Abstract
Introduction
Fibroblast growth factor (FGF-23) is a novel phosphaturic factor. Current data suggest that serum phosphate, dietary phosphate and 1,25 dihydroxyvitamin D regulate circulating FGF-23 levels in vivo. We examined if hypogonadism-induced increases in serum phosphate are associated with increases in circulating FGF-23 in healthy men in the absence of dietary manipulation.
Materials and methods
25 healthy men were administered goserelin acetate (GnRH analog) 3.6 mg subcutaneously every 4 weeks for 12 weeks to induce acute testosterone and estrogen deficiency. Subjects consumed an ad libitum diet. Morning fasting blood and urine samples were collected to measure serum phosphate, serum intact FGF-23, PTH, and the maximum tubular reabsorption of phosphate (TmP/GFR) at baseline, week 4 and 12. The changes in serum FGF-23 and phosphate at weeks 4 and 12 were compared to baseline using paired t-tests.
Results
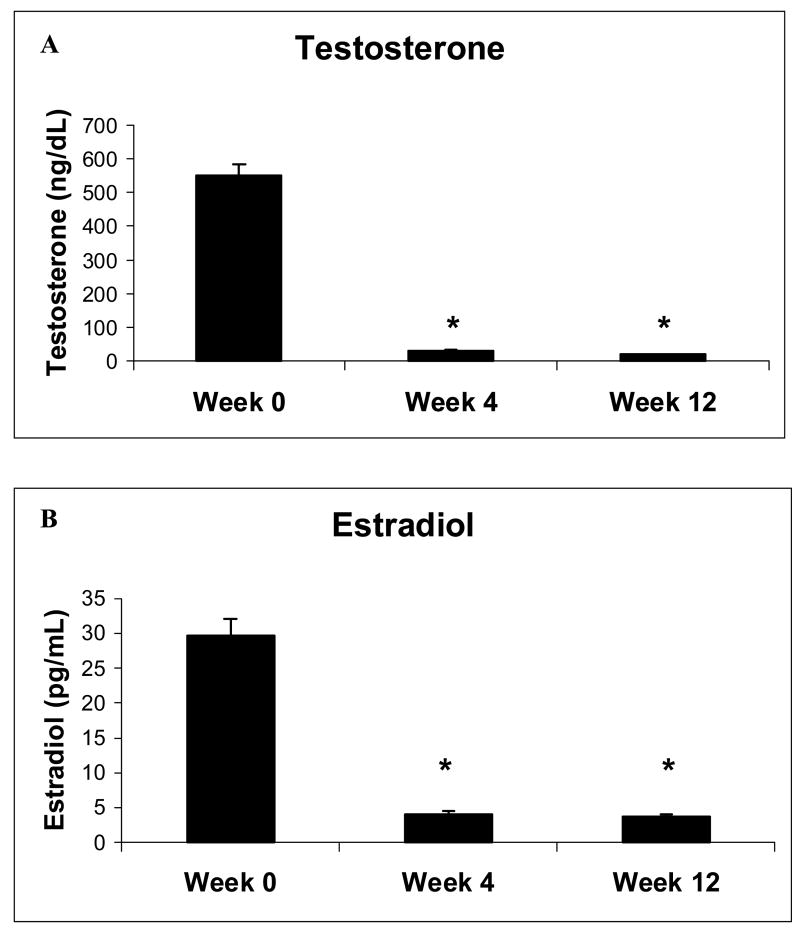
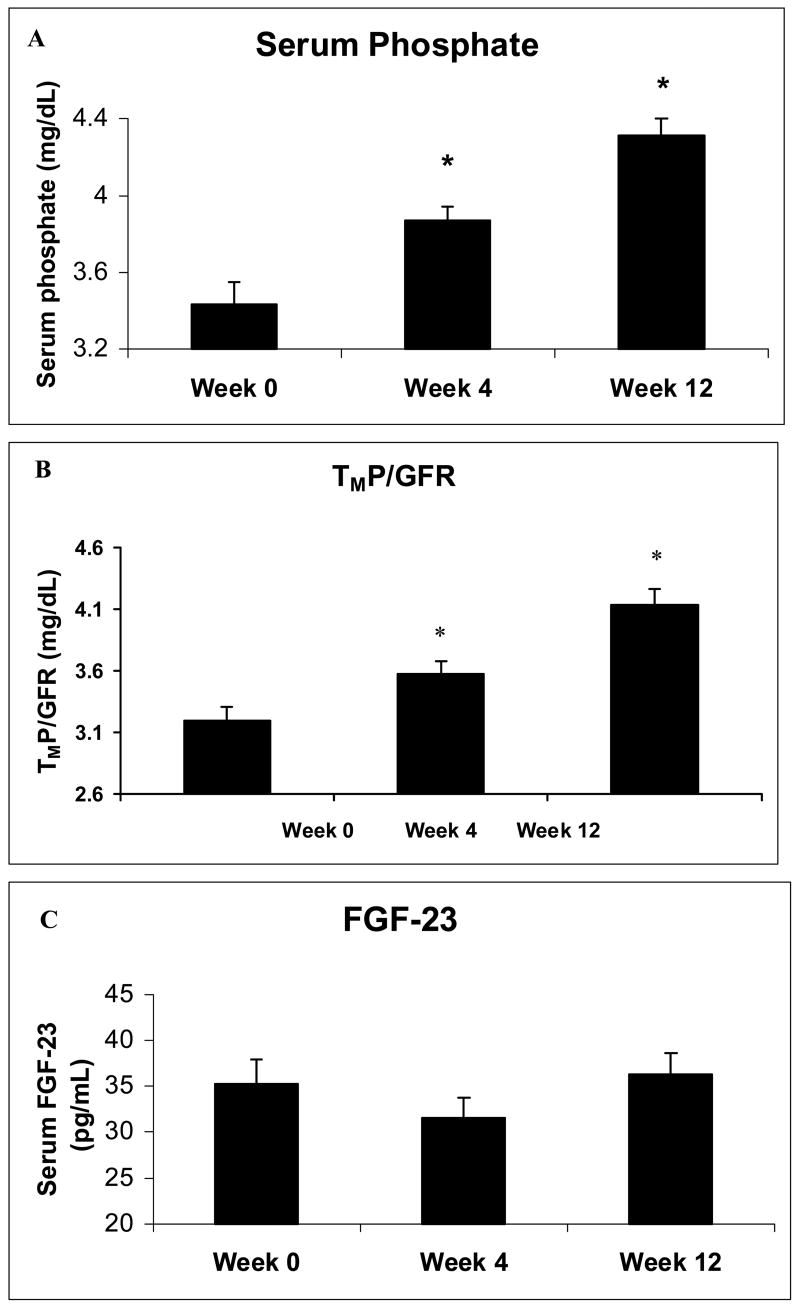
Goserelin therapy decreased mean serum testosterone levels from 543 ± 160 ng/dL to 33 ± 15 ng/dL at week 4 (p < 0.001), and to 20 ± 10 ng/dL at week 12 (p < 0.001). Serum phosphate increased significantly from 3.4 ± 0.6 mg/dL to 3.9 ± 0.4 mg/dL at week 4 (p = 0.002), and to 4.3 ± 0.4 mg/dL at week 12 (p < 0.001). TmP/GFR increased significantly from 3.2 ± 0.6 mg/dL to 3.6 ± 0.5 mg/dL at week 4 (p < 0.004), and to 4.1 ± 0.6 mg/dL at week 12 (p < 0.001). FGF-23 levels, however, did not change during the 12-week study.
Conclusions
Gonadal steroid deprivation increased serum phosphate levels in men but did not affect serum FGF-23 concentrations. The absence of any change in circulating FGF-23 suggests that supraphysiologic levels of serum phosphate may be required to stimulate circulating FGF-23 or that FGF-23 production is primarily sensitive to changes in dietary phosphate or 1,25 dihydroxyvitamin D within this physiologic serum phosphate range.
