Abstract
The obligate intracellular bacterium Chlamydia trachomatis requires iron in order to complete its developmental cycle. Addition of an iron-chelating drug, Desferal (deferoxamine mesylate), to infected cell culture causes Chlamydia to enter persistence. Here, we explore the ability of a stably-transfected cell line with inducible over-expression of the eukaryotic iron efflux protein ferroportin to starve C. trachomatis serovar E for iron. Ferroportin-induced iron removal is perhaps a more direct method of removing iron from the intracellular compartment versus exposure to an exogenous chemical chelator. Following induction, ferroportin-green fluorescent protein (Fpn-GFP) was detected in the plasma membrane, and cells expressing Fpn-GFP remained viable throughout the timescale required for Chlamydia to complete its developmental cycle. Following Fpn-GFP induction in infected cells, chlamydial infectivity remained unchanged, indicating chlamydiae were not in persistence. Ferritin levels indicate only a small decrease in cellular iron following Fpn-GFP expression relative to cultures exposed to Desferal. These data indicate that expression of Fpn-GFP in chlamydiae-infected cells is not capable of reducing iron below the threshold concentration needed to cause chlamydiae to enter persistence.
Keywords: Chlamydia trachomatis, Persistence, Iron restriction, Ferroportin
1. Introduction
Chlamydia trachomatis serovar E, an obligate intracellular bacterium, is the leading cause of bacterial sexually transmitted disease in the United States. C. trachomatis causes chronic, often asymptomatic infections, and can lead to more serious sequelae including endometritis and pelvic inflammatory disease.
Members of the genus Chlamydia (and the reclassified Chlamydophila) exhibit a unique biphasic developmental cycle, switching between the intracellular, replicative reticulate body (RB) and the extracellular, metabolically inert elementary body (EB). The canonical developmental cycle is interrupted when chlamydiae enter persistence. While the formulation of a firm molecular definition is ongoing, chlamydial persistence is characterized by a non-culturable, but viable, state in which RB do not undergo maturation into EB. Cellular division ceases, RB become enlarged, and transcription and protein expression profiles are altered. Several inducers of persistence have been observed, the most studied being tryptophan deprivation via interferon-γ exposure and incubation in the presence of penicillin. Persistence is a phenomenon of medical importance, since persistent chlamydiae are refractory to antibiotics and may upregulate destructive virulence factors.
Iron starvation has been observed to induce persistence in chlamydiae. Upon depletion of intracellular iron stores with the iron-chelating drug Desferal (deferoxamine mesylate), chlamydiae demonstrate delayed development, decreased infectivity of progeny EB, and the appearance of enlarged RB, which is reversible upon supplementing the medium with an iron source [1-4]. The existence of an iron-dependent repressor [5, 6] and differential transcription and protein expression profiles [3, 4, 7, 8] further illustrate the requirement for Chlamydia for iron.
The reliance of chlamydiae on iron availability is not surprising, as iron is the most important metal in biological systems and is required by nearly all organisms studied. This ubiquitous reliance of life on iron has evolved because the chemical properties of iron allow it to function as a biocatalyst and electron carrier when incorporated into proteins. While life depends on iron, iron in an aerobic environment poses some difficulties. Ferric iron (the form predominating in an aerobic environment) has a solubility of only 10−18 M at pH 7.0, and free iron can generate damaging free radicals via the Fenton reaction [9]. Thus, organisms tightly sequester iron with high-affinity binding proteins. Major iron-binding proteins in mammals include transferrin, lactoferrin, heme, and ferritin.
Because free iron is so scarce in the host, iron availability is a primary limitation to bacterial colonization, and many pathogens possess virulence factors regulated by iron availability, including siderophores, receptors for iron-binding proteins, iron transporters, and exotoxins [10, 11].
It is clear that iron availability fluctuates in the endometrium of menstruating women [12-15] and, thus, chlamydiae inhabiting this environment experience a modulation in iron availability. The importance of characterizing iron-restricted chlamydiae lies not only in elucidating mechanisms with which chlamydiae acquire iron from within its intracellular environment, but also in identification of iron deprivation-associated virulence factors involved in chlamydial pathogenesis.
