Abstract
Objective
To investigate the duration of postictal impairment of consciousness and the factors that affect it.
Patients and methods
90 children aged 1–16 years (37 male, 53 female, median age 6 years), attending the accident and emergency department, and inpatients of Leeds General Infirmary, Leeds, UK, who had experienced seizures involving impairment of consciousness. Interventions—hourly modified paediatric coma scores were determined, until a coma score of 15 was obtained. Linear regression analysis was used to determine the factors influencing recovery time.
Results
49 children were excluded owing to incomplete coma scoring, lost notes and refusal of consent. Median time for full recovery of consciousness was 38 min (0.63 h, range 0.05–17 h). Median recovery time was 18 min (0.3 h, range 0.05–9 h) from febrile seizures, which was significantly shorter than for seizures of other aetiologies (p<0.05), 1.35 h (range 0.07–13.13 h) from idiopathic seizures, 1.25 h (0.07–12.1 h) from remote symptomatic seizures and 4.57 h (0.25–17 h) from acute symptomatic seizures. Median recovery time after the use of benzodiazepines was 3.46 h (range 0.08–14.25 h), and was significantly longer (p<0.05) than for seizures not treated with benzodiazepines (median 0.47 h, range 0.05–17 h). Age, sex, seizure type and duration did not significantly affect recovery time.
Conclusions
Most children experiencing febrile seizures recover within 30 min. An acute symptomatic aetiology should be considered if recovery takes >1 h.
Many types of epileptic seizure are characterised by impaired awareness or responsiveness to external stimuli, or interference with cognitive functions such as memory. Until recently, such impairments were subsumed under the term “impaired consciousness”. The Diagnostic Scheme of the International League Against Epilepsy (ILAE) criticised the use of this term partly because at a fundamental level there is great dispute as to what is meant by consciousness, and at a practical level because of difficulties in measuring and assessing it.1 Nevertheless, the term continues to be widely used and most clinicians and lay people have little difficulty in recognising impaired consciousness. Indeed, in the 1989 seizure classification of the ILAE, it was the principal feature used in the subdivision of focal (partial) seizures into simple (without impairment of consciousness) and complex (with impairment of consciousness).2
The most widely used system for assessing and monitoring consciousness is the Glasgow Coma Scale. This measures consciousness according to eye opening, speech and motor response, and scores are from 3 (minimum score) to 15 (fully conscious). The Modified Paediatric Coma Scale is a version of the Glasgow Coma Scale modified for use in young children.3
After some epileptic seizures in which consciousness has been impaired, recovery is extremely rapid—for example, typical absences. For others, particularly generalised tonic–clonic seizures (GTCS) and complex focal seizures, there is a characteristic postictal period in which the patient's consciousness remains depressed. Clinical observation suggests that there is considerable variation in the duration of this period both between individuals and in the same individual after different seizures. However, there are no published quantitative data on the duration of postictal impairment of consciousness in children (or adults) or analysis of the factors affecting it.
One textbook states that many patients fall asleep postictally and may be unrousable for several hours, and that postictal confusion after generalised and focal seizures can last from 5–10 min up to hours, days or rarely 1–2 weeks.4 Another textbook on epilepsy suggested that the period is generally shorter in children and that “most children do not go through the post‐ictal phase of coma, confusion, headache and sleep, but recover in a few minutes”.5 Conversely, a paediatric textbook suggested that after GTCS, a child typically remains in deep sleep for 30 min to 2 h, although drowsiness after febrile seizures is usually brief.6
In many cases, a lack of quantitative data on the duration of recovery will be of little consequence. However, postictal impaired consciousness is not always a consequence of the seizure itself. Occasionally, it is a symptom of an evolving central nervous system (CNS) infection, such as meningitis or encephalitis, or a metabolic disorder. It may also arise as a consequence of head trauma causing or caused by the seizure. Occasionally, it may be due to non‐convulsive status epilepticus.
The clinician caring for a child after an epileptic seizure clearly needs to know how long postictal impairment of consciousness can reasonably be attributed to the seizure itself and when other causes should be actively considered. Such information is required to construct evidence‐based guidelines on the management of children after seizures.