Keywords: Fibroblast growth factor 23; phosphate; hypogonadism; homeostasis; 1,25 dihydroxyvitamin D
Introduction
Fibroblast growth factor 23 (FGF-23) is a novel phosphate regulating hormone originally identified in patients with renal phosphate wasting disorders (1, 16, 38, 49, 51). Ablation of the FGF-23 gene in mice results in hyperphosphatemia, increased 1,25 dihydroxyvitamin D levels and increased mortality (37, 42), while over-expression of the FGF-23 gene leads to hypophosphatemia and inappropriately low 1,25 dihydroxyvitamin D levels (18, 40). Together these results suggest that FGF-23 is essential for normal phosphate and vitamin D metabolism. FGF-23 is detectable in healthy individuals (8, 10, 16, 19, 51), and studies suggest that dietary phosphate, serum phosphate and 1,25 dihydroxyvitamin D regulate its production (8, 10, 22, 29). The mechanism by which FGF-23 production is stimulated, however, is yet to be fully defined.
Serum phosphate increases when gonadal steroids are withdrawn in both men (12, 23, 43, 44, 48) and women (9, 11, 21, 24, 30, 35). This increase in serum phosphate levels may be secondary to increased bone resorption (7, 11, 43, 48), increased renal reabsorption of phosphate (9, 11, 21, 25, 30, 35, 43), or both. In this study, we sought to determine if the hypogonadism-induced change in serum phosphate levels are associated with changes in serum FGF-23. Specifically, we wished to test the hypothesis that GnRH-agonist therapy would increase serum phosphate and serum FGF-23 levels in young healthy men in the absence of dietary manipulation.