Although Desferal creates an iron-restricted environment for chlamydiae, addition of an exogenous chemical may have pleiotropic effects on the host cell. In order to use a more elegant and direct system for starving chlamydiae for iron, we explored the use of a cell line with inducible iron starvation via over-expression of the eukaryotic iron efflux protein ferroportin. Ferroportin (IREG1, MTP, Slc 11a3) is the major iron exporter of enterocytes, macrophages, and hepatocytes, and functions to secrete intracellular iron into the bloodstream [16, 17]. With the idea that cells over-expressing ferroportin could be utilized in chlamydial iron studies, we obtained a stably-transfected cell line with an inducible gene expressing a ferroportin-green fluorescent protein fusion (Fpn-GFP). This cell line was capable of generating a drop in intracellular iron level following induction of Fpn-GFP, measured by a decrease in ferritin, the major intracellular iron storage protein [18].
2. Materials and methods
2.1. Cell culture
Human Embryonic Kidney cells (HEK293) and a stably transfected cell line expressing a ferroportin-green fluorescent protein fusion (HEK293-Fpn-GFP) were used to examine the effect of Fpn-GFP expression on chlamydial infectivity. In brief, the HEK293-Fpn-GFP cell line has two introduced elements: a stably expressed ecdysone receptor and an ecdysone inducible promoter upstream of the recombinant ferroportin gene fused with a GFP tag. The ecdysone analog, ponasterone A, is used to induce expression of Fpn-GFP.
HEK293 cells were maintained in Dulbecco's Modified Essential Medium (D-MEM) supplemented with 10% (vol/vol) Fetal Bovine Serum (FBS), 2 mM GlutaMax (Gibco), 1 μg/ml ciprofloxacin, 100 units/ml penicillin, and 0.1 mg/ml streptomycin. Medium for HEK293-Fpn-GFP cells is formulated as above, along with 0.4 mg/ml Zeocin (Invitrogen) and 1 mg/ml G418. Media supplemented with 10 μg/ml ponasterone A (A.G. Scientific, San Diego, CA) was used for induction of Fpn-GFP expression. HEK293, HEK293-Fpn-GFP, and C. trachomatis innoculum were confirmed to be free from Mycoplasma contamination by the VenorGem PCR test (Sigma-Aldrich).
2.2. Fluorescence microscopy
HEK293 and HEK293-Fpn-GFP cells were grown to subconfluency on 6-well Costar Transwell filter insert plates (Corning), and medium was replaced with medium plus or minus ponasterone. After 12, 24, or 36 hours (h), filter inserts were washed, cut out, and fixed with fresh 4% (wt/vol) paraformaldehyde for 30 minutes. After washing, filters were mounted on glass slides with Fluoromount-G (Southern Biotech, Birmingham, AL). A FITC band pass filter was used to visualize GFP fluorescence on a Zeiss Axiovert S 100 microscope. Images were captured with a Zeiss AxioCam digital camera using AxioCam software v1.1.6. Identical exposure settings were used for all images (exposure time=1 second, contrast=3, resolution=1280×1024).
2.3. Live/dead cell viability assay
Eukaryotic cell death was measured via the LIVE/DEAD Viability/Cytotoxicity Kit (Molecular Probes) at 12, 24, and 36 h following addition of ponasterone A. Controls included HEK293 cells without ponasterone. Host cells were grown in duplicate on Costar filters and processed according to the protocol. The previously described fluorescence microscope, camera, and capture software were used with a FITC long pass filter. The percentage dead cells of the total cells was determined by counting six fields per filter.
