Abstract
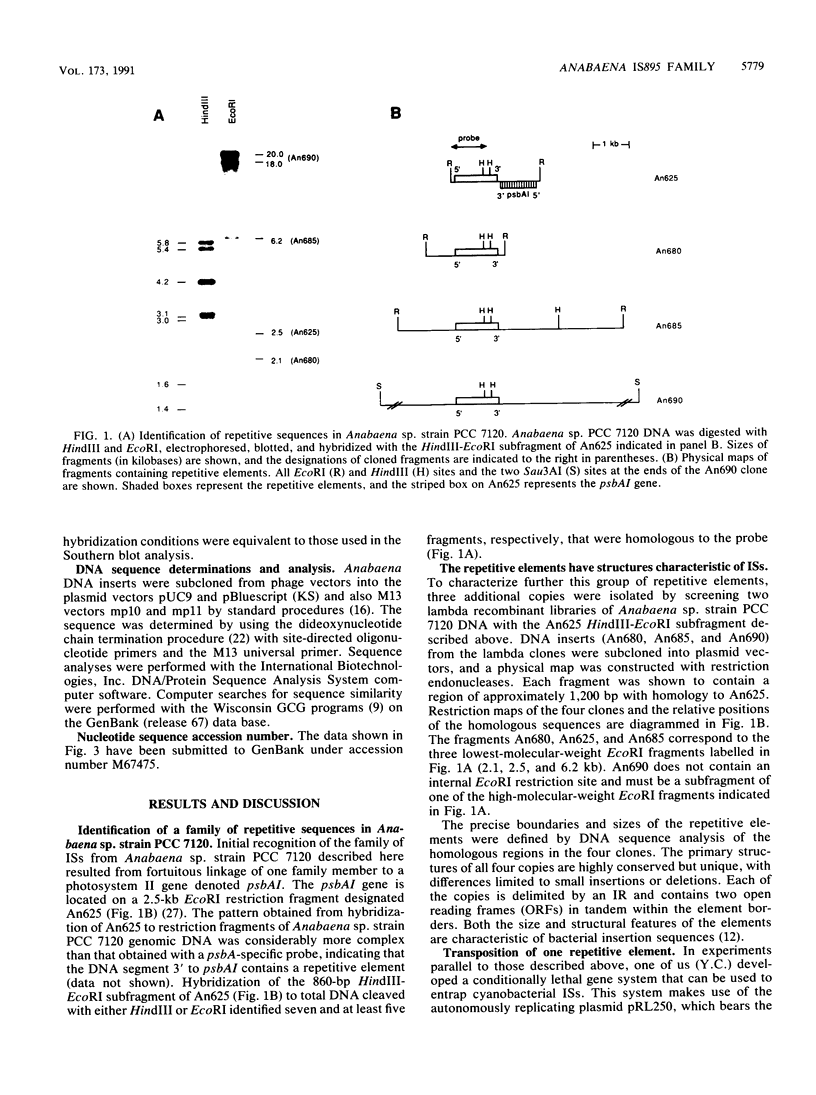
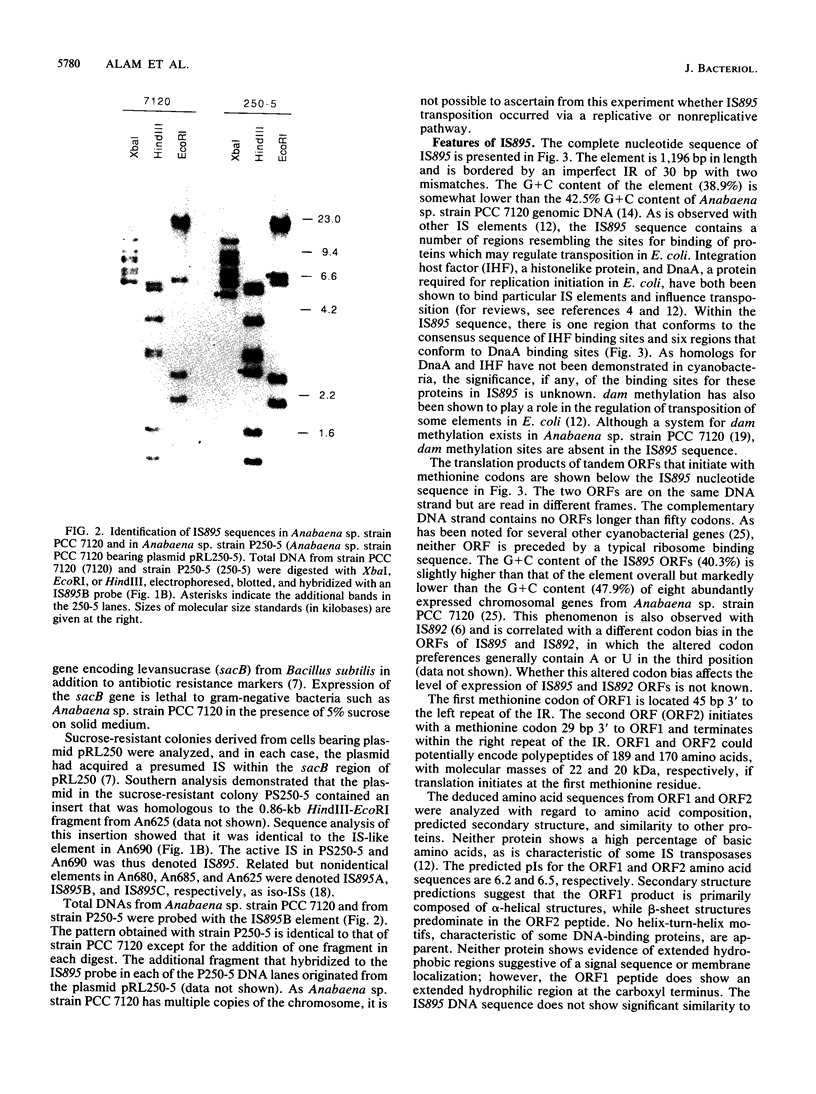
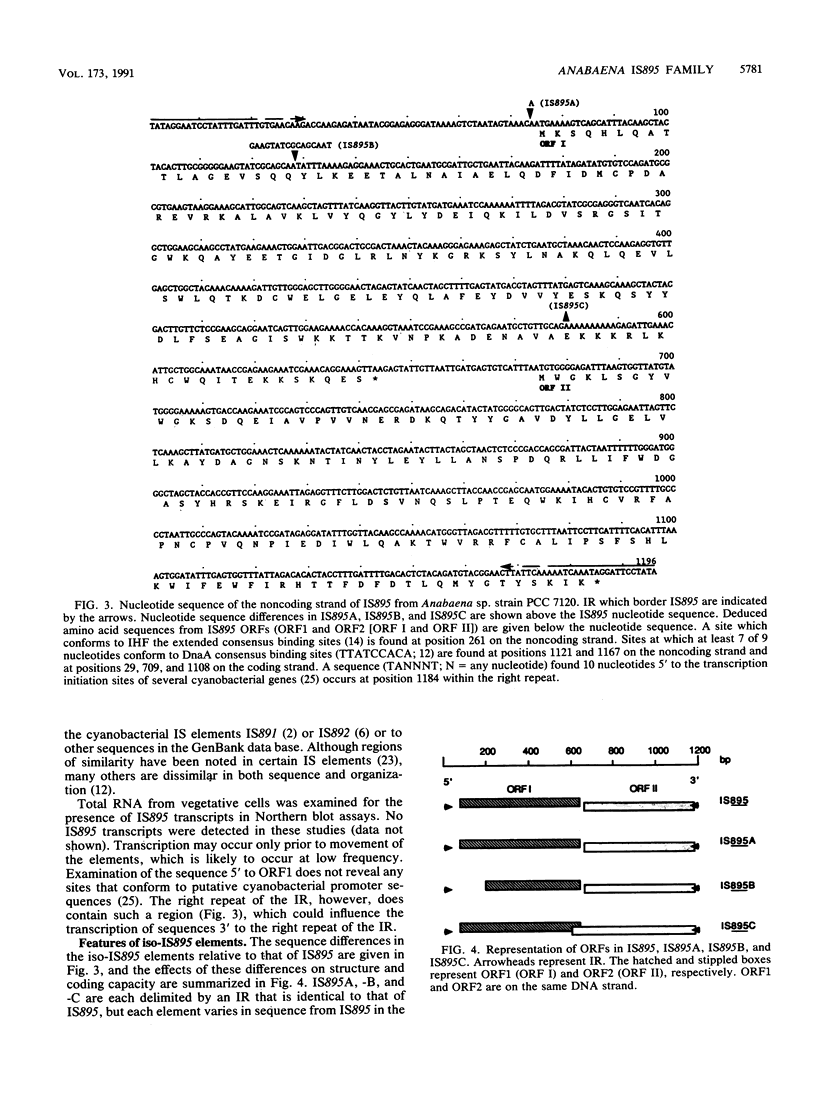
A family of repetitive elements from the cyanobacterium Anabaena sp. strain PCC 7120 was identified through the proximity of one element to the psbAI gene. Four members of this seven-member family were isolated and shown to have structures characteristic of bacterial insertion sequences. Each element is approximately 1,200 bp in length, is delimited by a 30-bp inverted repeat, and contains two open reading frames in tandem on the same DNA strand. The four copies differ from each other by small insertions or deletions, some of which alter the open reading frames. By using a system designed to trap insertion elements, one of the elements, denoted IS895, was shown to be mobile. The target site was not duplicated upon insertion of the element. Two other filamentous cyanobacterial strains were also found to contain sequences homologous to IS895.
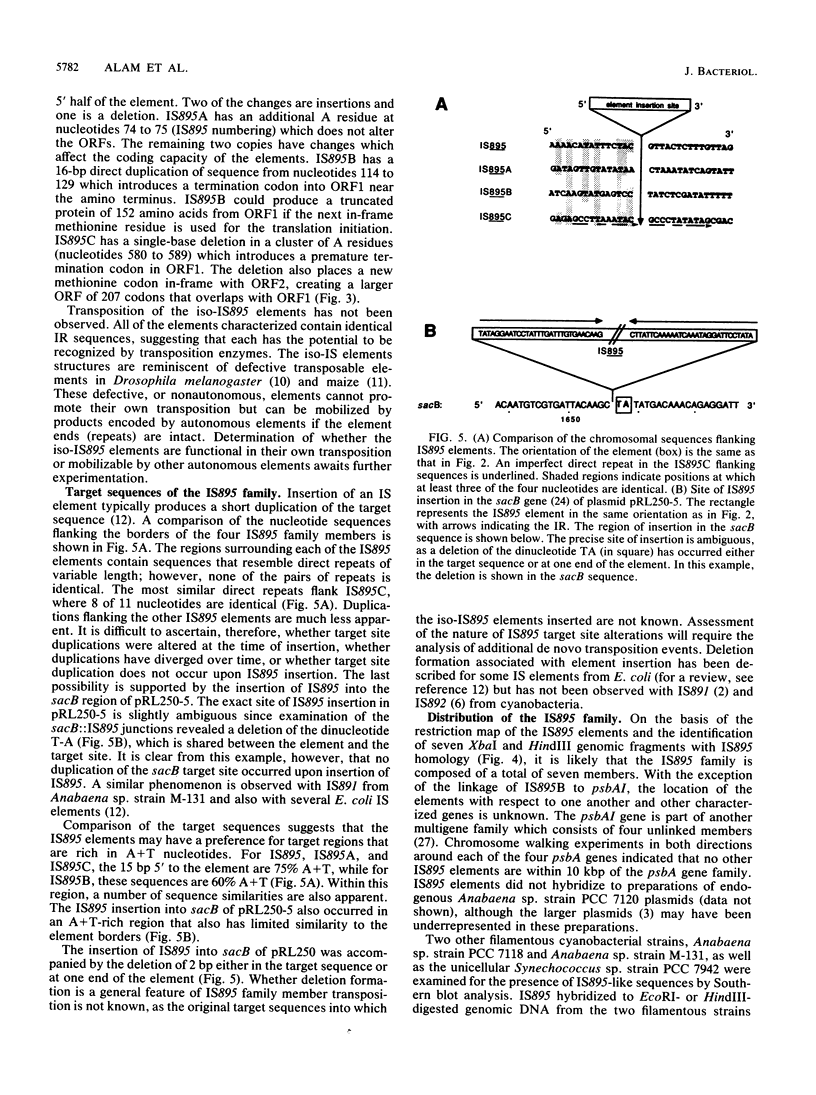