Subjects and Methods
Study Subjects
25 subjects (ages 20–44) were recruited as part of a larger study investigating the effects of androgens and estrogens on bone and mineral metabolism in men (20). Subjects were required to have normal serum testosterone, estradiol, lutenizing hormone, and follicle stimulating hormone levels. Additionally, they were required to have normal renal and hepatic function, and normal bone mineral density of the lumbar spine, femoral neck, and total hip. Subjects were excluded from participating if they had any history of congenital or acquired disorders in mineral metabolism (osteomalacia, vitamin D deficiency, hyperparathyroidism, Paget’s disease), recent fracture or immobilization, hyperthyroidism, cardiopulmonary disease, malignancy, benign prostatic hyperplasia, major psychiatric disease, or drug or alcohol abuse. Subjects were also excluded if they were using any drugs known to interact with the study medications or any drugs known to affect mineral metabolism (such as anticoagulants, gonadal steroids, glucocorticoids, anticonvulsants, and suppressive doses of thyroxine, lithium, bisphosphonates, calcitonin, or sodium fluoride). The study was approved by the Human Research Committee of Partners HealthCare Systems, and all subjects provided written informed consent.
Study Protocol
Subjects received the GnRH analog goserelin acetate (Zoladex; AstraZeneca, Wilmington, DE) 3.6 mg by subcutaneous injection every 4 weeks for 12 weeks. The effect of this medication was to induce acute and severe testosterone and estrogen deficiency. Subjects whose diets did not include at least 1000 mg elemental calcium daily were encouraged to increase calcium intake by either diet or calcium supplements. Otherwise, subjects consumed an ad libitum diet. Subjects were seen on the Mallinckrodt General Clinical Research Center (GCRC) at 4-week intervals for a total of 12 weeks (weeks 0, 4, 8, and 12). At each visit, a GCRC nurse administered the goserelin injection. Morning fasting blood and second void urine samples were collected before study medication was administered at each visit to assess hormone levels and biochemical markers.
Laboratory Methods
All testing was performed on previously unthawed samples that were stored at −80° F. Serum testosterone was measured by radioimmunoassay (RIA) using a commercial kit (Diagnostic Products, Los Angeles, CA) with a sensitivity of 4 ng/dL, and an intraassay coefficient of variation (CV) of 5% within the normal range and 18% in the castrate range, and an interassay CV of 7–12%. Serum estradiol was measured using an ultrasensitive competitive RIA after extraction and chromatographic purification (Nichols Institute, San Juan Capistrano, CA). The sensitivity of this assay is 2 pg/mL, and the intra- and interassay CVs are 5–6% and 6–13%, respectively. Serum intact FGF-23 was measured with a commercial kit that detects only the intact FGF-23 peptide (Kainos, Tokyo, Japan). This immunometric assay has a sensitivity of 3 pg/mL. The intra- and interassay CVs are ≤3% and ≤4%, respectively. Serum and urine phosphate were measured by colorimetric method (Roche Diagnostics, Indianopolis, IN) with intra- and interassay CVs of < 2 % and < 4%, respectively. Serum intact parathyroid hormone (PTH) was measured by a two-site immunoradiometric assay (Nichols Institute) with sensitivity of 1 pg/mL and intra- and interassay CVs of 2–3% and 6%, respectively. Serum 25 hydroxyvitamin D was measured using an extraction double-antibody RIA (DiaSorin, Stillwater, MN) with a sensitivity of 1.5 ng/mL and intra- and interassay CVs of 9–13% and 8–11%, respectively. Serum 1,25 dihydroxyvitamin D was measured by RIA (DiaSorin, Stillwater, MN) with intra- and interassay CVs of 7–11% and 11–15%, respectively. Serum N-telopeptide was measured using a competitive inhibition enzyme immunoassay (Osteomark, Ostex International, Inc., Seattle, WA) with sensitivity of 1nm BCE and intra- and interassay CVs of 6% and 9%, respectively. The maximum tubular reabsorption of phosphate corrected for glomerular filtration rate (TmP/GFR), a measure of renal phosphate excretion, was calculated using the Bijvoet formula (6).
Study Endpoints
The primary study endpoints were the change in serum phosphate and serum FGF-23 levels. Secondary endpoints included the change in serum PTH and 1,25 dihydroxyvitamin D levels, and the change in TmP/GFR.
Statistical Analysis
All data are expressed as the mean ± standard deviation unless specified otherwise. For all variables, the week 4 and 12 values were compared to the baseline visit using paired t-tests. Pearson correlation coefficients were calculated to determine the relationship between serum testosterone, estradiol, FGF-23, phosphate, and TmP/GFR. P values less than or equal to 0.05 were considered statistically significant (two-sided).
Results
The baseline characteristics of the 25 subjects are described in Table 1. Notably, the mean serum testosterone level was at the mid-point of the eugonadal range. The mean serum intact FGF-23 level was consistent with normative data from our group (8), and others (51) .
TABLE 1.
