Abstract
Background
Population‐based studies of childhood cancer incidence, survival and mortality make an important contribution to monitoring the successful implementation of new treatment guidelines and to understanding the epidemiology of these diseases.
Methods
We analysed incidence and survival data for cancers diagnosed in children under 15 years of age in the Republic of Ireland during 1994–2000 (the first 7 years of National Cancer Registry coverage), and longer term mortality trends.
Results
World age‐standardised incidence rates in Ireland averaged 142 cases per million children per year, slightly higher than the European average and slightly lower than the US average, although differences varied by diagnostic group. Observed 5‐year survival in Ireland (79% overall) was slightly higher than European and US averages, and was significantly higher for acute non‐lymphocytic leukaemia (67%) and (compared with the USA) significantly lower for Hodgkin lymphoma (83%). No significant increases in incidence rates were evident from the available 7 years' data, either overall or for particular diagnostic groups. Rates of childhood cancer mortality have declined markedly since the 1950s.
Conclusions
Data presented here are in line with other developed countries and suggest major improvements in treatment and consequent survival.
Cancer may be relatively rare in childhood compared to later in life but is one of the more frequent causes of non‐traumatic deaths in children in Ireland.1 As new medical treatments are developed, cancer in children is no longer equated with death, and more children survive and continue normal lives after treatment. Population‐based studies of mortality and survival can indicate if childhood malignancies are being treated successfully and, in particular, can reveal whether new treatment guidelines are being successfully implemented at the population level.2 There is also major interest in the epidemiology of childhood malignancies, in part because of their social impact.
Earlier published analyses of childhood cancer in Ireland have considered the incidence of cancers diagnosed in Ireland and Northern Ireland during 1994–1996,3 the incidence and mortality from childhood leukaemia,4 and trends in childhood cancer mortality.5 Here, we present the most comprehensive study to date of childhood cancer incidence and mortality in Ireland (average 850 000 children aged 0–14, 1994–2000) and national survival estimates for the first time, to assess if expected improvements in outcome have occurred and to provide a baseline for further monitoring. Comparisons are made with recent data for other European countries and the USA.
Methods
Incidence data
All cancer cases newly diagnosed in patients under 15 years of age were extracted from National Cancer Registry (NCR) data for 1994–2000. Data are primarily abstracted from hospital and pathology records by trained NCR staff (Tumour Registration Officers) and include all malignant and in situ neoplasms, neoplasms of uncertain behaviour and (for intracranial and intracranial sites) benign tumours.3 Particular care was taken to exclude prevalent cases originally diagnosed before 1994, although a few such cases may have been mistakenly registered. Data were coded to the International Classification of Childhood Cancer (ICCC),6 which includes all malignant cases and some non‐malignant intraspinal and intracranial tumours (see table 1). Incidence data for Europe were extracted from ACCIS (Automated Childhood Cancer Information System, http://www‐dep.iarc.fr/accis.htm),7 and for the USA from the SEER database (http://seer.cancer.gov/seerstat).8
Table 1 Numbers and world age‐standardised incidence rates (per million children aged 0–14 per year) of childhood cancers in Ireland, 1994–2000, and comparison with European (ACCIS)* and US (SEER)8 data.
| Diagnostic group | Ireland | Europe | USA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1994–2000 | 1990s | 1994–2000 | |||||||
| Male | Female | Total | WASR | WASR | |||||
| Cases | WASR | Cases | WASR | Cases | WASR | ||||
| (%) | (95% CI) | (%) | (95% CI) | (%) | (95% CI) | (range)† | (95% CI) | ||
| – | Total malignant cancers | 418 | 143.0 | 353 | 127.1 | 771 | 135.1 | – | 149.0 |
| (98) | (129 to 157) | (98) | (113 to 141) | (98) | (125 to 142) | (144 to 154) | |||
| I–XII | Total ICCC‐coded cancers | 425 | 144.9 | 362 | 130.0 | 787 | 137.5 | 130.9 | – |
