detrimental effect on ecological and population health. It is just over 40 years since wildlife studies first suggested that environmental chemicals could be interacting with hormone systems,1 a hypothesis which has since been consolidated and debated both in the popular press and in the scientific literature. Concerns about environmentally mediated endocrine toxicity have also captured the attention of many national and international health organisations,2 as well as lobbying groups such as Greenpeace and the World Wildlife Fund (UK). In recognition of the possibility of an emerging public health threat, the European Commission3 has identified endocrine disrupting chemicals (EDCs) (table 1) as an important public health issue and is currently supporting a number of research initiatives.
Table 1 Endocrine disrupting chemicals (EDCs).
| 1. Definition: “any exogenous substance or material that alters the function(s) of the endocrine system and consequently causes adverse health effects in a intact organism, its progeny or its (sub)population”2 |
| 2. EDCs include certain synthetic man‐made chemicals, but also many naturally occurring plant compounds (table 2) |
| 3. EDCs may act as hormone system agonists or antagonists (or both) |
| 4. EDCs interfere with hormone action by a variety of mechanisms: |
| a. Hormone receptor binding |
| b. Hormone production and synthesis |
| c. Hormone transport |
| d. Hormone metabolism and excretion |
| 5. EDCs come from a large variety of sources and include: |
| a. Pesticides, herbicides, and pharmaceutical agents |
| b. Cosmetics, sunscreens, and plastic formulations |
| 6. Many chemicals have yet to be tested for any EDC activity |
Although there is a wealth of laboratory based studies that demonstrate and delineate the range of toxic effects that can be induced following exposure to certain chemicals, extrapolating these observations to the natural environment and to human populations has been difficult. To date the clearest evidence that chemicals can act as endocrine disrupting agents has been derived from observations made in the wildlife setting where reports of deleterious effects on the reproductive system have predominated. However, insufficient epidemiological and laboratory data currently exist to provide an accurate assessment of the risks to public health posed by EDCs in the relatively low ambient doses normally found in the environment. There is indirect evidence from secular changes in childhood growth and reproductive development which suggests exposure to these agents may be significantly influencing human health trends.
Endocrine disrupting chemicals: an outline
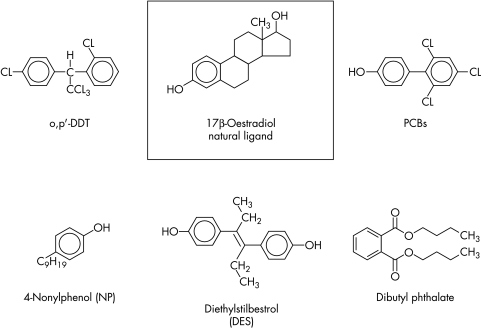
There are now more than 100 000 man‐made chemicals on the market. Only a relatively small subset of chemicals has been identified as having endocrine disrupting potential. The focus is on a relatively select group of man‐made compounds including the organochlorine pesticides, the polychlorinated biphenyls (PCBs), bisphenol A (BPA), the alklyphenols, and the phthalates, as well as a group of naturally occurring plant compounds known as the phytoestrogens (table 2). The majority of these compounds have been studied with respect to their ability to mimic the actions of oestrogen (behaving as so called xenoestrogens). However, it is recognised that many EDCs may interfere with other endocrine axes, including the testis, adrenal, and thyroid hormone systems. Some chemicals have effects on more than one endocrine system, a phenomenon that may be concentration or tissue dependent or both. Many EDCs also bear little structural resemblance to the natural hormone with which they interfere (fig 1) but are capable of inducing agonistic or antagonistic effects via a number of different mechanisms (table 1).