Baseline Characteristics
| Age (years) | 30.1 ± 5.6 |
| BMI (kg/m2) | 26 ± 4 |
| Testosterone (ng/dL) | 543 ± 160 |
| Estradiol (pg/mL) | 30 ± 13 |
| FGF-23 (pg/mL) | 35 ± 13 |
| Phosphate (mg/dL) | 3.4 ± 0.6 |
| TmP/GFR (mg/dL) | 3.2 ± 0.6 |
| Calcium (mg/dL) | 9.5 ± 0.2 |
| Parathyroid hormone (pg/mL) | 34 ± 18 |
| 25 hydroxyvitamin D (ng/mL) | 26 ± 10 |
| 1,25 dihydroxyvitamin D (pg/mL) | 41 ± 15 |
| Creatinine (mg/dL) | 1.0 ± 0.1 |
Systeme International conversion factors: for testosterone (nmol/L) is 0.03467; for estradiol (pmol/L) is 3.671; for phosphate (mmol/L) is 0.3229; for calcium (mmol/L) is 0.2495; for PTH (ng/L) is 1; for 25 hydroxyvitamin D (nmol/L) is 2.496; for 1,25 dihydroxyvitamin D (pmol/L) is 2.4; and for creatinine (μmol/L) is 88.4.
Gonadal steroids
Administration of goserelin induced severe gonadal steroid deficiency in all subjects (Figure 1). Specifically, mean serum testosterone decreased from 543 ± 160 ng/dL to 33 ± 15 ng/dL at week 4 (p < 0.001), and to 20 ± 10 ng/dL at week 12 (p < 0.001) (Figure 1a). Mean serum estradiol decreased from 30 ± 13 pg/mL to 4 ± 2 pg/mL at both weeks 4 and 12 (p < 0.001) (Figure 1b).
FIG. 1.
Mean (± SE) (a) testosterone and (b) estradiol levels at weeks 0, 4 and 12.
* p < 0.001 as compared to week 0.
Serum phosphate, TmP/GFR and FGF-23
After gonadal steroid withdrawal, mean serum phosphate increased from 3.4 ± 0.6 mg/dL to 3.9 ± 0.4 mg/dL at week 4 (p = 0.002), and to 4.3 ± 0.4 mg/dL at week 12 (p < 0.001) (Figure 2a). Additionally, TmP/GFR increased from 3.2 ± 0.6 mg/dL to 3.6 ± 0.5 mg/dL at week 4 (p < 0.004), and to 4.1 ± 0.6 mg/dL at week 12 (p < 0.001), reflecting increased renal phosphate reabsorption (Figure 2b). FGF-23 levels, however, did not change (Figure 2c).
FIG. 2.
Mean (± SE) (a) serum phosphate, (b) serum FGF-23 and (c) maximum tubular reabsorption of phosphate corrected for glomerular filtration rate at weeks 0, 4 and 12.
* p < 0.01 as compared to week 0.
Serum calcium, N-telopeptide, PTH and 1,25 dihydroxyvitamin D
Mean serum calcium was unchanged at week 4 but increased from 9.5 ± 0.2 mg/dL to 9.7 ± 0.3 mg/dL by week 12 (p < 0.02). This change in serum calcium was associated with a significant increase in serum N-telopeptide, a marker of bone resorption, at week 12 (p < 0.001) and decrease in serum PTH from 34 ± 18 pg/mL to 23 ± 11 pg/mL at week 12 (p = 0.003); there was no significant change in either serum N-telopeptide or PTH at week 4 as compared to baseline. Similarly, mean serum 1,25 dihydroxyvitamin D was unchanged at week 4 but decreased from 41 ± 15 pg/mL to 33 ± 13 pg/mL at week 12 (p = 0.02).
Univariate and multivariate analyses
We analyzed the relationship between serum phosphate and FGF-23 by univariate and multivariate analysis. While we observed an association between serum phosphate and FGF-23 at week 4 of gonadal steroidal withdrawal (R = 0.52, p = 0.0072), this association disappeared with multivariate analysis after controlling for calcium, PTH, 1,25 dihydroxyvitamin D and testosterone. Interestingly, however, 1,25 dihydroxyvitamin D was a significant predictor of circulating FGF-23 levels at week 4 (p = 0.0575) and at week 12 (p = 0.0357). We also observed that with univariate analysis, serum testosterone was negatively associated with serum phosphate levels at baseline (R = −0.44, p = 0.03) and estradiol was positively associated with serum phosphate at week 4 (R = −0.41, p = 0.04). Finally, there was a positive association between serum phosphate and TmP/GFR at each time point (R = 0.69, p = 0.0001 at baseline and week 12, R = 0.60, p = 0.0015 at week 4). There were, however, no other statistically significant associations amongst our variables of interest with univariate analysis.
Discussion
To our knowledge, this is the first study to examine the changes in circulating FGF-23 levels associated with hypogonadism-induced increases in serum phosphate levels. Specifically, these results demonstrate that acute gonadal steroid withdrawal increased serum phosphate levels and renal tubular reabsorption of phosphate significantly but did not stimulate FGF-23 production.