| (100) | (131 to 159) | (100) | (116 to 144) | (100) | (128 to 147) | (102 to 158) | – | ||
| I | Leukaemias‡ | 124 | 45.1 | 113 | 43.0 | 237 | 44.0 | 42.4 | 50.9 |
| (29) | (37 to 53) | (31) | (35 to 51) | (30) | (38 to 50) | (31 to 54) | (48 to 54) | ||
| Ia | Lymphoid leukaemia | 101 | 37.6 | 89 | 33.9 | 190 | 35.7 | 40.9 | |
| (24) | (30 to 45) | (25) | (27 to 41) | (24) | (30 to 40) | (38 to 43) | |||
| Ib | Acute non‐lymphocytic | 18 | 5.7 | 20 | 7.9 | 38 | 6.8 | 7.9 | |
| leukaemia | (4.2) | (3.0 to 8.4) | (5.5) | (4.4 to 11) | (4.8) | (4.6 to 9.0) | (6.9 to 9.0) | ||
| Ic | Chronic myeloid leukaemia | 2 | 0.7 | 2 | 0.5 | 4 | 0.6 | 0.9 | |
| (0.5) | (0.0 to 1.6) | (0.5) | (0.0 to 1.3) | (0.5) | (0.0 to 1.2) | (0.5 to 1.2) | |||
| Ie | Unspecified leukaemias | 3 | 1.1 | 2 | 0.7 | 5 | 0.9 | 0.9 | |
| (0.7) | (0.0 to 2.4) | (0.5) | (0.0 to 1.7) | (0.6) | (0.1 to 1.7) | (0.5 to 1.2) | |||
| II | Lymphomas and | 56 | 16.7 | 34 | 11.0 | 90 | 13.9 | 14.3 | 13.5 |
| reticuloendothelial neoplasms | (13.1) | (12 to 21) | (9.3) | (7.2 to 15) | (11) | (11 to 17) | (11 to 24) | (12 to 15) | |
| IIa | Hodgkin lymphoma | 22 | 6.1 | 9 | 2.4 | 31 | 4.3 | 4.9 | |
| (5.1) | (3.5 to 8.7) | (2.4) | (0.8 to 3.9) | (3.9) | (2.7 to 5.7) | (4.1 to 5.6) | |||
| IIb | Non‐Hodgkin lymphoma | 20 | 6.2 | 15 | 4.9 | 35 | 5.6 | 5.5 | |
| (4.7) | (3.4 to 9.0) | (4.1) | (2.3 to 7.4) | (4.4) | (3.6 to 7.4) | (4.7 to 6.4) | |||
| IIc | Burkitt lymphoma | 7 | 2.1 | 1 | 0.3 | 8 | 1.2 | 2.2 | |
| (1.6) | (0.4 to 3.7) | (0.2) | (0.0 to 1.0) | (1.0) | (0.3 to 2.0) | (1.7 to 2.8) | |||
| IId | Miscellaneous lymphoreticular | 0 | 0 | 2 | 0.9 | 2 | 0.4 | 0.3 | |
| neoplasms | (0.5) | (0.0 to 2.1) | (0.2) | (0.0 to 1.0) | (0.1 to 0.5) | ||||
| IIe | Unspecified lymphomas | 7 | 2.2 | 7 | 2.5 | 14 | 2.4 | 0.5 | |
| (1.6) | (0.5 to 3.9) | (1.9) | (0.5 to 4.3) | (1.7) | (1.0 to 3.6) | (0.3 to 0.8) | |||
| III | CNS and miscell intracranial | 117 | 38.5 | 98 | 33.9 | 215 | 36.2 | 28.1 | 30.4 |
| and intraspinal neoplasms | (27) | (31 to 46) | (27.0) | (27 to 41) | (27.3) | (31 to 41) | (14 to 43) | (28 to 32) | |
| IIIa | Ependymoma§ | 8 | 3.1 | 4 | 1.4 | 12 | 2.2 | 2.9 | |
| (1.8) | (0.9 to 5.1) | (1.1) | (0.0 to 2.7) | (1.5) | (0.9 to 3.4) | (2.3 to 3.5) | |||
| IIIb | Astrocytoma | 60 | 19.4 | 62 | 21.5 | 122 | 20.5 | 14.2 | |
| (14) | (14 to 24) | (17.1) | (16 to 27) | (15.5) | (17 to 24) | (13 to 16) | |||
| IIIc | Primitive neuroectodermal | 23 | 7.6 | 15 | 5.6 | 38 | 6.6 | 7.8 | |
| tumours | (5.4) | (4.4 to 11) | (4.1) | (2.7 to 8.5) | (4.8) | (4.4 to 8.7) | (6.8 to 8.8) | ||
| IIId | Other gliomas§ | 6 | 2.1 | 7 | 2.1 | 13 | 2.1 | 4.5 | |
| (1.4) | (0.3 to 3.7) | (1.9) | (0.5 to 3.6) | (1.6) | (0.9 to 3.2) | (3.7 to 5.3) | |||
| IIIe | Other specified§ | 13 | 4.1 | 7 | 2.2 | 20 | 3.2 | 0.4 | |
| (3.0) | (1.8 to 6.3) | (1.9) | (0.5 to 3.8) | (2.5) | (1.7 to 4.5) | (0.2 to 0.6) | |||
| IIIf | Unspecified | 7 | 2.1 | 3 | 1.2 | 10 | 1.7 | 0.6 | |
| intracranial/intraspinal§ | (1.6) | (0.5 to 3.7) | (0.8) | (0.0 to 2.4) | (1.2) | (0.6 to 2.7) | (0.3 to 0.9) | ||
| IV | Sympathetic nervous system | 25 | 9.7 | 12 | 5.0 | 37 | 7.3 | 9.8 | 11.3 |
| tumours | (5.8) | (5.8 to 13) | (3.3) | (2.1 to 7.8) | (4.7) | (4.9 to 9.7) | (2.3 to 17) | (10 to 13) | |
| IVa | Neuroblastoma and | 22 | 8.7 | 12 | 5.0 | 34 | 6.8 | 10.9 | |
| ganglioneuroblastoma | (5.1) | (5.0 to 12) | (3.3) | (2.1 to 7.8) | (4.3) | (4.5 to 9.1) | (9.6 to 12) | ||
| IVb | Other SNS tumours | 3 | 1.0 | 0 | 0 | 3 | 0.5 | 0.4 | |
| (0.7) | (0.0 to 2.1) | (0.3) | (0.0 to 1.0) | (0.2 to 0.6) | |||||
| V | Retinoblastoma | 9 | 3.8 | 7 | 3.1 | 16 | 3.4 | 3.8 | 5.3 |
| (2.1) | (1.3 to 6.2) | (1.9) | (0.8 to 5.4) | (2.0) | (1.7 to 5.1) | (1.6 to 8.3) | (4.4 to 6.2) | ||
| VI | Renal tumours‡ | 15 | 6.2 | 21 | 8.6 | 36 | 7.4 | 8.5 | 8.9 |
| (3.5) | (3.0 to 9.3) | (5.8) | (4.8 to 12) | (4.5) | (4.9 to 9.7) | (4.7 to 12) | (7.7 to 10) | ||
| VIa | Wilms tumour, rhadboid | 15 | 6.2 | 20 | 8.3 | 35 | 7.2 | 8.6 | |
| and clear cell sarcoma | (3.5) | (3.0 to 9.3) | (5.5) | (4.6 to 12) | (4.4) | (4.8 to 9.6) | (7.5 to 9.7) | ||
| VIb | Renal carcinoma | 0 | 0 | 1 | 0.3 | 1 | 0.1 | 0.2 | |
| (0.2) | (0.0 to 0.7) | (0.1) | (0.0 to 0.3) | (0.1 to 0.4) | |||||
| VII | Hepatic tumours | 1 | 0.3 | 3 | 1.3 | 4 | 0.8 | 2.3 | |
| (0.2) | (0.0 to 0.7) | (0.8) | (0.0 to 2.8) | (0.5) | (0.0 to 1.5) | (1.7 to 2.9) | |||