Table 2 Endocrine disrupting chemicals: example of the known principal categories, their sources, and main contamination routes in humans.
| Category | Example | Source | Contamination route |
|---|---|---|---|
| Pesticides | 2,4 Dichlorophen‐ | Herbicide | Foods: fruit/veg |
| oxyacetic acid | |||
| Hexachlorobenzene | Fungicides | Foods: fruit/veg, | |
| Tributyltin | cereals | ||
| Benomyl/carbendazium | Water | ||
| Vinclozolin | |||
| Malathion | Insecticides | Foods: fruit/veg | |
| Carbaryl | |||
| DDT (and | |||
| metabolites | |||
| DDE, DDD) | |||
| Aldrin | |||
| Industrial | Bisphenol A | Plastics | Plastic items |
| chemicals | Polystyrene | Drinks | |
| Foods: packaging | |||
| PCBs | Electrical: | Foods: fish/meat, | |
| waste byproducts | dairy items | ||
| Alkylphenols | Detergents | Household items | |
| Emulsifiers | Water | ||
| Fertilisers | Foods: fish | ||
| Phthalates esters | Plastics | Plastic items | |
| Drinks | |||
| Foods: packaging | |||
| Natural | Phytoestrogens, | Soya | Food/diet |
| plant | eg genistein, | Legumes/ | |
| compounds | coumestrol | beans |
Figure 1 Example of the chemical structure of suspected endocrine disrupting agents with known oestrogenic effects (xenoestrogens) (compared with the natural hormone ligand 17β‐oestradiol).
A number of chemicals with known or suspected endocrine disrupting activity are no longer approved or are restricted in their use. Nevertheless, some are known to be persistent in the environment and thus remain as general background pollutants. The organochlorine pesticides in particular are accumulating within the food chain and can be found widely distributed within fat‐containing foods of mammalian or marine origin. Direct dietary exposure is thought to be the most important route of contact with EDCs in humans, although significant exposure may also be achieved via routes other than food. These include air, drinking water, and particularly skin where contact with domestic chemicals present in household products, cosmetics, and clothing are thought to be relevant (table 2). Newborn infants and young children may also be potentially vulnerable to EDC exposure indirectly via the mother, with both transplacental and lactational routes of contamination recognised for certain chemicals.
Human health trends: environmental links
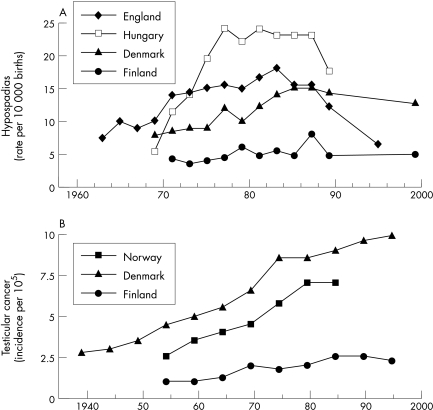
The current concerns about the effects of EDCs on humans are largely based on a series of observations which, when considered together, implicate these chemicals in a process that leads to deleterious effects in reproductive tract development and function. A number of adverse trends in male reproductive health have been observed in many developed countries including an increasing incidence of testicular cancer,4 a low and probably declining semen quality,5 and a high and possibly increasing frequency of undescended testes and hypospadias6 (fig 2). The relevance of environmental factors in the development of these problems is emphasised by the striking geographical variations reported between different countries, as well as the temporal changes in incidence rate that have occurred over the last 50 years. For example, epidemiological studies suggest that the rate of incidence of testicular cancer, cryptorchidism, hypospadias, and other male genital anomalies may be significantly lower in Finland compared to its relatively near Baltic neighbour Denmark. In a recently completed multinational project funded by the EU Framework V research program, the prevalence of cryptorchidism at birth in Denmark was observed to be at least fourfold higher than that observed in Finland.7 Similarly, the prevalence of hypospadias at birth was also much higher in Denmark (1.03%) compared with Finland (0.27%).8 Temporal changes in the incidence of male reproductive disorders were also observed in this study with a marked increase in the prevalence rate of cryptorchidism occurring over a 40 year period (1.8% in 1959–61 v 8.5% in 1997–01). This could not be accounted for by random fluctuations in prevalence rate, nor by differences in study design or by method of cryptorchidism ascertainment.7 In support of these observations, an increasing prevalence rate of cryptorchidism has also been seen in the UK, where over a 30 year period between the late 1950s and late 1980s the prevalence of cryptorchidism seems to have increased by 35%,9 although contemporary data suggest the rate may have stabilised in recent years.10
Figure 2 Secular, temporal, and geographical trends in (a) the prevalence of hypospadias at birth and (b) the age‐standardised incidence rate of testicular cancer in various European countries.