Our study aimed to determine how long children take to fully recover consciousness after epileptic seizures in which consciousness has been impaired and to investigate the factors affecting recovery time.
Methods
Leeds General Infirmary, Leeds, UK, is a large teaching hospital providing secondary paediatric care for part of the city of Leeds and tertiary paediatric services (including neurosciences) for the Yorkshire region (population 3.8 million). In the hospital, the Modified Paediatric Coma Scale is used to assess consciousness in children recovering from epileptic seizures.3 The standard procedure is for the coma score to be recorded at least at hourly intervals after seizures, until full recovery. Ambulance crews are required to assess consciousness using coma scores in children being transported to hospital after seizures.
Ethical approval was obtained from the local research ethics committee of the Leeds Teaching Hospitals. Before starting the study, the principal investigator provided further reinforcement to the hospital staff with regard to the policy on the postictal monitoring of children using the Modified Paediatric Coma Scale .
All children aged 12 months–16 years presenting to the accident and emergency department of Leeds General Infirmary after a suspected epileptic seizure during which consciousness had been impaired or those who had had an epileptic seizure with impairment of consciousness while an inpatient at one of the paediatric wards at Leeds General Infirmary, were eligible for inclusion in study. A prior diagnosis of epilepsy was not required. Participants were identified by the principal investigator interrogating databases and ward staff on a near daily basis over a 7‐month period from October 2002 to May 2003.
The medical, nursing and paramedic records were obtained for all identified participants, and were perused by the principal investigator. Participants were excluded at this stage if:
they did not meet the entry criteria (eg, the event was non‐epileptic);
their seizure had begun an hour before the first coma score was recorded, and they had fully regained consciousness at the time of the first coma score;
they had been intubated and ventilated.
The administration of rescue drugs for epilepsy was not an exclusion criterion.
Children were included in the study more than once if they had seizures on more than one occasion with full recovery in between, during the same or separate admissions.
A standard form was used to collect demographic details of each participant, along with information on their medical history and full details about their seizure. This included its manifestations, duration, and the type and timing of any rescue drugs given. Coma scores were extracted from the records.
The recovery time for each seizure was calculated as the time between the end of the seizure until a coma score of 15 was obtained.
Seizures were classified according to the 1989 ILAE classification,2 rather than the 2001 Diagnostic Scheme,1 reflecting the continued widespread usage of the ILAE classification in clinical areas where the study was conducted. Seizure aetiology was classified as febrile, remote symptomatic, acute symptomatic or idiopathic. A febrile seizure was defined as an epileptic seizure occurring in association with a pyrexia in a neurodevelopmentally normal child <5 years of age in the absence of a confirmed or suspected CNS infection. Pyrexia was defined as a temperature >37.5°C. In some cases, however, febrile seizures occurred out‐of‐hospital, hence temperature was not recorded. Remote symptomatic seizures were epileptic seizures considered to arise as a consequence of a previous known or suspected disorder of the CNS that is remote from the seizure itself. Acute symptomatic seizures were those resulting from acute damage to the CNS.
Median recovery times were calculated for the group as a whole and for subgroups defined by age, sex, seizure type, aetiology of seizure and according to whether rescue drugs for epilepsy had been given. Data were analysed using STATA software V.9.0, by including these variables in the linear regression model. Log transformation of the outcome was used to overcome skewness in the time to recovery outcome variable. Model goodness of fit was assessed by graphical inspection of the standardised residuals versus the fitted values, which showed a good random scatter using the log‐transformed outcome variable. The standard significance level of 5% was used.
Results
A total of 90 children were included in the study, of whom, 37 were boys (median age 2.5 years; range 1–16 years) and 53 were girls (median age 4.5 years; range 1–16 years). Ten of these patients were included twice after separate seizure episodes and five patients were included three times. The total number of seizure episodes analysed was 110 (68 girls and 42 boys). Seventeen of the episodes involved more than one seizure.
Forty nine other seizure episodes (28 boys and 21 girls) were potentially eligible but not included (31% exclusion rate). In 37 patients, this was because the coma scores were not recorded (n = 31) or were incompletely recorded (n = 6). In nine patients, the medical records could not be traced, in two patients, consent was refused and in one child, an unrelated fluctuation in consciousness was observed. Six other seizure episodes did not meet the entry criteria as patients were intubated and ventilated.