| VIIa | Hepatoblastoma | 0 | 0 | 2 | 0.9 | 2 | 0.4 | 2.0 | |
| (0.5) | (0.0 to 2.1) | (0.2) | (0.0 to 1.0) | (1.5 to 2.6) | |||||
| VIIb | Hepatic carcinoma | 1 | 0.3 | 0 | 0 | 1 | 0.1 | 0.3 | |
| (0.2) | (0.0 to 0.7) | (0.1) | (0.0 to 0.3) | (0.1 to 0.4) | |||||
| VIIc | Unspecified malignant | 0 | 0 | 1 | 0.4 | 1 | 0.2 | <0.1 | |
| hepatic tumours | (0.2) | (0.0 to 1.3) | (0.1) | (0.0 to 0.6) | (0.0 to 0.1) | ||||
| VIII | Malignant bone tumours‡ | 22 | 6.6 | 15 | 4.6 | 37 | 5.6 | 5.4 | 5.6 |
| (5.1) | (3.7 to 9.3) | (4.1) | (2.2 to 6.9) | (4.7) | (3.7 to 7.4) | (3.9 to 7.6) | (4.8 to 6.5) | ||
| VIIIa | Osteosarcoma | 10 | 2.7 | 7 | 2.0 | 17 | 2.3 | 3.3 | |
| (2.3) | (1.0 to 4.3) | (1.9) | (0.5 to 3.5) | (2.1) | (1.2 to 3.4) | (2.7 to 4.0) | |||
| VIIIc | Ewing sarcoma | 10 | 3.2 | 8 | 2.6 | 18 | 2.9 | 1.8 | |
| (2.3) | (1.1 to 5.2) | (2.2) | (0.7 to 4.4) | (2.2) | (1.5 to 4.2) | (1.3 to 2.3) | |||
| VIIIe | Unspecified malignant | 2 | 0.7 | 0 | 0 | 2 | 0.3 | <0.1 | |
| bone tumours | (0.4) | (0.0 to 1.6) | (0.2) | (0.0 to 0.8) | (0.0 to 0.1) | ||||
| IX | Soft tissue sarcomas‡ | 27 | 9.4 | 23 | 7.8 | 50 | 8.6 | 8.3 | 9.9 |
| (6.3) | (5.8 to 13) | (6.3) | (4.5 to 11) | (6.3) | (6.1 to 11) | (3.5 to 12) | (8.8 to 11) | ||
| IXa | Rhabdomyosarcoma and | 18 | 6.7 | 15 | 5.3 | 33 | 6.0 | 5.1 | |
| embryonal sarcoma | (4.2) | (3.5 to 9.8) | (4.1) | (2.5 to 8.0) | (4.1) | (3.9 to 8.1) | (4.2 to 5.9) | ||
| IXb | Fibrosarcomas and other | 2 | 0.6 | 5 | 1.4 | 7 | 1.0 | 1.8 | |
| fibromatous neoplasms | (0.4) | (0.0 to 1.5) | (1.3) | (0.1 to 2.6) | (0.8) | (0.2 to 1.7) | (1.3 to 2.3) | ||
| IXd | Other specified soft tissue | 5 | 1.5 | 3 | 1.1 | 8 | 1.3 | 2.2 | |
| sarcomas | (1.1) | (0.1 to 2.8) | (0.8) | (0.0 to 2.2) | (1.0) | (0.3 to 2.1) | (1.7 to 2.8) | ||
| IXe | Unspecified soft tissue | 2 | 0.6 | 0 | 0 | 2 | 0.3 | 0.8 | |
| sarcomas | (0.4) | (0.0 to 1.3) | (0.2) | (0.0 to 0.6) | (0.5 to 1.2) | ||||
| X | Germ cell to trophoblastic and | 13 | 4.3 | 14 | 5.1 | 27 | 4.7 | 4.0 | 5.5 |
| other gonadal neoplasms‡ | (3.0) | (1.9 to 6.7) | (3.8) | (2.3 to 7.8) | (3.4) | (2.9 to 6.5) | (1.3 to 5.1) | (4.7 to 6.4) | |
| Xa | Intracranial and intraspinal | 4 | 1.2 | 2 | 0.7 | 6 | 0.9 | 1.4 | |
| germ cell tumours§ | (0.9) | (0.0 to 2.2) | (0.5) | (0.0 to 1.7) | (0.7) | (0.1 to 1.6) | (1.0 to 1.8) | ||
| Xb | Other and unspecified non‐ | 2 | 0.7 | 5 | 2.2 | 7 | 1.5 | 1.7 | |
| gonadal germ cell tumours | (0.4) | (0.0 to 1.7) | (1.3) | (0.2 to 4.1) | (0.8) | (0.3 to 2.5) | (1.2 to 2.2) | ||
| Xc | Gonadal germ cell tumours | 7 | 2.4 | 7 | 2.2 | 14 | 2.3 | 2.2 | |
| (1.6) | (0.5 to 4.2) | (1.9) | (0.5 to 3.8) | (1.7) | (1.0 to 3.5) | (1.6 to 2.7) | |||
| XI | Carcinomas/other malignant | 9 | 2.3 | 18 | 5.0 | 27 | 3.7 | 3.4 | 4.9 |
| epithelial neoplasms | (2.1) | (0.7 to 3.7) | (4.9) | (2.6 to 7.3) | (3.4) | (2.2 to 5.0) | (1.2 to 25) | (4.1 to 5.7) | |
| XIa | Adrenocortical carcinoma | 1 | 0.3 | 0 | 0 | 1 | 0.1 | 0.2 | |
| (0.2) | (0.0 to 0.7) | (0.1) | (0.0 to 0.3) | (0.1 to 0.4) | |||||
| XIb | Thyroid carcinoma | 1 | 0.3 | 2 | 0.6 | 3 | 0.4 | 1.5 | |
| (0.2) | (0.0 to 0.7) | (0.5) | (0.0 to 1.4) | (0.3) | (0.0 to 0.9) | (1.1 to 2.0) | |||
| XIc | Nasopharyngeal carcinoma | 2 | 0.5 | 5 | 1.4 | 7 | 1.0 | 0.2 | |
| (0.4) | (0.0 to 1.2) | (1.3) | (0.0 to 0.7) | (0.8) | (0.0 to 0.8) | (0.1 to 0.4) | |||
| XId | Malignant melanoma | 2 | 0.5 | 5 | 1.4 | 7 | 1.0 | 1.6 | |
| (0.4) | (0.0 to 1.2) | (1.3) | (0.1 to 2.6) | (0.8) | (0.2 to 1.6) | (1.1 to 2.0) | |||
| XIe | Skin carcinoma | 0 | 0 | 3 | 0.8 | 3 | 0.4 | 0.1 | |
| (0.8) | (0.0 to 1.7) | (0.3) | (0.0 to 0.8) | (0.0 to 0.2) | |||||
| XIf | Other and unspecified | 3 | 0.8 | 7 | 1.9 | 10 | 1.4 | 1.3 | |
| carcinomas | (0.7) | (0.0 to 1.6) | (1.9) | (0.4 to 3.3) | (1.2) | (0.5 to 2.1) | (0.9 to 1.7) | ||
| XII | Other and unspecified | 7 | 2.2 | 4 | 1.5 | 11 | 1.9 | 1.2 | 0.5 |
| malignant neoplasms | (1.6) | (0.5 to 3.8) | (1.1) | (0.0 to 2.9) | (1.3) | (0.7 to 2.9) | (0.0 to 13) | (0.3 to 0.8) | |
| XIIa | Other specified malignant | 1 | 0.3 | 0 | 0 | 1 | 0.2 | 0.2 | |
| tumours | (0.2) | (0.0 to 0.9) | (0.1) | (0.0 to 0.4) | (0.0 to 0.4) | ||||
| XIIb | Other unspecified malignant | 6 | 1.9 | 4 | 1.5 | 10 | 1.7 | 0.3 | |
| tumours | (1.4) | (0.3 to 3.4) | (1.1) | (0.0 to 2.9) | (1.2) | (0.6 to 2.7) | (0.1 to 0.5) | ||
*Source: ACCIS database of childhood cancers in Europe, 1990s (http://www‐dep.iarc.fr/accis/data.htm).