Secular changes in human growth and physical development resulting in increased mean population height and earlier age of onset of puberty have more consistently been linked to changes occurring in the environment, and have been related to improvements occurring in socioeconomic conditions including heath services, hygiene, and nutrition. Nevertheless, there is some evidence to suggest that exposure to EDCs may also have an important role in explaining some of these trends. Early pubertal development has been a common observation in children moving from a developing to a developed country, and has been attributed to the transition from an underprivileged to a privileged environment. Rapid increases in body fat mass leading to increases in circulating concentrations of sex steroids, insulin‐like growth factor I (IGF‐I), and leptin have been proposed as the possible mechanism.11 Toxicological studies carried out on children migrating from developing to developed countries have led to speculation that exposure to endocrine active chemicals may have a role in the development of early puberty in some children.12
Endocrine disrupting chemicals: putative tissue effects
It has been argued that the seemingly rapid pace of increase in the incidence of male reproductive disorders over the last few decades favours environmental or lifestyle factors rather than the accumulation of susceptibility genes within the population as the most likely explanation for this phenomenon.13 In support of this hypothesis, it has been proposed that these disorders are part of a single common underlying entity known as the testicular dysgenesis syndrome (TDS).13 The aetiological basis for this condition is complex and is thought to be due to a combination of both genetic and environmental factors that result in the disruption of normal gonadal development during fetal life. The so called oestrogen hypothesis was the first to propose that environmental chemicals with oestrogen‐like actions could have adverse effects on male gonadal development and subsequent reproductive health.14 This has since been expanded to include environmental chemicals with anti‐androgen actions and it is now thought that an imbalance between androgen and oestrogen activity is the key mechanism by which exposure to EDCs results in the development of TDS and male reproductive tract abnormalities.15
Some chemicals with known EDC activity interact at a molecular level with oestrogen and androgen receptors thereby potentially interfering with sex hormone dependent regulation of specific developmental programming genes. Examples include the Hox genes, which play a pivotal role in the normal differentiation of the reproductive tract and are directly modulated by potent synthetic non‐steroidal oestrogen agents such as diethylstilbestrol (DES).16 The androgen‐oestrogen imbalance hypothesis explored using subsequent animal studies is beginning to indicate the significance of anti‐androgenic activity in endocrine disruption. Such activity reflects two broad mechanisms, those that act at the level of the androgen receptor as antagonists of the natural ligand, and those that are active in suppression of androgen biosynthesis. Chemicals with defined antagonist activity such as the pesticides vinclozolin, linuron, and DDE competitively inhibit binding to the androgen receptor,17 whereas some phthalate esters (for example, diethylhexyl phthalate (DEHP)) act as anti‐androgens without binding to this receptor.18 The resulting alteration in androgen dependent gene expression in experimental animals results in delayed puberty, hypospadias, and cryptorchidism in addition to other indicators of incomplete masculinisation.19 The anti‐androgenic activity of agents such as dibutylphthalate (DBP), octylphenol, and DEHP can be demonstrated in the male reproductive tract of in utero exposed rats and resemble the changes defined by the TDS in humans.20
Time of exposure to EDCs and gender specific changes have a significant bearing on prenatal and postnatal development. Thus phthalates administered prenatally impede testicular descent in rats,21 while exposure of young female rats to o,p′‐DDT results in an earlier age of onset of puberty.22 Similar prenatal exposures to PCBs cause an acceleration in postnatal growth rate and changes in the timing of onset of puberty when compared to non‐exposed control animals.23 Rats fed phytoestrogen rich food (for example, flax seed) during pregnancy also have accelerated postnatal growth and an earlier puberty.24 How exposure to endocrine active chemicals results in changes in childhood growth and the timing of puberty is unclear, but there is likely to be a relationship to a specific oestrogenic effect of certain EDC compounds. Chronic exposure to oestrogenic chemicals, such as the DDT related compounds, is thought to lead to direct stimulation of oestrogenic sensitive tissues peripherally and to the induction of pubertal changes in a gonadotrophin independent manner. Alternatively, exposure to these compounds may act centrally, resulting in earlier maturation of the neuroendocrine mechanisms responsible for the induction of the hypothalamic‐pituitary‐gonadal axis (that is, gonadotrophin dependent puberty). Studies in animals show evidence both in vitro and in vivo that exposure to EDCs with oestrogenic activity can have profound effects on hypothalamic gonadotrophin releasing hormone (GnRH) gene expression and on the survival and growth of GnRH secreting neurons.25
Endocrine disrupting chemicals: wildlife and human studies
Observations made in the wildlife setting support many of the above laboratory studies. For example, the chemical mixtures contained within paper mill effluents can be capable of masculinising female mosquitofish by binding to the androgen receptor and inducing androgen dependent gene expression.26 Under‐masculinisation of male alligators has also been linked to contamination of their natural Florida habitat with DDT,27 whilst the discovery of large numbers of intersex fish in English rivers has been attributed to contamination of the aquatic environment with oestrogenic compounds.28 One of the most notable wildlife examples of endocrine disruption has been observed in marine gastropods (whelks) exposed to tributyltin (TBT), a constituent of marine anti‐fouling paints, that results in the development of male sexual organs in genetically female whelks (that is, pseudohermaphroditism or imposex).29 The mechanism is presumably related to inhibition of the steroidogenic enzyme, aromatase, responsible for the conversion of androgens to oestrogen. In the EU, and increasingly in other areas of the world, the application of TBT‐containing anti‐fouling paints and other organotin agents has been prohibited since 2003, with a complete ban expected by 2008.