Most seizures were GTCS, including secondary GTCS (n = 79); other seizure types included complex focal (n = 20), clonic (n = 6) and tonic (n = 5). Fifty nine seizures were febrile, 25 were remote symptomatic, 17 were idiopathic and 7 were from acute symptomatic. Causes of acute symptomatic seizures were head injury (n = 2), post‐craniotomy meningitis (n = 2), hydrocephalus (n = 2) and drug overdose (diagnosis was known before the seizure, n = 1).
Rescue drugs for epilepsy were given in 25 patients (diazepam in 14, midazolam in 2, lorazepam in 1; 8 were given more than one benzodiazepine).
For the group as a whole, the median seizure duration was 3.5 min (range 20 s–1 h) and the median recovery time was 38 min (range 3 min–17 h). Table 1 shows details of seizure durations and recovery times for different subgroups of patients.
Table 1 Details of seizure duration and recovery time in different subgroups of patients.
| Variable | Subgroup | n | Seizure duration (min) | Recovery time (h) | ||
|---|---|---|---|---|---|---|
| Median | Range | Median | Range | |||
| Sex | Female | 68 | 3.5 | 0.03–60 | 0.74 | 0.07–17.00 |
| Male | 42 | 3.5 | 0.03–55 | 0.41 | 0.05–13.13 | |
| Seizure aetiology | Febrile | 59 | 2.5 | 0.03–40 | 0.30 | 0.05–9.00 |
| Remote symptomatic | 25 | 8.0 | 0.25–60 | 1.25 | 0.07–12.10 | |
| Idiopathic | 19 | 5.0 | 0.75–50 | 1.35 | 0.07–13.13 | |
| Acute symptomatic | 7 | 0.5 | 0.03–8.0 | 4.57 | 0.25–17.00 | |
| Seizure type | GTCS | 79 | 4.0 | 0.25–60 | 0.55 | 0.05–13.13 |
| Complex focal | 20 | 4.75 | 0.03–50 | 0.82 | 0.10–14.25 | |
| Clonic | 6 | 1.25 | 0.03–50 | 3.74 | 0.05–17.00 | |
| Tonic | 5 | 1.5 | 0.25–5 | 2.80 | 0.22–8.92 | |
| No of seizures | Single | 93 | 4.0 | 0.03–60 | 0.53 | 0.05–17.00 |
| Multiple | 17 | 1.0 | 0.03–50 | 4.00 | 0.10–14.25 | |
| Use of rescue drugs for epilepsy | No | 85 | 2.5 | 0.03–60 | 0.47 | 0.05–17.00 |
| Yes | 25 | 15.0 | 0.25–60 | 3.47 | 0.08–14.25 | |
GTCS, generalised tonic–clonic seizure.
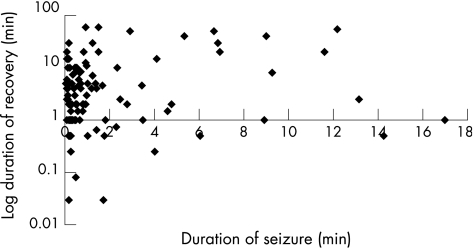
Linear regression analysis showed that aetiology of seizures and administration of rescue drugs were the only two covariates affecting recovery time, allowing for the effects of all the other variables (table 2). Age, sex, seizure duration, seizure type and the occurrence of multiple seizures during the same illness did not significantly affect recovery time independently of seizure aetiology and rescue drugs (figs 1–3).
Table 2 Linear regression analysis of the variables affecting time to recovery of consciousness.