†Range of 27 national estimates is shown for European rates.
‡There were no Irish cases in the following diagnostic subgroups (US rates <0.5 per million): Id (other specified leukaemias), VIc (unspecified malignant renal tumours), VIIIb (chondrosarcoma), VIIId (other specified malignant bone tumours), IXc (Kaposi sarcoma), Xd (gonadal carcinomas) or Xe (other and unspecified malignant gonadal tumours).
§Benign tumours or tumours of uncertain behaviour may be included in these groups (but US data exclude non‐malignant cases); among Irish cases, the proportion of non‐malignant cases was 2/12 in group IIIa, 0/13 in IIId, 16/20 in IIIe, 0/10 in IIIf and 1/6 in Xa.
WASR, rates standardised for age to the world standard population.
Mortality data
Official mortality statistics were obtained for the period 1953–2000,1 coded to the International Classification of Diseases, 9th edition (ICD‐9)9 or earlier editions. All childhood deaths attributed to malignant neoplasms were included in analyses here, but lack of tumour morphology data prevented coding to the ICCC scheme.
Rates and trends
Incidence and mortality rates were directly age standardised to the traditional world population standard (ratio 12:10:9 for ages 0–4:5–9:10–14), for comparison with European and US statistics (re‐computed to the same standard). Trends in rates were assessed using the Joinpoint statistical package (http://srab.cancer.gov/joinpoint/).10
Survival analysis
Observed (crude) survival was calculated by actuarial methods. For children in developed countries, this closely matches relative and cancer‐specific survival.2 Follow‐up was based on clinical records and on linkage to national death certificate data up to December 2002. Comparisons between patient groups were also made using Cox regression analysis. Comparisons with international data used results of the EUROCARE‐3 project (European cases diagnosed in 1990–1994 and followed to 1999),2 and of US SEER cancer registries (1994–2000 cases followed to 2002, analysed using Seer*Stat version 6.2.3, http://seer.cancer.gov/seerstat).8 For international comparisons, survival results were combined for males and females and, where data allowed, age standardised to the EUROCARE‐3 patient populations for specific diagnostic groups.2
Loss to follow‐up could not be directly measured, as follow‐up was based mainly on linkage to Irish death certificates. A few deaths may have occurred among any children whose families emigrated within 5 years of the cancer diagnosis, but any effect on survival estimates is likely to be small. Five years of potential follow‐up was available for most patients, but 23% had less than 4 years of follow‐up when censored (table 2).
Table 2 Summary of patient characteristics and data quality indicators for incident childhood cancers, Ireland, 1994–2000, and comparison with European (EUROCARE‐3)2 and US (SEER) data8.
| Ireland 1994–2000 | Europe 1990–94 | USA 1994–2000 | |||
|---|---|---|---|---|---|
| Cases | % | Mean (%) | National range (%) | % | |
| All cases | 787 | 100.0 | |||
| Boys | 425 | 54.0 | 56 | 49–70 | 54.4 |
| 0–4 years of age | 327 | 41.6 | 45 | 35–56 | 46.5 |
| 5–9 years of age | 208 | 26.4 | 26.4 | ||
| 10–14 years of age | 252 | 32.0 | 27.2 | ||
| Microscopic verification | 740 | 94.0 | 93 | 79–100 | 95.5 |
| Death certificate‐ or autopsy‐only* | 4 | 0.5 | 0.6 | 0–5.9 | 0.4 |
| Unspecified cases† | 44 | 5.6 | 3.4 | 0–14.9 | 2.2 |
| Lost to follow‐up‡ | NA | NA | 1.1 | 0–7.0 | |
| Alive with follow‐up <4 years | 183 | 23.2 | 6.9 | 0–22.2 | |
*Three death certificate‐only and one autopsy‐only cases were excluded from survival analyses.
†Unspecified cases: cases assigned to non‐specific ICCC categories Ie, IIe, IIIf, VIc, VIIc, VIIIe, IXe or XIIb (cf table 1).
‡Loss to follow‐up was not directly available for Irish cases, as follow‐up was based mainly on comprehensive death certificate linkage.
Ethics approval
The National Cancer Registry is statutorily empowered to collect incidence and follow‐up data on cancer patients in the Republic of Ireland, and to report non‐identifiable summary data on those patients. Identifiable data are subject to strict confidentiality and not released outside the NCR except to physicians and hospitals involved in treating specific patients. No further ethics approval was sought.
Results
Incidence
The age and sex composition of incident cases during 1994–2000 are summarised along with standard data quality indicators2,11 in table 2. In general, there was good agreement with European and US averages from the 1990s (EUROCARE‐3 and SEER data).2,8 The proportion of cases assigned to non‐specific diagnostic subgroups was higher in Ireland (5.6%) than in Europe overall (3.4%) or the US (2.2%), partly reflecting stricter rules in the Irish registry concerning the acceptability of non‐microscopic diagnoses.
A total of 787 incident cases of childhood cancer, including 771 malignant cases, were recorded in Ireland during the 7‐year period (table 1), which is about 112 per year (range 101–129). This excludes neoplasms that do not fall within the ICCC classification. There were 425 cases (54% of the total) in males and 362 (46%) in females, an annual average of 61 male and 52 female cases. The largest number of cases occurred in the 0–4‐year‐old (327) age group , followed by the 10–14‐ (252) and 5–9‐year‐old (208) age groups.