Evidence directly implicating EDCs in the aetiology of male reproductive tract problems and in secular trends in growth and development in humans is limited and circumstantial. DES, once commonly prescribed to pregnant women to prevent recurrent miscarriages, produces a number of toxic effects on the reproductive systems of both the mother and her offspring.30 Several studies have reported an increased incidence of urogenital tract anomalies in male offspring (hypospadias, cryptorchidism, micropenis) following prenatal exposure to DES, together with impaired semen quality in later life.31 In addition, epidemiological studies have reported an increased risk of genital malformations and cryptorchidism in children of agricultural workers exposed to high levels of pesticides.32 Children exposed in utero or postnatally to high concentrations of PCBs or DDE have been observed to be smaller at birth, to have reduced postnatal growth, and to achieve a lower final height and lean body mass when compared to controls.33 Several studies have also reported early puberty in children following exposure to xenoestrogen compounds, including an earlier age of onset of pubic hair development and age of menarche in breast fed girls exposed to high concentrations of polybrominated biphenyls in utero.34 Outbreaks of isolated premature breast development (premature thelarche) in girls and of bilateral gynaecomastia in boys have also been reported in well‐defined geographical areas where there has been evidence of contamination of the local food supplies with phytoestrogens.35 Concentrations of the organochloride pesticide p,p′‐DDE (a persistent derivative of DDT with known potent oestrogen agonist and androgen antagonist actions) are significantly higher in the blood of children migrating from developing countries who subsequently develop central precocious puberty in their adoptive country when compared to native (Belgium) patients.12 These observations have led to the hypothesis that the oestrogenic effects of p,p′‐DDE result in both maturation of the central mechanisms regulating GnRH secretion and suppression of hypothalamic‐pituitary‐gonadal axis activity through negative feedback inhibition; subsequent withdrawal from exposure to these EDCs (on migration) removes this inhibitory effect allowing puberty to progress. In contrast to these observations, however, chronic exposure to PCB compounds has been also been linked to the later onset of puberty in both boys and girls, with a doubling of serum PCB concentrations associated with a three to four times risk of delayed puberty.36
The first study purportedly linking prenatal EDC exposure directly with developmental genital changes in human offspring has recently been reported.37 The anogenital distance (AGD; a sensitive index of prenatal anti‐androgen exposure used by animal researchers) in male infants was significantly shorter in boys whose mothers had evidence of elevated phthalate levels during pregnancy. In addition, reduced AGD and higher maternal phthalate exposure was associated with impaired testicular descent. In support of these observations, significant dose dependent associations between neonatal exposure to phthalate monoesters in breast milk and reproductive hormone levels in boys at 3 months of age (62 cryptorchid v 68 healthy boys) have also been recently reported.38 High phthalate concentrations in mothers' breast milk were linked to lower serum testosterone levels and to higher LH:testosterone ratio in infants, although no correlation between phthalate exposure and cryptorchidism was observed. These preliminary findings are the first human data that are consistent with phthalate induced adverse effects on Leydig cell function and with the phthalate related syndrome of incomplete virilisation observed in prenatally exposed rodents.