Prior studies have shown that acute gonadal steroid withdrawal in men results in increased bone resorption and increased serum phosphate levels, as noted in this study (20, 43, 48). Animal studies have confirmed that hypogonadism-induced bone resorption results in loss of phosphate from mineralized bone (7). Additionally, as shown in this study, gonadal steroid withdrawal has also been associated with increased renal tubular reabsorption of phosphate (43), possibly due to the observed concomitant decrease in serum PTH levels. It is also possible that the increased serum phosphate levels associated with goserelin administration are secondary to altered gastrointestinal absorption of phosphate or cellular redistribution of phosphate.
Secreted FGF-23 is a 227 amino acid peptide that is presumed to be a low-level transcript in all tissues (1, 39). In situ hybridization studies in patients with McCune-Albright syndrome and fibrous dysplasia of the bone demonstrated that osteoprogenitor cells, osteoblasts and osteocytes are the main cellular sources of FGF-23 (31). The molecular mechanisms that explain FGF-23’s physiologic effects are under intense investigation (4, 47, 52); and the potential interplay between FGF-23 and other phosphaturic factors overexpressed in renal phosphate wasting disorders is of great interest (5, 15, 27, 32, 33). Additionally, the primary stimulus that triggers the production or release of FGF-23 is still largely unknown. Current evidence suggests that in addition to 1,25 dihydroxyvitamin D, both serum phosphate and dietary phosphate regulate circulating FGF-23 levels in vivo. In animal models of renal failure-induced hyperphosphatemia, serum phosphate is positively associated with serum FGF-23 (34); and lowering serum phosphate with the use of phosphate binders suppresses circulating FGF-23 (26). Studies by Perwad et al support the hypothesis that dietary phosphate regulates circulating FGF-23 (29). In studies of mice lacking the renal sodium-phosphate cotransporter and wildtype mice, they demonstrated an effect of dietary phosphate on circulating FGF-23 levels that was independent of alterations in serum phosphate. Finally, administration of 1,25 dihydroxyvitamin D to mice rapidly increases both circulating FGF-23 levels and FGF-23 transcription in bone (22), and notably in our multivariate analysis, 1,25 dihydroxyvitamin D was the only significant predictor of circulating FGF-23 levels.
There is less consensus in human studies concerning the impact of serum and dietary phosphate on circulating FGF-23 levels. In a study of 6 men, phosphate loading and deprivation did not impact circulating FGF-23 levels (19). In two studies of men (n=29 and n=13), as compared to the control diet, dietary phosphate deprivation decreased circulating FGF-23 levels significantly, but dietary phosphate loading did not change FGF-23 levels (2, 10). Serum phosphate, however, was not associated with circulating FGF-23 (10). Finally, in our study of 66 men and women, dietary phosphate loading increased and depletion decreased circulating FGF-23 levels significantly, and multivariate regression analysis suggested that both dietary phosphate and serum phosphate were independent predictors of FGF-23 (8). Nonetheless, the relationship between FGF-23 and serum phosphate in these dietary studies in humans is limited by the fact that serum phosphate levels did not change with dietary phosphate loading in any of the studies (2, 8, 10, 19). Thus, the current model is the first protocol to describe serum FGF-23 levels in the context of acute increases in serum phosphate in humans with normal physiology.
The present study’s finding of stable FGF-23 levels with hypogonadism-induced increased serum phosphate must be interpreted in light of a recent report by Gupta et al who demonstrated that patients with chronic hypoparathyroidism have both elevated serum phosphate and elevated circulating FGF-23 levels (13). The difference in these findings may be related to the supraphysiologic levels of serum phosphate in subjects with hypoparathyroidism. Specifically, it is possible that higher levels of serum phosphate are needed to stimulate an increase in circulating FGF-23 levels than those achieved in our hypogonadal subjects whose mean serum phosphate was 4.3 ± 0.4 mg/dL, as opposed to 5.6 ± 1.1 mg/dL in the hypoparathyroid patients reported in Gupta et al. Alternately it is possible that dietary phosphate or 1,25 dihydroxyvitamin D is the main regulator of circulating FGF-23 when serum phosphate is in the physiologic range as seen in our hypogonadal men. Finally, the different findings of these studies may be secondary to the absence of PTH in patients with hypoparathyroidism. The relationship between FGF-23 and PTH in normal human physiology is yet to be fully defined, however most data (36, 41, 46, 50) do not support that an association exists between the two hormones. Of note, however, some animal data (3, 18), but not all (36, 40) suggest that there may be a relationship between FGF-23 and PTH. There is also lack of consensus about the relationship between calcium and FGF-23 in human studies, where some studies find an association between FGF-23 and calcium (14, 17) and others (28, 45, 46) do not. We did not observe a relationship between calcium and FGF-23 or between PTH and FGF-23 with either univariate or multivariate analysis.