The most common childhood cancers were leukaemias (30%), tumours of the central nervous system and related tissues (CNS) (27%), and lymphomas and related neoplasms (11%). The others were mainly soft tissue sarcomas, and bone, renal and sympathetic nervous system tumours (table 1).
The world age‐standardised incidence rates in 1994–2000 averaged 145 cases per million for males, 130 for females and 137.5 overall (table 1). The crude incidence rate was 132 cases per million. Age‐standardised incidence rates in Ireland were slightly higher (by about 5%) than the European average (131 per million, national range 102–158), but, based on invasive cases, lower (by about 9%) than the US SEER average for the same period (table 1). For the main diagnostic groups, differences from European and US rates were, in general, fairly minor. The most notable (absolute and relative) difference was perhaps for CNS and intracranial tumours (group III), with Irish rates about 29% higher than the European average and 19% higher than the US figure. However, based on malignant cases only, rates of CNS/intracranial tumours in Ireland were 9% higher than US SEER rates, which exclude non‐malignant tumours; it is possible also that non‐malignant tumours were under‐recorded in the European ACCIS data.
Age‐standardised incidence rates for total childhood cancers showed no significant trend in Ireland during 1994–2000, with an average annual change of +0.1% overall (95% CI −8.2% to +15.2%, p = 0.528). Among specific ICCC groups, the only significant trend was a decrease in the rate for CNS tumours (group III) by 6.9% per year overall (−12.0% to −1.5%, p = 0.0011). The latter trend largely reflected higher case numbers in 1994 and 1995, particularly astrocytomas (subgroup IIIb) in both years and primitive neuroectodermal tumours (IIIc) in 1995. For most other major cancer groups (I, II, IV, V, VI, IX, X and XI), rates appeared to be increasing (by 2–12% per year overall), while rates for malignant bone tumours (group VIII) appeared to be decreasing, but none of these trends were statistically significant. In part, this may reflect the short period for which trends could be assessed, the small numbers of cases involved and resultant large year‐to‐year fluctuations (fig 1). For leukaemias, for example, the annual number of cases ranged from 25 to 44.
Figure 1 Annual variation in numbers of cases of major childhood cancers in Ireland, 1994–2000.
Survival
Overall 5‐year survival for malignant cancers among children under 15 was 79% for males and 78% for females (table 3). Equivalent 5‐year survival for ICCC‐coded cancers, including some non‐malignant intracranial or intraspinal cases, was again about 79%. For the major diagnostic groups, estimates generally ranged from 65% to 85% (tables 3 and 4). In the three largest groups, 5‐year survival averaged 79% for leukaemias, 77–89% for lymphomas (male‐female range) and 73–74% for CNS tumours, although it was only 44–47% for one CNS subgroup (primitive neuroectodermal tumours).
Table 3 Observed 5‐year survival rates (not standardised by age) for neoplasms diagnosed in 1994–2000 among patients aged 0–14 years at diagnosis, followed to 31 December 2002.
| Diagnostic group | Cases, n | 5‐Year survival (%) and 95% CI | ||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||
| Invasive cancers: total | 417 | 351 | 79.2 | 74.8 to 82.9 | 78.3 | 73.5 to 82.4 |
| ICCC groups I–XII: total | 424 | 359 | 79.5 | 75.1 to 83.2 | 78.8 | 74.1 to 82.8 |
| I. Leukaemia | 124 | 113 | 78.8 | 69.8 to 85.4 | 79.0 | 70.1 to 85.6 |
| Ia. Lymphoid leukaemia | 99 | 87 | 80.8 | 70.6 to 87.7 | 82.1 | 72.1 to 88.8 |
| Ib. Acute non‐lymphocytic leukaemia | 20 | 22 | 75.0 | 50.0 to 88.7 | 67.7 | 43.9 to 83.2 |
| II. Lymphomas, etc | 56 | 34 | 88.7 | 76.3 to 94.8 | 76.9 | 56.9 to 88.5 |
| IIa. Hodgkin lymphoma | 22 | 9 | 90.4 | 66.8 to 97.5 | 63.6 | 23.5 to 86.8 |
| IIb. Non‐Hodgkin lymphoma† | 20 | 15 | 95.0 | 69.5 to 99.3 | 78.4 | 46.5 to 92.6 |
| III. CNS/intracranial/intraspinal neoplasms* | 117 | 98 | 74.4 | 65.1 to 81.5 | 73.4 | 63.5 to 81.1 |
| IIIb. Astrocytoma | 60 | 62 | 83.3 | 71.2 to 90.7 | 80.6 | 68.4 to 88.5 |
| IIIc. Primitive neuroectodermal tumours | 23 | 15 | 43.9 | 22.1 to 63.9 | 46.7 | 21.2 to 68.7 |
| IIIe. Other specified intracranial/spinal* | 13 | 7 | 92.3 | 56.6 to 98.9 | 100 | 53.8 to 100 |
| IV. Sympathetic nervous system tumours | 25 | 12 | 79.7 | 57.8 to 91.0 | 82.9 | 47.2 to 95.4 |
| IVa. Neuroblastoma | 22 | 12 | 76.9 | 53.1 to 90.0 | 82.9 | 47.2 to 95.4 |
| VI. Renal tumours | 15 | 21 | 100 | 77.4 to 100 | 95.2 | 70.7 to 99.3 |
| VIa. Wilms tumour, etc | 15 | 20 | 100 | 77.4 to 100 | 95.0 | 69.5 to 99.3 |
| VIII. Malignant bone tumours | 22 | 15 | 76.9 | 53.0 to 89.7 | 80.0 | 50.0 to 93.1 |
| VIIIa. Osteosarcoma | 10 | 7 | 70.0 | 32.9 to 89.2 | 85.7 | 33.4 to 97.9 |
| VIIIc. Ewing sarcoma | 10 | 8 | 79.4 | 39.6 to 94.4 | 75.0 | 31.5 to 93.1 |
| IX. Soft tissue sarcomas | 27 | 23 | 73.3 | 48.2 to 87.6 | 61.0 | 36.1 to 78.6 |
| IXa. Rhabdomyosarcoma, etc | 18 | 15 | 72.7 | 41.1 to 89.2 | 55.8 | 26.1 to 77.7 |
| X. Germ cell and other gonadal tumours* | 13 | 13 | 67.9 | 35.0 to 86.7 | 92.3 | 56.6 to 98.9 |
| XI. Carcinomas, etc | 9 | 18 | 77.8 | 36.5 to 94.2 | 100 | 78.9 to 100 |
Survival is presented for all malignant cancers and for major International Classification of Childhood Cancers (ICCC) groups or subgroups.