2.4. Chlamydial infectivity
Cultures of HEK293 and HEK293-Fpn-GFP cells were grown in triplicate in 25 cm2 tissue culture flasks (Corning). All antibiotics were removed 24 h prior to infection. C. trachomatis serovar E/UW-5/CX was used in all infections. The host cells were infected with C. trachomatis inoculum titrated to infect 80% of cells and were subsequently exposed to ponasterone at 36 hours post-infection (hpi). Controls consisted of chlamydiae-infected cells incubated in medium without ponasterone A. EB were harvested at 64 hpi, and were diluted 1:10 and used to infect fresh host cells grown on glass coverslips. Six fields per replicate were counted to calculate percent infectivity.
2.5. TEM analysis of chlamydial morphology
Samples were collected for thin-section transmission electron microscopy (TEM). Host cells were infected as above, and immediately exposed to medium with or without ponasterone (HEK293-Fpn-GFP cells) or with or without 50 μM Desferal (HEK293 cells). At 48 hpi, cells were processed and embedded in Epon-Araldite 812 resin (Polysciences, Inc.) as described previously [19]. Gold thin sections were cut on an Ultracut T Microtome (Leica) and examined using a Tecnai 10 (FEI) transmission electron microscope operating at 60-80 kV.
2.6. Ferritin assay
The Spectro Ferritin Assay (Ramco Laboratories, Stafford, TX), an enzyme immunoassay procedure, was used to measure ferritin content of samples. HEK293-Fpn-GFP cells were incubated in flasks with medium with or without ponasterone A for 48 hours, and HEK293 cells were incubated in medium with 0, 5, 10, 25, or 50 μM Desferal (Sigma-Aldrich) for 48 hours. Following incubation, cell monolayers were washed three times in Dulbecco's PBS and collected in lysis buffer (150 mM NaCl, 10 mM Tris [pH 7.4], 1% (vol/vol) Triton X-100, and a general protease inhibitor cocktail [Sigma-Aldrich]). Ten to twenty μg of protein was loaded into each well, in triplicate, in 96-well plates. The protein concentration of samples was determined by BCA assay (Pierce) and was used to standardize ferritin concentrations for variations in protein load. The entire experiment was repeated three times, with at least three replicates per run. A two-tailed Student's t-test was used to determine significance for a change in ferritin concentration.
3. Results
3.1. Timeframe for Fpn-GFP expression
In order to coordinate Fpn-GFP expression with the chlamydial developmental cycle, it was necessary to establish the timecourse of Fpn-GFP expression in transfected HEK293 cells following addition of the inducer, ponasterone A. Fluorescence microscopy was used to detect the green fluorescence of Fpn-GFP following induction. Fluorescence was clearly visible beginning 12 hours following induction, reached a maximum at 24 hours, and was stable through 36 hours (Fig. 1). Green fluorescence was also visible in the plasma membrane of the transfected cells, the correct final location of this protein. Addition of ponasterone A to the HEK293 cell line showed only background fluorescence similar to uninduced cells.
Fig. 1.
Fpn-GFP expression is visible by green fluorescence at the plasma membrane of induced HEK293-Fpn-GFP cells. HEK293-Fpn-GFP (A, B) and untransfected HEK293 (C, D) cells were incubated in medium containing the inducer ponasterone A for 12, 24, and 36 hours, along with a negative control lacking ponasterone A. Images were taken with fluorescence (A, C) and light (B, D) microscopy. Bar = 50 μm.
3.2. Host cell viability
The effect of Fpn-GFP expression on cell viability was unknown, and since healthy host cells are an obvious necessity for chlamydial development, cell viability for the remainder of the chlamydial developmental cycle following induction with ponasterone A needed to be verified. Incubation of either the HEK293 or the HEK293-Fpn-GFP cell lines with ponasterone A through 36 hours had no effect on viability (Fig. 2). The HEK293 cell population had an average of 6.5% dead cells (standard deviation [s.d.] 2.9) across all incubation times, while the HEK293-Fpn-GFP had an average of 28.5% dead cells (s.d. 5.4). Since the data showed there is no increase in cell death as a result of Fpn-GFP expression, this rules out the possibility that differences in infectivity after induction could be the result of host cell death.
Fig. 2.