There are limitations to our study. First, prior data suggest that the half life of serum intact FGF-23 is 30 minutes (51). Thus it is possible that there were transient changes in serum FGF-23 levels with withdrawal of gonadal steroids that were not detected due to the timing of our blood draws. However, since the serum phosphate levels were consistently higher than the baseline values, we would anticipate that the FGF-23 levels would be consistently elevated if serum phosphate was stimulating FGF-23 in this physiologic phosphate range. Second, we only measured the maximum tubular reabsorption of phosphate corrected for glomerular filtration rate and did not measure 24-hour urinary phosphate excretion. While it is possible that there were more dramatic changes in 24-hour urinary phosphate excretion with withdrawal of gonadal steroids, prior data suggest similar associations between changes in circulating FGF-23 and either 24-hour urinary phosphate measures or single urinary measures of renal tubular handling of phosphate (8, 10). Third, we did not control dietary phosphate intake over the intervention. Thus it is possible that increased or decreased dietary phosphate intake between subjects could have obscured any change in circulating FGF-23 levels with the withdrawal of gonadal steroids. However, we assessed the change in serum phosphate and FGF-23 by paired t-tests and since most individuals maintain a relatively constant diet, the impact of inter-subject variation in dietary phosphate would be minimized. Finally, in this model, it is impossible to differentiate the impact of the increase in serum phosphate from that of the decrease in 1,25 dihydroxyvitamin D on circulating FGF-23 levels. Given the data that 1,25 dihydroxyvitamin D stimulates increased FGF-23 transcription (22) it is possible that the observed decrease in circulating 1,25 dihydroxyvitamin D in this model may have obscured any potential increase in FGF-23 stimulated by the observed increase in serum phosphate.
In summary, this study demonstrates that hypogonadism-induced changes in serum phosphate levels are not accompanied by changes in circulating FGF-23 levels. These data are surprising and hypothesis generating given prior evidence that serum phosphate regulates circulating FGF-23. Together with prior data, this observation suggests that the putative “phosphate sensor”, which stimulates the release of circulating FGF-23, may either respond primarily to changes in dietary phosphate or 1,25 dihydroxyvitamin D, or may require supraphysiologic serum phosphate levels to affect FGF-23 production.
Acknowledgments
Funding: National Institute of Health grants K23-RR-16310 (to BZL) and M01-RR-01066 (to the Mallinckrodt General Clinical Research Center at Massachusetts General Hospital); the Massachusetts General Hospital Physician-Scientist Development Award (to SMB)
We are grateful to the staff of the Mallinckrodt General Clinical Research Center for implementation of the study protocol, Carrie Whelan for the performance of the FGF-23 assays, and Douglas Hayden for the performance of statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.ADHR-Consortium Autosomal dominant hypophosphatemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 2.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91:3144–9. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 3.Bai X, Miao D, Li J, Goltzman D, Karaplis AC. Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology. 2004;145:5269–79. doi: 10.1210/en.2004-0233. [DOI] [PubMed] [Google Scholar]
- 4.Baum M, Schiavi S, Dwarakanath V, Quigley R. Effect of fibroblast growth factor-23 on phosphate transport in proximal tubules. Kidney Int. 2005;68:1148–53. doi: 10.1111/j.1523-1755.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 5.Berndt T, Craig TA, Bowe AE. Secreted frizzled related protein-4 is a potent tumor derived phosphaturic agent. J Clin Invest. 2003;112:785–794. doi: 10.1172/JCI18563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bijvoet OL. Relation of plasma phosphate concentration to renal tubular reabsorption of phosphate. Clin Sci. 1969;37:23–36. [PubMed] [Google Scholar]
- 7.Broulik PD, Rosenkrancova J, Ruzicka P, Sedlacek R. Effect of alendronate administration on bone mineral density and bone strength in castrated rats. Horm Metab Res. 2005;37:414–8. doi: 10.1055/s-2005-870234. [DOI] [PubMed] [Google Scholar]
- 8.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21:1187–96. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 9.Falch JA, Gautvik KM. A longitudinal study of pre- and postmenopausal changes in calcium metabolism. Bone. 1988;9:15–9. doi: 10.1016/8756-3282(88)90022-1. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005;90:1519–24. doi: 10.1210/jc.2004-1039. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher JC, Riggs BL, DeLuca HF. Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. J Clin Endocrinol Metab. 1980;51:1359–64. doi: 10.1210/jcem-51-6-1359. [DOI] [PubMed] [Google Scholar]