*ICCC groups III and X include some intracranial or intraspinal tumours of benign or uncertain behaviour.
†Note that “lymphoma NOS” (ICD‐O morphology code 9590/3) is not included under non‐Hodgkin lymphoma in the ICCC classification.
Where observed survival is 100%, the “rule of three” is used to estimate the upper 95% CI for mortality,12 thus the lower 95% CI for survival = 100*1−(3/n), where n = number of patients with complete follow‐up (or the weighted annual average number of patients, adjusted for censoring, across the follow‐up period).
Table 4 Comparison of observed 5‐year survival rates for childhood cancer cases between Ireland, other European countries2 and the USA8.
| Diagnostic group | Ireland | Europe (20 countries) | Nordic countries†† | USA‡‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1994–2000 | 1990–94 | 1990–94 | 1994–2000 | |||||||
| n | Survival (%) | 95% CI | Survival (%) | 95% CI | Range† | Survival (%) | 95% CI | Survival (%) | 95% CI | |
| Invasive cancers: total | 768 | 79.0 | 76.0 to 82.0 | 71.8 | 70.7 to 72.8 | 44.9 to 90.1 | 75.7 | 74.9 to 76.4 | ||
| ICCC groups I–XII: total* | 783 | 79.4 | 76.5 to 82.4 | – | – | – | – | – | ||
| Ia. Lymphoid leukaemia | 186 | 81.7 | 75.9 to 87.6 | 79 | 77 to 81 | 47 to 100 | 84.8 | 82.1 to 87.7 | 83.5 | 82.3 to 84.8 |
| Ib. Acute non‐lymphocytic leukaemia | 42 | 70.1 | 56.6 to 83.6 | 48 | 41 to 49 | 17 to 70 | 61.8 | 51.9 to 73.5 | 48.6 | 44.9 to 52.3 |
| IIa. Hodgkin lymphoma | 31 | 82.9‡ | 63.6 to 92.6 | 94 | 88 to 97 | 73 to 100 | 93.3 | 87.8 to 99.3 | 94.9 | 92.9 to 96.8 |
| IIb. Non‐Hodgkin lymphoma | 35 | 87.9‡ | 70.7 to 95.3 | 79 | 73 to 84 | 42 to 92 | 79.4 | 72.8 to 86.6 | 81.0 | 77.8 to 84.2 |
| III. CNS tumours* | 197 | 71.2 | 64.8 to 77.6 | – | – | – | 73.0 | 69.6 to 76.6 | 67.4 | 65.5 to 69.2 |
| IIIa. Ependymoma* | 10 | 59.2‡ | 24.2 to 82.5 | 55 | 49 to 62 | 36 to 73 | – | – | 65.4 | 59.2 to 71.6 |
| IIIb. Astrocytoma | 122 | 81.3 | 74.4 to 88.2 | 79 | 74 to 83 | 63 to 89 | – | – | 79.8 | 77.6 to 82.0 |
| IIIc. Primitive neuroectodermal tumours | 38 | 48.6 | 32.7 to 64.5 | 50 | 46 to 55 | 11 to 100 | – | – | 62.8 | 59.2 to 66.4 |
| IVa. Neuroblastoma | 34 | 73.0 | 56.4 to 89.6 | 62§ | 57 to 66 | 41 to 68 | 62.0 | 54.6 to 71.2 | 61.7§ | 58.3 to 65.0 |
| V. Retinoblastoma | 16 | 100‡ | 76.5 to 100 | 91¶ | 81 to 96 | 60 to 100 | – | – | 94.9¶ | 92.3 to 97.5 |
| VIa. Wilms tumour, etc. | 35 | 97.1‡ | 81.4 to 103 | 84 | 80 to 87 | 70 to 100 | 91.9 | 87.5 to 96.5 | 85.6 | 83.0 to 88.2 |
| VIIIa. Osteosarcoma (bone) | 13 | 68.8** | 36.7 to 87.0 | 66** | 57 to 64 | 13 to 82 | – | – | 64.0** | 57.7 to 70.3 |
| VIIIc. Ewing sarcoma (bone) | 18 | 75.5 | 52.4 to 98.7 | 69 | 62 to 75 | 31 to 86 | – | – | 65.4 | 58.3 to 72.6 |
| IXa. Rhabdomyosarcoma, etc | 33 | 64.5 | 46.5 to 82.4 | 67 | 62 to 72 | 39 to 100 | – | – | 64.0 | 59.7 to 68.4 |
Survival rates are age standardised to the EUROCARE‐3 patient population for each ICCC group unless otherwise noted, and comparisons are not shown for cancers with <10 Irish cases or for those not published for Europe.2
*These diagnostic groups strictly include some CNS or intracranial tumours of benign or uncertain behaviour, but as non‐malignant cases are not included in the European or US survival data quoted here, invasive cases have also been excluded from Irish data used in these comparisons.
†Inter‐country range of European estimates (1990–94) is quoted where available (countries with >4 cases; Gatta et al2).
‡Irish survival estimates for these diagnostic groups are not age standardised because of insufficient data (or 100% survival in some age groups).
§European survival estimate for neuroblastoma is age standardised using ages 0, 1–4 and 5–14.
¶European survival estimate for retinoblastoma is age standardised using ages 0 and 1–4 only.
**Survival estimates for osteosarcoma are not age standardised and are restricted to age 10–14.
††“Nordic countries” as presented by Gatta et al2 comprise Finland, Iceland, Norway and Sweden (but not Denmark).
‡‡US SEER data.8
Combined EUROCARE results for western or north‐western Europe were not published separately, but EUROCARE survival figures for the Nordic countries2 approach or exceed those for Ireland (table 4).
Differences in survival between males and females were generally slight (table 3). Cox regression analysis did not confirm any significant gender differences for any diagnostic group or overall (adjusted for age group and major diagnostic group): hazard ratio = 1.07 (95% CI 0.78 to 1.47, p = 0.655) for females vs males.