Endocrine disrupting chemicals: the future strategy
So far the evidence from toxicological studies carried out in animals and from observations made in selected wildlife and human populations have raised more questions than have been answered concerning the health effects and significance of EDCs in our environment. Epidemiological data suggest that certain populations have experienced a rise in reproductive health disorders and have observed secular changes in the growth and pubertal development of children, yet establishing a clear link with EDCs has not yet been possible. Other possible explanations for the observed health trends are at least as plausible, including improvements in medical surveillance techniques and changes in medical practice that have resulted in better case ascertainment and improved data records. Furthermore, most of the evidence of EDC effects based on laboratory experiments and epidemiological studies relates to levels far in excess of those encountered in the general environment.
These issues highlight two important questions that are central to assessing and monitoring the health risks posed by EDCs. Firstly, to what degree and extent are human and wildlife populations exposed to EDCs, and secondly what are the characteristics of low dose effects from the concentrations likely in the environment? Addressing these questions is likely to prove too difficult due to some inadequacies in current research methodology and strategy. It is recognised that most European populations are exposed to low levels of a number of chemical compounds with known and potential endocrine disrupting activity, but the full array of EDCs present in the environment remains largely unknown. Much of the focus to date has been on monitoring a relatively small number of persistent organochlorine compounds, while less attention is paid to the much larger number of non‐persistent pollutants with which humans are likely to come into contact. Most risk assessments for chemicals are not designed to evaluate endocrine disrupting activity at low concentrations and are based on the assumption of a threshold dose below which there are no observed effects (the so called NOAEL – no observed adverse effect level). This NOAEL principle may not be tenable when dealing with EDCs given that many of these hormonally active chemical compounds may still be biologically active at concentrations previously not considered toxic. In addition, safety testing programmes will need to take into consideration that some EDCs may not have a simple, classical, linear dose‐response relationship and that for some compounds an inverted U‐shaped dose‐response curve exists.39 Also many EDCs may be acting in concert with naturally occurring hormones to produce their adverse effects.
EDC assessment is further complicated when the effects of simultaneous, multiple exposure to chemicals is considered, as mixture effects can occur even when each component is present at a dose that individually does not produce any effect. Mixture effects are usually thought of in relation to the effects that are expected to occur based on the known potency of individual components. If the observed effects are larger than expected the mixture effect is considered synergistic, if smaller than expected it is antagonistic, and if expectations are met the individual components of the mixture are thought to be acting in an additive way. There is no consensus on how mixture additivity effects should be assessed and quantified, although it is generally agreed that single chemicals present in low, ineffective concentrations cannot be taken to signal absence of risk. Depending on how many similarly acting chemicals are also present, the assumption of no hazard can be entirely wrong. Finally, many testing guidelines are based on the reproductive consequences of EDC exposure and do not take into account the effects of EDCs on other hormone systems and tissues, nor do they allow for differential threshold effects at various developmental stages.
The current body of evidence has resulted in some progress towards a ban in the use of certain chemical agents. For example, several of the phthalates (DEHP, DBP, BBP (butylbenzyl phthalate)) are now prohibited in Europe, whilst others are strictly regulated (for example, DINP (diisononyl phthalate), DIDP (diisodecyl phthalate), and DNOP (dioctyl phthalate)) and are banned only from use in toys and childcare articles that can be put in children's mouths. There remain wide gaps in knowledge about the health effects of EDCs on wildlife and human populations and the shortcomings of current monitoring and chemical safety testing procedures strongly argue in favour of the need for further research. This situation has been highlighted at a recent meeting of international experts and scientists and a consensus statement was been published in 2005 outlining future research priorities (table 3).40 In Europe, a co‐ordinated programme of research has been established that should be able to address some of the major research questions that many believe need to be urgently answered if the protection of human and wildlife health in Europe is to be ensured. The creation of CREDO (Cluster of Research into Endocrine Disruption in EurOpe) is one such initiative. Supported by the European Commission, CREDO has the specific task of exploiting European research potential and of facilitating effective research collaborations across the many different research institutions and disciplines that have an interest in EDCs. Currently CREDO is co‐ordinating four core projects across Europe with a total budget of 20 million Euros at its disposal, with further funded projects likely to come on board at some point in the near future (table 4).