Cell death following addition of ponasterone A as a function of time. HEK293 and HEK293-Fpn-GFP were incubated with 10 μg/ml ponasterone A for 12, 24, or 36 hours or in the absense of inducer. Cell death was calculated as a percentage of dead cells to total cells measured using a live/dead assay.
3.3. Infectivity of progeny EB harvested from induced versus uninduced cells
Decreased infectivity is a hallmark of chlamydial iron restriction. In order to evaluate the ability of Fpn-GFP expression to starve chlamydiae for iron, an infectivity assay was conducted to detect a change in infectivity in EB harvested from induced cells versus uninduced cells. Since iron restriction interrupts the normal chlamydial developmental cycle, it was hypothesized that a Fpn-GFP-mediated drop in intracellular iron would significantly decrease infectivity of EB; however, no drop in infectivity was seen between EB harvested from induced and uninduced HEK293-Fpn-GFP cells (Fig. 3). The only difference in infectivity was between EB harvested from HEK293-Fpn-GFP and HEK293 cells. Average infectivity of EB harvested from HEK293-Fpn-GFP (with and without addition of ponasterone A) was 25% (s.d. 6) versus 39% (s.d. 7) for HEK293.
Fig. 3.

Expression of Fpn-GFP in induced HEK293-Fpn-GFP infected cells does not reduce infectivity of progeny elementary bodies (EB). EB were harvested from infected host cells grown in the presence or absence of inducer, and infectivity was titrated. Chlamydial infectivity is expressed as a ratio to HEK293 minus inducer.
3.4. Analysis of chlamydial morphology
We also examined infected cells at 48 hours post infection (hpi) by thin section transmission electron microscopy. Persistent inclusions are marked by abberant morphology, including enlarged RB and membranous blebbing [3]. Contrasting with infected HEK293 cells containing normal chlamydial inclusions (Fig. 4A), HEK293 cells exposed to Desferal showed the beginnings of this characteristic morphology (Fig. 4B,C,D). HEK293-Fpn-GFP cells plus (4E) or minus ponasterone A (4F) induction showed no notable differences in chlamydial morphology, although inclusions consistently appeared less robust compared to those in HEK293 cells, regardless of ponasterone exposure.
Fig. 4.
Transmission Electron Microscopy (TEM) visualization of chlamydial inclusions plus or minus exposure to 50 μM Desferal or with or without Fpn-GFP induction. (A) Typical mid-cycle inclusions in HEK293 cells. (B,C,D) Desferal-exposed HEK293 cells with inclusions exhibiting abherent morphology, including enlarged reticulate bodies (RB), smaller inclusions, and membranous blebs. Image D shows a high magnification view of chlamydial envelope ghosts and membranous ghosts. Inclusions in HEK293-Fpn-GFP cells uninduced (E) and induced (F) show similar morphology. Bars: (A,B,D,E,F) 5 μm; (C) 2 μm.
3.5. Quantitation of intracellular ferritin following Desferal exposure or induction of Fpn-GFP
Because progeny EB from induced HEK293-Fpn-GFP cells did not show the anticipated drop in infectivity, we compared the change in ferritin level in cells exposed to Desferal, which is known to result in decreased chlamydial infectivity, to that of cells expressing Fpn-GFP. To assure Desferal does not induce toxicity in HEK293 cells, the Live/dead assay was used to verify there was no increase in cell death following 48 h exposure to 50 μM Desferal versus mock-exposed cells. Expression of ferritin, the primary intracellular iron storage protein, is tightly regulated by iron level, and is, therefore, a useful target for measuring relative iron levels.
HEK293 cells were exposed to various concentrations of Desferal for 48 hours. Ferritin content showed a dose-responsive drop in relation to Desferal concentration, falling from 28.2 ng/ml (in cells not exposed to Desferal) to 3.2 ng/ml (in cells exposed to 50 μM desferal (Fig. 5A). Each increase in Desferal concentration led to a statistically significant drop in ferritin level (p< 0.01). Following incubation of HEK293-Fpn-GFP cells for 48 hours, average ferritin levels dropped from 49.3 ng/ml (s.d. 10.4) to 38.2 ng/ml (s.d. 8.3), a change that is not statistically significant (Fig. 5B). It is notable that ferritin levels naturally fluctuate, which likely explain the disparity between overall ferritin levels between HEK293 and HEK293-Fpn-GFP cells.