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft I., Wolk C. P. Characterization of an insertion sequence (IS891) of novel structure from the cyanobacterium Anabaena sp. strain M-131. J Bacteriol. 1989 Nov;171(11):5949–5954. doi: 10.1128/jb.171.11.5949-5954.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft I., Wolk C. P., Oren E. V. Physical and genetic maps of the genome of the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1989 Nov;171(11):5940–5948. doi: 10.1128/jb.171.11.5940-5948.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y. P., Wolk C. P. Use of a conditionally lethal gene in Anabaena sp. strain PCC 7120 to select for double recombinants and to entrap insertion sequences. J Bacteriol. 1990 Jun;172(6):3138–3145. doi: 10.1128/jb.172.6.3138-3145.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y. Characterization of insertion sequence IS892 and related elements from the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1991 Sep;173(18):5771–5777. doi: 10.1128/jb.173.18.5771-5777.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S. E., Haselkorn R. Isolation and sequence of the gene for the large subunit of ribulose-1,5-bisphosphate carboxylase from the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1835–1839. doi: 10.1073/pnas.80.7.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J. W., Robinson S. J., Haselkorn R. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature. 1985 Apr 4;314(6010):419–423. doi: 10.1038/314419a0. [DOI] [PubMed] [Google Scholar]
- Goodrich J. A., Schwartz M. L., McClure W. R. Searching for and predicting the activity of sites for DNA binding proteins: compilation and analysis of the binding sites for Escherichia coli integration host factor (IHF). Nucleic Acids Res. 1990 Sep 11;18(17):4993–5000. doi: 10.1093/nar/18.17.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo H., Nyman K., Doroszkiewicz W., Ohtsubo E. Multiple copies of iso-insertion sequences of IS1 in Shigella dysenteriae chromosome. Nature. 1981 Aug 13;292(5824):640–643. doi: 10.1038/292640a0. [DOI] [PubMed] [Google Scholar]