| Variable | Coefficient (anti‐log) | 95% CI | p>[t] |
|---|---|---|---|
| Duration (min) | 1.02 | 1.00 to 1.04 | 0.10 |
| Age | 1.03 | 0.96 to 1.10 | 0.46 |
| Sex (female) | 0.81 | 0.50 to 1.30 | 0.38 |
| Acute symptomatic | 6.88 | 2.33 to 20.34 | 0.001 |
| Idiopathic | 2.49 | 1.06 to 5.86 | 0.04 |
| Remote symptomatic | 1.98 | 0.87 to 4.50 | 0.10 |
| Use of emergency drugs | 2.28 | 1.19 to 4.36 | 0.01 |
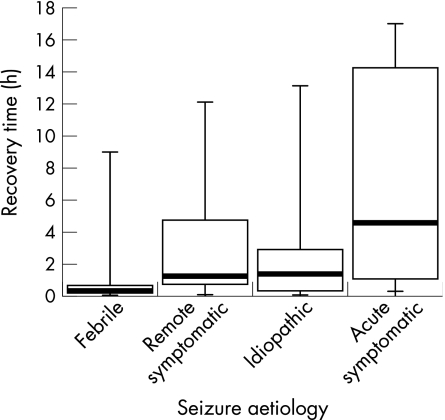
Figure 1 Recovery time (h) versus seizure aetiology.
Figure 2 Log duration of recovery (min) versus duration of seizure (min).
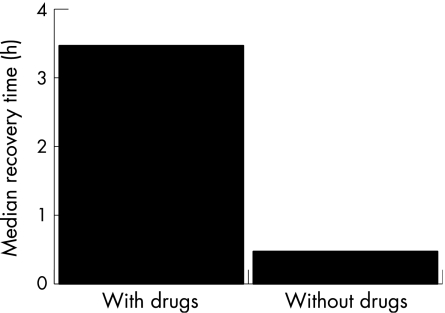
Figure 3 Median recovery time (h) versus rescue drugs (with drugs and without drugs).
Figure 1 shows the recovery times (median, range and interquartile range) from seizures of different aetiology. Median recovery time was 18 min (range 3 min–9 h) from febrile seizures, 1.25 h (range 4 min–12.10 h) from remote symptomatic seizures, 1.35 h (range 4 min–13.13 h) from idiopathic seizures and 4.57 h (range 0.25–17.00 h) acute symptomatic seizures. Regression analysis showed that children experiencing acute symptomatic seizures took almost seven times longer to recover than those experiencing febrile seizures (taken as the baseline). It took children 2.5 times longer to recover from idiopathic seizures and twice as long to recover from remote symptomatic seizures compared with febrile seizures (table 2).
Children who received rescue drugs for epilepsy had a median recovery time of 3.46 h (range 5 min–14.25 h), which was 2.28 times longer than for children who were not treated with drugs and had a median recovery time of 28 min (range 3 min–17 h).
Discussion
As far as we know, this is the first study to prospectively investigate recovery time after epileptic seizures in children. Most children regain consciousness within 30–40 min, although some take many hours, without sinister cause. The two variables affecting recovery were seizure aetiology and administration of rescue drugs for epilepsy. Children recover most quickly from febrile seizures and most slowly from acute symptomatic seizures. These findings are in keeping with clinical experience and the known pharmacology of benzodiazepines. However, it was surprising that seizure duration did not significantly affect recovery time; clinical dogma is that it does.7
This study cannot be said to represent the whole population of children with epileptic seizures. Firstly, many children do not attend hospital after seizures. Secondly, the number of acute symptomatic seizures shows the influence of undertaking the study in a neurosciences centre. However, that most seizures were febrile reflects the high prevalence of such seizures in children. The median duration of febrile seizures was 2.5 min, consistent with previous reports. To obtain a representative population, a community study would be required. This would be near impossible, considering the difficulties in accurately assessing consciousness.
By allowing seizure durations to be estimated by observers and by including children whose coma scores were recorded hourly (rather than, eg, at 15‐min intervals), we ensured that most eligible children were included. If we had insisted on accurate timing of seizures and 15‐min coma scores, most children would have been excluded. However, parents are notoriously inaccurate and overestimate seizure duration. This may partly explain why seizure duration was found not to correlate with recovery time. The use of hourly coma scores means, inevitably, that actual recovery times will be shorter than the figures given.
The study had a high exclusion rate owing to failures to take postictal coma scores. It may be that these children were fully recovered, and staff neglected to document this or to record coma scores. This would further reduce actual recovery times. Lack of coma scoring may be a reflection of a general lack of awareness of the potential significance of postictal impaired consciousness.