Fig. 5.
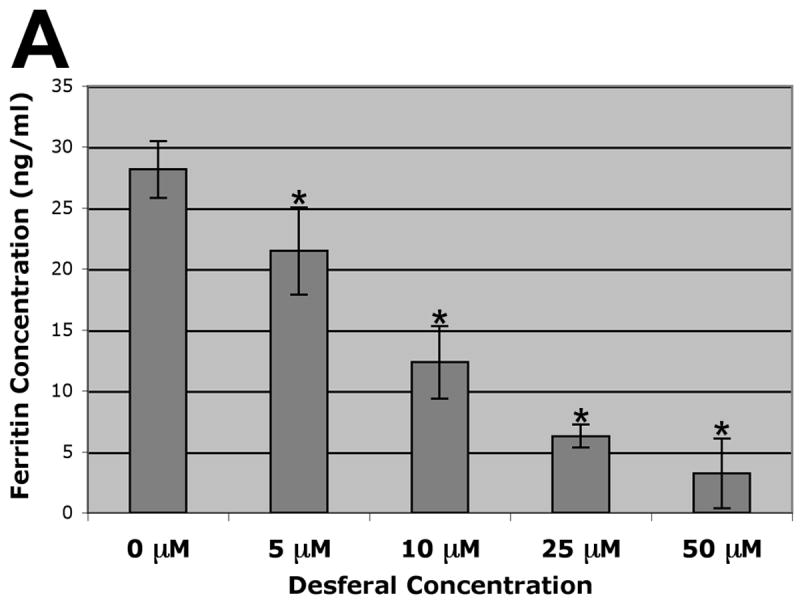

Ferritin content of host cells following exposure to Desferal or expression of Fpn-GFP. (A) Ferritin levels of HEK293 cells following 48 h exposure to 0, 5, 10, 25, or 50 μM Desferal. Asterisk indicates a significant change (p < 0.01). (B) Ferritin levels of HEK293-Fpn-GFP cells, incubated plus or minus the inducing agent ponasterone A for 48 h.
4. Discussion
Because there is no established genetic system in Chlamydia, transfected eukaryotic host cells represent one way to use genetic tools with this intracellular bacterium. In many expression systems, tetracycline is used as the inducing agent. While the primary effect in other applications is a decreased risk for bacterial contamination of the culture, tetracycline is lethal for Chlamydia. In this study, we found no decrease in chlamydial infectivity when the inducer ponasterone was incubated with infected HEK293 cells. Thus, ponasterone-inducible systems are a viable option for creating host genetic systems for Chlamydia, and this is the first study known to these authors to use a ponasterone-inducible system in Chlamydia.
Currently, the addition of the iron-chelating drug Desferal is the sole approach used to study iron starvation in Chlamydia, and this method has proved capable of causing chlamydiae to enter a persistent state. While Desferal is apparently effective in starving chlamydiae for iron, it would be advantageous to have an additional method for starving chlamydiae of iron, as the addition of a chemical agent may have pleiotropic effects on cell viability and protein expression. For example, Desferal was found to penetrate and cause direct damage to Pneumocystis carinii, independent of an iron effect, and is now being considered as a treatment for patients with Pneumocystis infections due to its toxic effect on the pathogen [20].
Over-expression of eukaryotic ferroportin was successfully used to starve a facultative intracellular pathogen of iron in at least one other study. In a transient ferroportin expression system, a drop in available iron was successful in limiting intracellular growth of Salmonella enterica [21].