Table 3 The Prague Declaration on Endocrine Disruption (www.edenresearch.info/declaration.html).
| Summary of research priorities |
|---|
| 1. To ascertain the full array of EDCs |
| • Development of new chemical analytical methods |
| • Development and validation of bioassay techniques |
| • Establishment of human and wildlife reference tissue “Bio‐banks” |
| 2. To elucidate possible modes of action of EDCs |
| • Effects of EDCs on novel target tissues |
| • Effects on wider array of cellular signaling pathways |
| ‐ in particular those closely linked to major disease conditions |
| including metabolic syndrome, obesity, and heart disease |
| 3. To develop new assays and screening methods for EDCs |
| • Utilising modern technologies: |
| ‐ genomics, proteomics, bioinformatics, and metabonomics |
| 4. To determine mechanisms by which EDCs are involved in human disease |
| • Taking into account the complexity of the effect and exposure |
| scenario: |
| ‐ multiple tissue targets |
| ‐ exposure to multiple contaminants |
| ‐ low exposure levels |
| ‐ long exposure time |
| 5. To determine the significance and impact of EDC mixture effects |
| • Relationships between exposure time and dose |
| • Effects of sequential exposure to several chemicals |
| 6. To determine the consequences of endocrine disruption on wildlife and ecosystems |
| • Emphasis on better linkage of laboratory and field investigations, |
| considering a broad coverage of vertebrate and invertebrate groups |
| 7. To link effects seen at organism level to population‐level and ecosystems effects |
| • Application of rigorous human epidemiology methodology to the |
| wildlife arena |
| 8. To develop special programs of EDC health impact surveillance |
| • Detection of possible effects of EDCs on the newborn child giving |
| rise to problems in childhood and adulthood |
Table 4 The European CREDO initiative: summary of the current core research programmes (www.credocluster.info).
| Core projects 2005 |
|---|
| 1. EDEN |
| Endocrine disruption research. Exploring novel endpoints, exposure, low‐dose and mixture‐effects in humans, aquatic wildlife, and laboratory animals (www.edenresearch.info) |
| 2. EURISKED |
| Multi‐organic risk assessment of selected endocrine disrupters (www.eurisked.org) |
| 3. COMPRENDO |
| Comparative research on endocrine disrupters, phylogenetic approach, and common principles focusing on androgenic/anti‐androgenic compounds (www.comprendo‐project.org) |
| 4. FIRE |
| Risk assessment of brominated flame retardants as suspected endocrine disrupters for human and wildlife health (www.rivm.nl/fire) |
The magnitude of any adverse effects from exposure to EDCs in the environment remains unknown. Only the coordination of targeted toxicological studies in animals, including the application of the newer technologies of proteomics and metabonomics, with unbiased eco‐epidemiological studies will lead to better understanding of causation. It is a general principle that the developing fetus and young child are more susceptible to the adverse effects of toxic chemicals whatever their nature. Consequently, practical measures (for example, washing fresh fruit and vegetables before consumption) to reduce levels of exposure to chemicals to as low as is reasonably practical (the ALARP principle) are important actions to take to safeguard the population from the soup of man‐made chemicals.
Electronic‐database information
The following web sites have been mentioned in this article: EDEN, www.edenresearch.info; credo, www.credocluster.info; EURISKED, www.eurisked. org; COMPRENDO, www.comprendo‐project.org; and FIRE, www.rivm.nl/fire.
Copyright ©2006 BMJ Publishing Group & Royal College of Paediatrics and Child Health
Abbreviations
AGD - anogenital distance
DBP - dibutylphthalate
DEHP - diethylhexyl phthalate
DES - diethylstilbestrol
EDC - endocrine disrupting chemical
GnRH - gonadotrophin releasing hormone
NOAEL - no observed adverse effect level
PCBs - polychlorinated biphenyls
TBT - tributyltin
TDS - testicular dysgenesis syndrome
Footnotes
Competing interests: CLA has no competing interests. IAH is the current chairman of the Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (Food Standards Agency, UK)
The following web sites have been mentioned in this article: EDEN, www.edenresearch.info; credo, www.credocluster.info; EURISKED, www.eurisked. org; COMPRENDO, www.comprendo‐project.org; and FIRE, www.rivm.nl/fire.