- Padhy R. N., Hottat F. G., Coene M. M., Hoet P. P. Restriction analysis and quantitative estimation of methylated bases of filamentous and unicellular cyanobacterial DNAs. J Bacteriol. 1988 Apr;170(4):1934–1939. doi: 10.1128/jb.170.4.1934-1939.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D., Mazur B. J., Haselkorn R. Isolation and physical mapping of nitrogen fixation genes from the cyanobacterium Anabaena 7120. J Biol Chem. 1982 Nov 10;257(21):13157–13163. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E., Kröger M., Rak B. IS150: distribution, nucleotide sequence and phylogenetic relationships of a new E. coli insertion element. Nucleic Acids Res. 1988 Jul 25;16(14B):6789–6802. doi: 10.1093/nar/16.14.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Le Coq D., Aymerich S., Gonzy-Tréboul G., Gay P. The DNA sequence of the gene for the secreted Bacillus subtilis enzyme levansucrase and its genetic control sites. Mol Gen Genet. 1985;200(2):220–228. doi: 10.1007/BF00425427. [DOI] [PubMed] [Google Scholar]
- Vrba J. M., Curtis S. E. Characterization of a four-member psbA gene family from the cyanobacterium Anabaena PCC 7120. Plant Mol Biol. 1990 Jan;14(1):81–92. doi: 10.1007/BF00015657. [DOI] [PubMed] [Google Scholar]