The goal of this project was to evaluate use of the HEK293-Fpn-GFP cell line to create an iron-starved environment following infection with Chlamydia. Ultimately, we found that the Fpn-GFP system used in the present study was not capable of reducing iron below the threshold level chlamydiae require for normal development. Infectivity of EB harvested from induced host cells did not demonstrate a drop in infectivity, and, upon comparing ferritin levels in ponasterone-induced HEK293-Fpn-GFP cells with HEK293 cells exposed to Desferal, we found that Fpn-GFP induction did not lead to a statistically significant decrease in host cell ferritin.
It is not surprising that Desferal produces a greater drop in ferritin level than Fpn-GFP expression. Desferal is a high affinity intracellular iron chelator that can strip iron from all intracellular iron stores, ferritin being the major source. In iron studies where it is undesirable to cause the chlamydiae to enter persistence through complete iron restriction, the HEK293-Fpn-GFP cell line may be useful for generating a small decrease in intracellular iron.
The data from Chlosta et al. (2006) suggests either Salmonella is more sensitive to iron restriction than is Chlamydia, or the transient HeLa cell expression system used by Chlosta et al. created a greater drop in intracellular iron than did the HEK293-Fpn-GFP system used in the current study. Although Chlosta et al. detected a decrease in iron availability using a Salmonella iron-sensing promoter assay, they did not use a quantitative measure of intracellular iron. This would have allowed comparison of the relative efficacy of iron efflux in their Fpn expression system to that of the HEK293-Fpn-GFP system used in this study.
The HEK293-Fpn-GFP cells used in this study were previously used to elucidate the relationship between ferroportin and the peptide hormone hepcidin [18]. The experimental procedure used by Nemeth et al. (2004) included supplementing the medium with iron prior to Fpn-GFP expression. Expression of Fpn-GFP caused ferritin levels to return to the pre-supplementation level, a much greater decrease than seen in the current study. This may mean that the Fpn-GFP model requires a higher initial concentration of intracellular iron for effective efflux. Because the critical factor for restricting iron from an intracellular pathogen is iron level rather than the change in iron level, supplementing the iron level prior to Fpn-GFP induction would have been ineffective in this study. Thus, we conclude inducing HEK293-Fpn-GFP is ineffective in producing an iron-restricted environment during C. trachomatis serovar E infection such that the chlamydial developmental cycle is altered.
Acknowledgments
We appreciate the gift of the HEK293 and the HEK293-Fpn-GFP cell lines from Jerry Kaplan and his laboratory (University of Utah). We also acknowledge the use of the Electron Microscope Core Facility at the J.H. Quillen College of Medicine and the assistance of Judy Whittimore. This work was supported by Public Health Services grant AI040915 through the National Institutes of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Al-Younes HM, Rudel T, Brinkmann V, Szczepek AJ, Meyer TF. Low iron availability modulates the course of Chlamydia pneumoniae infection. Cell Microbiol. 2001;3:427–437. doi: 10.1046/j.1462-5822.2001.00125.x. [DOI] [PubMed] [Google Scholar]
- 2.Freidank HM, Billing H, Wiedmann-Al-Ahmad M. Influence of iron restriction on Chlamydia pneumoniae and C. trachomatis. J Med Microbiol. 2001;50:223–227. doi: 10.1099/0022-1317-50-3-223. [DOI] [PubMed] [Google Scholar]