References
- 1.Carson R.Silent spring. New York: Houghton Mifflin, 1962
- 2.Damstra T, Barlow S, Bergman A.et al, eds. Global assessment of the state‐of‐the‐science of endocrine disruptors. Geneva: The International Programme on Chemical Safety (IPCS), World Health Organisation, 2002
- 3.European Commission Communication from the Commission to the Council and the European Parliament on the implementation of the Community Strategy for Endocrine Disrupters ‐ a range of substances suspected of interfering with hormone systems of humans and wildlife. Report No. COM(2001)262 final. Brussels: European Commission, 2001
- 4.Moller H. Trends in sex‐ratio, testicular cancer and male reproductive hazards: are they connected? APMIS 1998106(1)232–8 discussion 238–9. [DOI] [PubMed] [Google Scholar]
- 5.Andersen A G, Jensen T K, Carlsen E.et al High frequency of sub‐optimal semen quality in an unselected population of young men. Hum Reprod 200015(2)366–372. [DOI] [PubMed] [Google Scholar]
- 6.Paulozzi L J, Erickson J D, Jackson R J. Hypospadias trends in two US surveillance systems. Pediatrics 1997100(5)831–834. [DOI] [PubMed] [Google Scholar]
- 7.Boisen K A, Kaleva M, Main K M.et al Difference in prevalence of congenital cryptorchidism in infants between two Nordic countries. Lancet 2004363(9417)1264–1269. [DOI] [PubMed] [Google Scholar]
- 8.Boisen K A, Chellakooty M, Schmidt I M.et al Hypospadias in a cohort of 1072 Danish newborn boys: prevalence and relationship to placental weight, anthropometrical measurements at birth, and reproductive hormone levels at three months of age. J Clin Endocrinol Metab 200590(7)4041–4046. [DOI] [PubMed] [Google Scholar]
- 9.John Radcliffe Hospital Group Cryptorchidism: a prospective study of 7500 consecutive male births, 1984–88. Arch Dis Child 199267892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Acerini C L, Forbes K, Tucker P.et al Incidence and natural history of cryptorchidism from birth to 2 years ‐ a prospective, longitudinal cohort study. Horm Res 200462(suppl 2)17 [Google Scholar]
- 11.Parent A S, Teilmann G, Juul A.et al The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev 200324(5)668–693. [DOI] [PubMed] [Google Scholar]
- 12.Krstevska‐Konstantinova M, Charlier C, Craen M.et al Sexual precocity after immigration from developing countries to Belgium: evidence of previous exposure to organochlorine pesticides. Hum Reprod 200116(5)1020–1026. [DOI] [PubMed] [Google Scholar]
- 13.Skakkebaek N E, Rajpert‐De Meyts E, Main K M. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 200116(5)972–978. [DOI] [PubMed] [Google Scholar]
- 14.Sharpe R M, Skakkebaek N E. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet 1993341(8857)1392–1395. [DOI] [PubMed] [Google Scholar]
- 15.Williams K, Fisher J S, Turner K J.et al Relationship between expression of sex steroid receptors and structure of the seminal vesicles after neonatal treatment of rats with potent or weak estrogens. Environ Health Perspect 2001109(12)1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Block K, Kardana A, Igarashi P.et al In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing mullerian system. FASEB J 200014(9)1101–1108. [DOI] [PubMed] [Google Scholar]
- 17.Lambright C, Ostby J, Bobseine K.et al Cellular and molecular mechanisms of action of linuron: an antiandrogenic herbicide that produces reproductive malformations in male rats. Toxicol Sci 200056(2)389–399. [DOI] [PubMed] [Google Scholar]
- 18.Parks L G, Ostby J S, Lambright C R.et al The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci 200058(2)339–349. [DOI] [PubMed] [Google Scholar]
- 19.Gray L E, Ostby J, Furr J.et al Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update 20017(3)248–264. [DOI] [PubMed] [Google Scholar]
- 20.Fisher J S, Macpherson S, Marchetti N.et al Human ‘testicular dysgenesis syndrome': a possible model using in‐utero exposure of the rat to dibutyl phthalate. Hum Reprod 200318(7)1383–1394. [DOI] [PubMed] [Google Scholar]