In general, age‐adjusted 5‐year survival for Irish cases closely matched or slightly exceeded recent European (EUROCARE‐3) and US (SEER) averages for the same diagnostic groups (table 4). Formal statistical comparison was not possible, but (based on non‐overlap of 95% confidence intervals) Irish survival estimates were significantly higher than European estimates for invasive cancers as a whole (79% for Ireland vs 72% for Europe), and European and US estimates for ANLL (acute non‐lymphocytic leukaemia; 67% vs 48% and 49%, respectively) (table 4). For Hodgkin lymphoma, survival was significantly lower for Irish than US cases (83% vs 95%). For neuroblastoma, survival of Irish cases averaged 73%, compared with 62% for both EUROCARE‐3 and US SEER data, but differences were not statistically significant.
Mortality
A total of 144 children (87 male and 57 female) died from malignant cancer in Ireland during 1994–2000, approximately 21 per year. The mortality/incidence ratio was 0.19 overall, 0.21 for males and 0.16 for females. Numbers of deaths over the 7 years were highest in the age group 10–14 years (56), followed by 0–4 years (46) and 5–9 years (40). The crude mortality rate averaged 20.4 per million per year overall, 28.4 in males and 19.6 in females, while the age‐standardised rates were 30.3 (95% CI 23.8 to 36.8) in males and 19.6 (95% CI 14.4 to 24.8) in females.
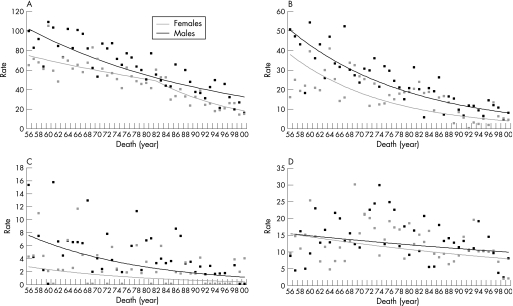
Apparent short‐term trends in mortality during 1994–2000, which showed a significant decline, by an average of 13% per year for males (p = 0.023) but no significant trend for females (p = 0.695), may be uninformative because of the small numbers of deaths involved. However, age‐standardised total cancer mortality rates showed clear and significant downward trends for both sexes during 1956–2000 (fig 2). For males the trend was best modelled as a steady reduction, by 2.6% per annum during 1956–2000 (95% CI −3.1% to −2.1%, p<0.001), while for females the best fit to the data was an initial decline of 1.6% per annum during 1956–82 (95% CI −2.5% to −0.7%, p<0.001) followed by a steeper decline of 5.8% per annum during 1982–2000 (95% CI −7.9% to −3.6%, p<0.001). Of the three largest subgroups of childhood cancers, deaths from leukaemias and lymphomas also showed obvious declines during 1956–2000 (fig 2), by about 4% or 5% per annum, although the statistical significance of trends could not be assessed because of sparse data (no deaths in some years). For cancers of the brain and CNS, long‐term trends were less obvious but suggested a decline by about 1% per year (fig 2).
Figure 2 Trends in annual childhood cancer mortality in Ireland (world age‐standardised rates per million), 1956–2000: (A) all malignant cancers (ICD‐9 codes 140–208); (B) leukaemias (ICD‐9 codes 204–208); (C) lymphomas (ICD‐9 codes 200–202); (D) brain and central nervous system (ICD‐9 codes 191–192).
Discussion
In this study, we have quantified the childhood cancer burden in Ireland, in terms of incidence, survival and mortality, and compared these measures with international findings. Overall incidence and survival were comparable to European and US averages. No significant trends in incidence were apparent over the main study period (1994–2000), apart from an apparent decline for CNS tumours, but mortality has shown major long‐term reductions. These results are reassuring in that they show no unexpected patterns or trends, and survival and mortality broadly match findings from other western populations.
Overall incidence of childhood cancer in Ireland was (for ICCC‐coded cases) about 5% higher than the European average (http://www‐dep.iarc.fr/accis/data.htm), but (for invasive cases) about 9% lower than the US average,8 over similar periods. Slightly more recent coverage in the Irish data possibly accounts for some of the difference from the European average. For specific diagnostic groups, differences from European and US rates were generally minor. Based on examination of annual incidence data, the apparently high rate of CNS/intracranial tumours (group III) in Ireland partly reflects high recorded rates of astrocytoma and ependymoma in the period 1994–95. This might suggest inclusion of some prevalent cases for those years, but similar temporal patterns were not seen for other diagnostic groups. Higher Irish than US rates for group III partly reflect the fact that US data did not include non‐malignant tumours, and it may be that non‐malignant tumours were not comprehensively included in European data. In common with Europe as a whole,7 the overall incidence rate of childhood cancer in Ireland was slightly higher (by about 10–12%) in males than in females, although this varied between cancer types.
Mortality rates from childhood cancer were far lower than incidence rates, reflecting high survival rates, which in general were at least as high as European and US averages.2,8 Survival comparisons made here with international data are for fully malignant cases only, for all populations, and inclusion of non‐malignant tumours would increase Irish survival figures only slightly (cf table 4). However, the European data represent cases diagnosed, on average, 5 years earlier than the Irish cases, they include poorer survival for eastern European countries, and Irish survival rates might have been over‐estimated slightly if any unreported deaths occurred outside Ireland. Nevertheless, the high survival rates and long‐term decreases in mortality in Ireland clearly imply effective modern treatments, and are consistent with improvements in survival rates and declines in mortality rates seen elsewhere in Europe.13,14
Relatively high 5‐year survival (67%) was recorded in Ireland for ANLL compared with EUROCARE‐3 results for 1990–94 (48% overall or 62% for Nordic countries)2 and US SEER results for 1994–2000 (49%).8 The high recorded survival for Ireland is considered to reflect the inclusion of the majority of childhood acute myeloid leukaemia (AML) cases in relevant trial protocols. Re‐checks of death certificate matching confirm that no ANLL deaths within Ireland have been missed, and restriction of results to AML or to cases with a full 5 years of potential follow‐up does not appreciably change the survival results. The UK Medical Research Council AML 12 trial (since 1995) reported a 5‐year survival of 66% for enrolled paediatric cases of AML, the main cell type in this group, and noted that improvements in supportive care contributed to improved survival compared with the earlier AML 10 trial.15
Childhood cancers, although rare, have a high social impact. Continued monitoring of incidence, treatment and survival is thus important, and public concerns about possible geographic variation4 may, on occasion, require investigation. There is evidence from the European ACCIS study that the incidence of childhood cancers, in almost all diagnostic groups, has increased since the 1970s.7 In the USA, modest increases in childhood cancer incidence during the period 1975–95, mainly during the 1980s, were considered most likely to reflect “diagnostic improvements or reporting changes”.16 The small annual numbers of cases in Ireland and the short period for which full cancer registration has been in place (since 1994) make it difficult, as yet, to provide firm assessments of trends in incidence and survival. But the results presented here are based on complete national coverage and (along with long‐term mortality data) provide an important baseline for monitoring of further progress against childhood cancer in Ireland.