- 3.Raulston JE. Response of Chlamydia trachomatis serovar E to iron restriction in vitro and evidence for iron-regulated chlamydial proteins. Infect Immun. 1997;65:4539–4547. doi: 10.1128/iai.65.11.4539-4547.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goellner S, Schubert E, Liebler-Tenorio E, Hotzel H, Saluz HP, Sachse K. Transcriptional response patterns of Chlamydophila psittaci in different in vitro models of persistent infection. Infect Immun. 2006;74:4801–4808. doi: 10.1128/IAI.01487-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wyllie S, Raulston JE. Identifying regulators of transcription in an obligate intracellular pathogen: a metal-dependent repressor in Chlamydia trachomatis. Mol Microbiol. 2001;40:1027–1036. doi: 10.1046/j.1365-2958.2001.02453.x. [DOI] [PubMed] [Google Scholar]
- 6.Rau A, Wyllie S, Whittimore J, Raulston JE. Identification of Chlamydia trachomatis genomic sequences recognized by chlamydial divalent cation-dependent regulator A (DcrA) J Bacteriol. 2005;187:443–448. doi: 10.1128/JB.187.2.443-448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukhopadhyay S, Miller RD, Sullivan ED, Theodoropoulos C, Mathews SA, Timms P, Summersgill JT. Protein expression profiles of Chlamydia pneumoniae in models of persistence versus those of heat shock stress response. Infect Immun. 2006;74:3853–3863. doi: 10.1128/IAI.02104-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wehrl W, Meyer TF, Jungblut PR, Muller EC, Szczepek AJ. Action and reaction: Chlamydophila pneumoniae proteome alteration in a persistent infection induced by iron deficiency. Proteomics. 2004;4:2969–2981. doi: 10.1002/pmic.200400917. [DOI] [PubMed] [Google Scholar]
- 9.Andrews SC, Robinson AK, Rodriguez-Quinones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003;27:215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 10.Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Annu Rev Microbiol. 2000;54:881–941. doi: 10.1146/annurev.micro.54.1.881. [DOI] [PubMed] [Google Scholar]
- 11.Wooldridge KG, Williams PH. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev. 1993;12:325–348. doi: 10.1111/j.1574-6976.1993.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JE, Leone PA, Miller WC, Chen C, Hobbs MM, Sparling PF. Selection for expression of the gonococcal hemoglobin receptor during menses. J Infect Dis. 2001;184:1621–1623. doi: 10.1086/324564. [DOI] [PubMed] [Google Scholar]
- 13.Andrews NC. Iron homeostasis: insights from genetics and animal models. Nat Rev Genet. 2000;1:208–217. doi: 10.1038/35042073. [DOI] [PubMed] [Google Scholar]
- 14.Cohen MS, Britigan BE, French M, Bean K. Preliminary observations on lactoferrin secretion in human vaginal mucus: variation during the menstrual cycle, evidence of hormonal regulation, and implications for infection with Neisseria gonorrhoeae. Am J Obstet Gynecol. 1987;157:1122–1125. doi: 10.1016/s0002-9378(87)80274-0. [DOI] [PubMed] [Google Scholar]
- 15.Kelver ME, Kaul A, Nowicki B, Findley WE, Hutchens TW, Nagamani M. Estrogen regulation of lactoferrin expression in human endometrium. Am J Reprod Immunol. 1996;36:243–247. doi: 10.1111/j.1600-0897.1996.tb00171.x. [DOI] [PubMed] [Google Scholar]
- 16.Levenson CW, Tassabehji NM. Iron and ageing: an introduction to iron regulatory mechanisms. Ageing Res Rev. 2004;3:251–263. doi: 10.1016/j.arr.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Ganz T. Cellular iron: ferroportin is the only way out. Cell Metab. 2005;1:155–157. doi: 10.1016/j.cmet.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 19.Wyrick P, Choong J, Knight S, Goyeau D, Stuart ES, Macdonald A. Chlamydia trachomatis antigens on the surface of infected human endometrial epithelial cells. Immun Infect Dis. 1994;4:131–141. [Google Scholar]
- 20.Clarkson AB, Jr, Turkel-Parrella D, Williams JH, Chen LC, Gordon T, Merali S. Action of deferoxamine against Pneumocystis carinii. Antimicrobial Agents & Chemotherapy. 2001;45:3560–3565. doi: 10.1128/AAC.45.12.3560-3565.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chlosta S, Fishman DS, Harrington L, Johnson EE, Knutson MD, Wessling-Resnick M, Cherayil BJ. The iron efflux protein ferroportin regulates the intracellular growth of Salmonella enterica. Infection & Immunity. 2006;74:3065–3067. doi: 10.1128/IAI.74.5.3065-3067.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]