- 21.Shono T, Kai H, Suita S.et al Time‐specific effects of mono‐n‐butyl phthalate on the transabdominal descent of the testis in rat fetuses. BJU Int 200086(1)121–125. [DOI] [PubMed] [Google Scholar]
- 22.Gellert R J, Heinrichs W L. Effects of DDT homologs administered to female rats during the perinatal period. Biol Neonate 197526(3–4)283–290. [DOI] [PubMed] [Google Scholar]
- 23.Sager D B, Girard D M. Long‐term effects on reproductive parameters in female rats after translactational exposure to PCBs. Environ Res 199466(1)52–76. [DOI] [PubMed] [Google Scholar]
- 24.Tou J C, Chen J, Thompson L U. Flaxseed and its lignan precursor, secoisolariciresinol diglycoside, affect pregnancy outcome and reproductive development in rats. J Nutr 1998128(11)1861–1868. [DOI] [PubMed] [Google Scholar]
- 25.Gore A C. Environmental toxicant effects on neuroendocrine function. Endocrine 200114(2)235–246. [DOI] [PubMed] [Google Scholar]
- 26.Parks L G, Lambright C S, Orlando E F.et al Masculinization of female mosquitofish in Kraft mill effluent‐contaminated Fenholloway River water is associated with androgen receptor agonist activity. Toxicol Sci 200162(2)257–267. [DOI] [PubMed] [Google Scholar]
- 27.Guillette L J, Jr, Gross T S, Masson G R.et al Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspect 1994102(8)680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthiessen P, Sumpter J P. Effects of estrogenic substances in the aquatic environment. EXS 199886319–335. [DOI] [PubMed] [Google Scholar]
- 29.Santos M M, Castro L F, Vieira M N.et al New insights into the mechanism of imposex induction in the dogwhelk Nucella lapillus. Comp Biochem Physiol C Toxicol Pharmacol 2005141(1)101–109. [DOI] [PubMed] [Google Scholar]
- 30.Swan S H. Intrauterine exposure to diethylstilbestrol: long‐term effects in humans. APMIS 2000108(12)793–804. [DOI] [PubMed] [Google Scholar]
- 31.Gill W B, Schumacher G F, Bibbo M.et al Association of diethylstilbestrol exposure in utero with cryptorchidism, testicular hypoplasia and semen abnormalities. J Urol 1979122(1)36–39. [DOI] [PubMed] [Google Scholar]
- 32.Weidner I S, Moller H, Jensen T K.et al Cryptorchidism and hypospadias in sons of gardeners and farmers. Environ Health Perspect 1998106(12)793–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gladen B C, Ragan N B, Rogan W J. Pubertal growth and development and prenatal and lactational exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene. J Pediatr 2000136(4)490–496. [DOI] [PubMed] [Google Scholar]
- 34.Blanck H M, Marcus M, Tolbert P E.et al Age at menarche and tanner stage in girls exposed in utero and postnatally to polybrominated biphenyl. Epidemiology 200011(6)641–647. [DOI] [PubMed] [Google Scholar]
- 35.Freni‐Titulaer L W, Cordero J F, Haddock L.et al Premature thelarche in Puerto Rico. A search for environmental factors. Am J Dis Child 1986140(12)1263–1267. [DOI] [PubMed] [Google Scholar]
- 36.Den Hond E, Roels H A, Hoppenbrouwers K.et al Sexual maturation in relation to polychlorinated aromatic hydrocarbons: Sharpe and Skakkebaek's hypothesis revisited. Environ Health Perspect 2002110(8)771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swan S H, Main K M, Liu F.et al Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect 2005113(8)1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Main K M, Mortensen G K, Kaleva M M.et al Human breast milk contaminations with phthalates and alterations of endogenous reproductive hormones in three‐month‐old infants. Environ Health Perspect. (in press) (online 7 September 2005, see http://ehp.niehs.nih.gov/docs/2005/8075/abstract.html ) [DOI] [PMC free article] [PubMed]
- 39.Nagel S C, vom Saal F S, Thayer K A.et al Relative binding affinity‐serum modified access (RBA‐SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Environ Health Perspect 199710570–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulte‐Oehlmann U. The Prague Declaration on Endocrine Disruption. Environ Sci Pollut Res Int 200512(4)188. [PubMed] [Google Scholar]