- 12.Goldray D, Weisman Y, Jaccard N, Merdler C, Chen J, Matzkin H. Decreased bone density in elderly men treated with the gonadotropin-releasing hormone agonist decapeptyl (D-Trp6-GnRH. J Clin Endocrinol Metab. 1993;76:288–90. doi: 10.1210/jcem.76.2.7679397. [DOI] [PubMed] [Google Scholar]
- 13.Gupta A, Winer K, Econs MJ, Marx SJ, Collins MT. FGF-23 is elevated by chronic hyperphosphatemia. J Clin Endocrinol Metab. 2004;89:4489–92. doi: 10.1210/jc.2004-0724. [DOI] [PubMed] [Google Scholar]
- 14.Imanishi Y, Kawata T, Kobayashi K, Ishimura E, Miki T, Arnold A, Inaba M, Nishizawa Y. Elevated expression of FGF23 in bone in primary hyperparathyroidism in vivo. J Bone Miner Res. 2004;19:S133 Abstr. [Google Scholar]
- 15.Jan de Beur SM, Finnegan RB, Vassiliadis J. Tumors associated with oncogenic osteomalacia express genes important in bone and mineral metabolism. J Bone Miner Res. 2002;17:1102–110. doi: 10.1359/jbmr.2002.17.6.1102. [DOI] [PubMed] [Google Scholar]
- 16.Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Juppner H. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–63. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi K, Imanishi Y, Miyauchi A, Onoda N, Kawata T, Tahara H, Goto H, Miki T, Ishimura E, Sugimoto T, Ishikawa T, Inaba M, Nishizawa Y. Regulation of plasma fibroblast growth factor 23 by calcium in primary hyperparathyroidism. Eur J Endocrinol. 2006;154:93–9. doi: 10.1530/eje.1.02053. [DOI] [PubMed] [Google Scholar]
- 18.Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Juppner H, Jonsson KB. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–94. doi: 10.1210/en.2003-1768. [DOI] [PubMed] [Google Scholar]
- 19.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–9. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 20.Leder BZ, LeBlanc KM, Schoenfeld DA, Eastell R, Finkelstein JS. Differential effects of androgens and estrogens on bone turnover in normal men. J Clin Endocrinol Metab. 2003;88:204–10. doi: 10.1210/jc.2002-021036. [DOI] [PubMed] [Google Scholar]
- 21.Lindsay R, Coutts JR, Hart DM. The effect of endogenous oestrogen on plasma and urinary calcium and phosphate in oophorectomized women. Clin Endocrinol (Oxf) 1977;6:87–93. doi: 10.1111/j.1365-2265.1977.tb01999.x. [DOI] [PubMed] [Google Scholar]
- 22.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–15. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 23.Maillefert JF, Sibilia J, Michel F, Saussine C, Javier RM, Tavernier C. Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol. 1999;161:1219–22. [PubMed] [Google Scholar]
- 24.Masse PG, Dosy J, Jougleux JL, Caissie M, Howell DS. Bone mineral density and metabolism at an early stage of menopause when estrogen and calcium supplement are not used and without the interference of major confounding variables. J Am Coll Nutr. 2005;24:354–60. doi: 10.1080/07315724.2005.10719485. [DOI] [PubMed] [Google Scholar]
- 25.Morris HA, Porter SJ, Durbridge TC, Moore RJ, Need AG, Nordin BE. Effects of oophorectomy on biochemical and bone variables in the rat. Bone Miner. 1992;18:133–42. doi: 10.1016/0169-6009(92)90853-6. [DOI] [PubMed] [Google Scholar]
- 26.Nagano N, Miyata S, Abe M, Kobayashi N, Wakita S, Yamashita T, Wada M. Effect of manipulating serum phosphorus with phosphate binder on circulating PTH and FGF23 in renal failure rats. Kidney Int. 2006 doi: 10.1038/sj.ki.5000020. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien SP, Bowe AE, Byrne A, Weber W, Pragnell M, Kumar R, Schiavi SC. FGF23 and FRP4 internalize sodium-phosphate co-transporter (NaPi2a) in opposum kidney proximal tubule cells. J Bone Miner Res. 2003;18:S330. [Google Scholar]
- 28.Pande S, Ritter CS, Schiavi SC, Brown AJ. FGF-23 in chronic kidney disease and post renal transplantation. J Bone Miner Res. 2003;18:S293(Abstr.). [Google Scholar]
- 29.Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin d metabolism in mice. Endocrinology. 2005;146:5358–64. doi: 10.1210/en.2005-0777. [DOI] [PubMed] [Google Scholar]
- 30.Ralston SH, Fogelman I, Leggate J, Hart DM, Farrish E, Fletcher CD, McIntosh W, Barlow D. Effect of subdermal oestrogen and oestrogen/testosterone implants on calcium and phosphorus homeostasis after oophorectomy. Maturitas. 1984;6:341–4. doi: 10.1016/0378-5122(84)90006-9. [DOI] [PubMed] [Google Scholar]