Although the clinical and public health message from the present study is reassuring, it is vital to continue to quantify the childhood cancer burden in Ireland. This will help to estimate care needs, plan interventions and quantify use of appropriate cancer treatments. Monitoring of possible late effects on growth, puberty and reproduction, cardiac and thyroid health, and on cognitive and psychosocial outcomes for survivors is also needed.17,18 Estimation of childhood cancer prevalence and inclusion of adolescents in survival analyses would also be useful. Continued collaboration with international studies of childhood cancer will help ensure that monitoring and research is carried out appropriately and placed in a wider context.
What is already known on this topic
Improved treatment methods have resulted in marked improvements in survival rates, and consequent reductions in mortality rates, for childhood cancers in developed countries.
There is evidence that the incidence rates of most major diagnostic groups of childhood cancers have increased in Europe since the 1970s.
What this study adds
National survival rates and comprehensive data on incidence for childhood cancer in the Republic of Ireland are presented for the first time.
High recent survival rates and major long‐term reductions in mortality rates for childhood cancer in Ireland are consistent with survival and treatment improvements documented elsewhere in Europe.
Acknowledgements
We thank the tumour registration officers and other staff of the National Cancer Registry, the Central Statistics Office for providing mortality data, and hospitals and pathology labs for providing the Registry with access to case data. Dr Riccardo Capocaccia (Istituto Superiore di Sanità, Rome) provided details of EUROCARE‐3 standard patient population structures for age adjustment of survival data. Client‐server access to SEER data was provided by the US National Cancer Institute's DCCPS Surveillance Research Program, Cancer Statistics Branch.
Abbreviations
ACCIS - Automated Childhood Cancer Information System
AML - acute myeloid leukaemia
ANLL - acute non‐lymphocytic leukaemia
ICCC - International Classification of Childhood Cancer
ICD‐9 - International Classification of Diseases, 9th edition
NCR - National Cancer Registry
Footnotes
Funding: The National Cancer Registry is funded by the Department of Health and Children, Dublin.
Competing interests: None.
References
- 1.Central Statistics Office Annual report on vital statistics [years 1956 to 2000]. Dublin: Government Publications Office, 1957–2001
- 2.Gatta G, Corazziari I, Mangani C.et al Childhood cancer survival in Europe. Ann Oncol 200314(Suppl 5)v119–v127. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Registry Cancer in Ireland, 1994–1998. Incidence, mortality, treatment and survival. Cork: National Cancer Registry (Ireland) 2001:19–30. http://www.ncri.ie/pubs/report‐1998.shtml (accessed 5 August 2007)
- 4.Herity B, Daly L, Breatnach F.et al Childhood leukaemia in Ireland. Ir Med J 19928550–52. [PubMed] [Google Scholar]
- 5.Kelleher M J, Corcoran P, Keohane B.et al Suicide, road traffic and cancer deaths among the young in Ireland. Ir Med J 19958896–98. [PubMed] [Google Scholar]
- 6.Kramárová E, Stiller C A, Ferlay J.et alInternational classification of childhood cancer 1996. IARC Technical Report No. 29. Lyon: IARC, 1996
- 7.Steliarova‐Foucher E, Stiller C, Kaatsch P.et al Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCIS project): an epidemiological study. Lancet 20043642097–2105. [DOI] [PubMed] [Google Scholar]
- 8.SEER Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence ‐ SEER 18 Regs, Nov 2004 Sub (1973–2002 varying) ‐ linked to county attributes ‐ total U.S., 1969–2002 counties. Bethesda, MD: National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, 2004
- 9.World Health Organization International statistical classification of diseases and related health problems, 9th revision. Geneva: World Health Organization, 1997
- 10.Kim H‐J, Fay M P, Feuer E J.et al Permutation tests for Joinpoint regression with applications to cancer rates. Stat Med 200019335–351. [DOI] [PubMed] [Google Scholar]
- 11.Parkin D M, Chen V W, Ferlay J.et alComparability and quality control in cancer registration. IARC Technical Report No.19. Lyon: IARC, 1994
- 12.van Belle G.Statistical rules of thumb. New York: Wiley‐Interscience, 200249–50.
- 13.Levi F, La Vecchia C, Negri E.et al Childhood cancer mortality in Europe, 1955–1995. Eur J Cancer 200137785–809. [DOI] [PubMed] [Google Scholar]
- 14.Gatta G, Capocaccia R, Stiller C A.et al Childhood cancer survival trends in Europe: a EUROCARE Working Group study. J Clin Oncol 2005233742–3751. [DOI] [PubMed] [Google Scholar]
- 15.Gibson B E, Wheatley K, Hann I M.et al Treatment strategy and long‐term results in paediatric patients treated in consecutive UK AML trials. Leukaemia 2004192130–2138. [DOI] [PubMed] [Google Scholar]
- 16.Linet M S, Ries L A, Smith M A.et al Cancer surveillance series: recent trends in childhood cancer incidence and mortality in the United States. J Natl Cancer Inst 1999911051–1058. [DOI] [PubMed] [Google Scholar]
- 17.Scottish Intercollegiate Guidelines Network Long term follow up of survivors of childhood cancer. Edinburgh: Scottish Intercollegiate Guidelines Network, Royal College of Physicians, 2004. http://www.sign.ac.uk/guidelines/fulltext/76/index.html (accessed 5 August 2007)
- 18.Children's Oncology Group Long‐term follow‐up guidelines for survivors of childhood, adolescent, and young adult cancers. Version 1.2. Arcadia, CA: Children's Oncology Group 2004. http://www.survivorshipguidelines.org/ (accessed 5 August 2007)