One curious aspect of the population was that there were more girls than boys, contrary to most epidemiological studies. We have no satisfactory explanation for this, and it is not completely explained by a higher number of male exclusions or the fact that more girls were included more than once. Also, surprisingly, there were no children with acute symptomatic seizures due to meningitis (without prior craniotomy). Again, we have no clear explanation for this, but we are dealing with this in future studies.
It is generally thought that failure to recover as expected from an epileptic seizure should prompt consideration of a sinister aetiology, such as intracranial infection or raised intracranial pressure. Currently, there are no data on which to base these expectations. This study goes some way to deal with this deficiency. It confirms that children without a previously known cause for seizures generally take longer to recover from acute symptomatic seizures than from febrile or idiopathic seizures. Moreover, most children are fully conscious 30 min after a febrile seizure, and 90% recover within an hour. Therefore, if a child with a suspected febrile seizure has not fully recovered within an hour, we recommend that they are investigated or treated for an acute symptomatic seizure. As a depressed conscious level is considered a contraindication to a lumbar puncture, such children should probably receive prophylactic antibiotic and antiviral agents, and undergo a computed tomography scan, pending consideration for lumbar puncture. Advice to parents or primary‐care practitioners should reflect these considerations. A pragmatic approach might expect children to recover quickly from febrile seizures. If their consciousness is still depressed after 30 min, they should be urgently referred to hospital.
Recovery from idiopathic seizures was also shorter than for acute symptomatic seizures. However, the difference was not as great. This may reflect the smaller numbers of children with idiopathic seizures. More work has to be done before it is possible to recommend a time at which an acute symptomatic cause should be suspected.
Notwithstanding previous comments on the lack of a clear relationship between seizure duration and recovery time, this study suggests that any relationship that exists may not be as strong as often supposed. The clinician should, therefore, not be falsely lulled into relative inaction if a child is found to be recovering slowly on the basis that the seizure was long. This study suggests that prolonged recovery is likely to reflect an acute symptomatic aetiology. Similarly, clinicians should not give undue weight to considerations such as age of the child or seizure type when making management decisions on a child who has not fully regained consciousness after an epileptic seizure.
What is already known on this topic
It is generally thought that the time taken to recover full consciousness after epileptic seizures may be short (minutes) or prolonged (hours), and that children recover quickly from febrile seizures.
However, there are no published data on this topic.
What this study adds
This study confirms the variability in the time taken to recover full consciousness after epileptic seizures.
It shows that the main factors influencing recovery time are the aetiology of the seizure and the administration of rescue drugs for epilepsy.
Rescue drugs are increasingly given at home and by paramedics during epileptic seizures. As expected, this considerably prolongs recovery time. However, it would be dangerous to ascribe a prolonged recovery to drugs without careful consideration of the clinical circumstances. When drugs are given to children with recurrent seizures, it may be possible to ascribe an individualised expected recovery time. In others, it would seem prudent to disregard the drugs given when making decisions on management, accepting that there will inevitably be several children who will be investigated or treated for conditions such as meningitis, who are subsequently shown to have febrile or idiopathic seizures.
Abbreviations
CNS - central nervous system
GTCS - generalised tonic–clonic seizure
ILAE - International League Against Epilepsy
Footnotes
Competing interests: None.
References
- 1.Engel J. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia 200142796–803. [DOI] [PubMed] [Google Scholar]
- 2.Commission on Classification and Terminology of the International League Against Epilepsy Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 198930389–399. [DOI] [PubMed] [Google Scholar]
- 3.Tatman A, Warren A, Williams A.et al Development of a modified paediatric coma scale in intensive care clinical practice. Arch Dis Child 199777519–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hopkins A, Shorvon S, Cascino G.Epilepsy. 2nd edn. London: Chapman & Hall Medical, 1995
- 5.Brown J K. Fits in children. In: Laidlaw J, Richens A, eds. A textbook of epilepsy. 2nd edn. Edinburgh: Churchill Livingstone, 198234–96.
- 6.Johnston M V. Seizures in childhood. In: Behrman RE, Kliegman RM, Jenson HB, eds. Nelson textbook of pediatrics. 17th edn. Philadelphia: WB Saunders, 20041993–2009.
- 7.Browne T R, Homes G L.Handbook of epilepsy. 2nd edn. Philadelphia: Lippincott, Williams & Wilkins, 2000