- 31.Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, Waguespack S, Gupta A, Hannon T, Econs MJ, Bianco P, Gehron Robey P. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112:683–92. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rowe PS, de Zoysa PA, Dong R, Wang HR, White KE, Econs MJ, Oudet CL. MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics. 2000;67:54–68. doi: 10.1006/geno.2000.6235. [DOI] [PubMed] [Google Scholar]
- 33.Rowe PS, Kumagai Y, Gutierrez G, Garrett IR, Blacher R, Rosen D, Cundy J, Navvab S, Chen D, Drezner MK, Quarles LD, Mundy GR. MEPE has the properties of an osteoblastic phosphatonin and minhibin. Bone. 2004;34:303–19. doi: 10.1016/j.bone.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N. Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem. 2005;280:2543–9. doi: 10.1074/jbc.M408903200. [DOI] [PubMed] [Google Scholar]
- 35.Selby PL, Peacock M, Barkworth SA, Brown WB, Taylor GA. Early effects of ethinyloestradiol and norethisterone treatment in post-menopausal women on bone resorption and calcium regulating hormones. Clin Sci (Lond) 1985;69:265–71. doi: 10.1042/cs0690265. [DOI] [PubMed] [Google Scholar]
- 36.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–35. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 37.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of FGF23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–8. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98:6500–5. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia.[see comment] Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6500–5. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun. 2004;314:409–14. doi: 10.1016/j.bbrc.2003.12.102. [DOI] [PubMed] [Google Scholar]
- 41.Singh RJ, Kumar R. Fibroblast growth factor 23 concentrations in humoral hypercalcemia of malignancy and hyperparathyroidism. Mayo Clin Proc. 2003;78:826–9. doi: 10.4065/78.7.826. [DOI] [PubMed] [Google Scholar]
- 42.Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, Juppner H, Lanske B. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–32. doi: 10.1016/j.matbio.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stepan JJ, Lachman M, Zverina J, Pacovsky V, Baylink DJ. Castrated men exhibit bone loss: effect of calcitonin treatment on biochemical indices of bone remodeling. J Clin Endocrinol Metab. 1989;69:523–7. doi: 10.1210/jcem-69-3-523. [DOI] [PubMed] [Google Scholar]
- 44.Tan MO, Yilmaz C, Uygur MC, Duyur B, Erol D. Effects of combined androgen blockade on bone metabolism and density in men with locally advanced prostate cancer. Int Urol Nephrol. 2002;34:75–9. doi: 10.1023/a:1021358912734. [DOI] [PubMed] [Google Scholar]
- 45.Tebben P, Singh RJ, Bungum AO, Kumar R. Elevated Serum Fibroblast Growth Factor 23 in Patients with Small Cell Lung Cancer with Normal Serum Phosphorus. J Bone Miner Res. 2004;19:S121(Abstr.). [Google Scholar]
- 46.Tebben PJ, Singh RJ, Clarke BL, Kumar R. Fibroblast growth factor 23, parathyroid hormone, and 1alpha,25-dihydroxyvitamin D in surgically treated primary hyperparathyroidism. Mayo Clin Proc. 2004;79:1508–13. doi: 10.4065/79.12.1508. [DOI] [PubMed] [Google Scholar]
- 47.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Yasutake J, Fujita T, Fukumoto S, Yamashita T. Identification of Essential Moclecule Responsible for Tissue Specific FGF23. J Bone Miner Res. 2005;20:S345 (abstract). [Google Scholar]
- 48.Wang C, Swerdloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G, Matsumoto AM, Weber T, Berman N. Effects of transdermal testosterone gel on bone turnover markers and bone mineral density in hypogonadal men. Clin Endocrinol (Oxf) 2001;54:739–50. doi: 10.1046/j.1365-2265.2001.01271.x. [DOI] [PubMed] [Google Scholar]
- 49.White KE, Jonsson KB, Carn G, Hampson G, Spector TD, Mannstadt M, Lorenz-Depiereux B, Miyauchi A, Yang IM, Ljunggren O, Meitinger T, Strom TM, Juppner H, Econs MJ. The autosomal dominant hypophosphatemic rickets (ADHR) gene is a secreted polypeptide overexpressed by tumors that cause phosphate wasting. J Clin Endocrinol Metab. 2001;86:497–500. doi: 10.1210/jcem.86.2.7408. [DOI] [PubMed] [Google Scholar]
- 50.Yamashita H, Yamashita T, Miyamoto M, Shigematsu T, Kazama JJ, Shimada T, Yamazaki Y, Fukumoto S, Fukagaw M, Noguchi S. Fibroblast growth factor (FGF)-23 in patients with primary hyperparathyroidism. Eur J Endocrinol. 2004;151:55–60. doi: 10.1530/eje.0.1510055. [DOI] [PubMed] [Google Scholar]
- 51.Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87:4957–60. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 52.Yu X, Ibrahimi OA, Goetz R, Zhang F, Davis SI, Garringer HJ, Linhardt RJ, Ornitz DM, Mohammadi M, White KE. Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology. 2005;146:4647–56. doi: 10.1210/en.2005-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]