Abstract
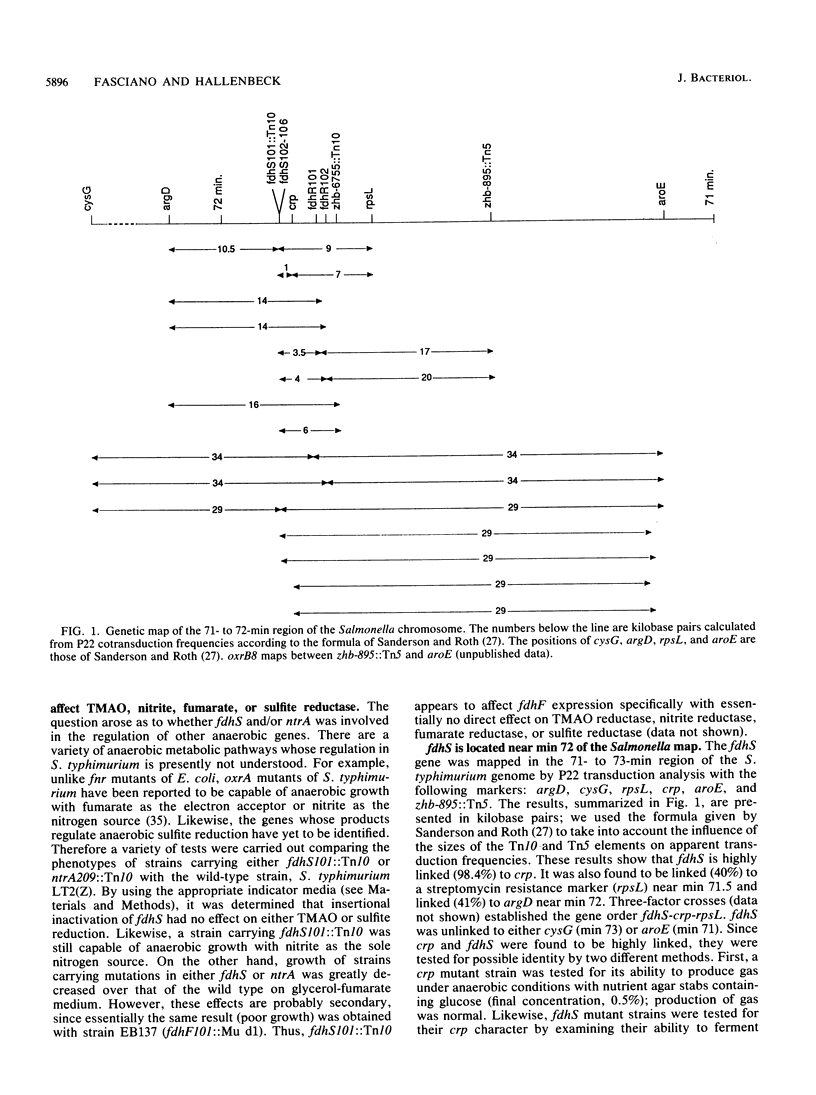
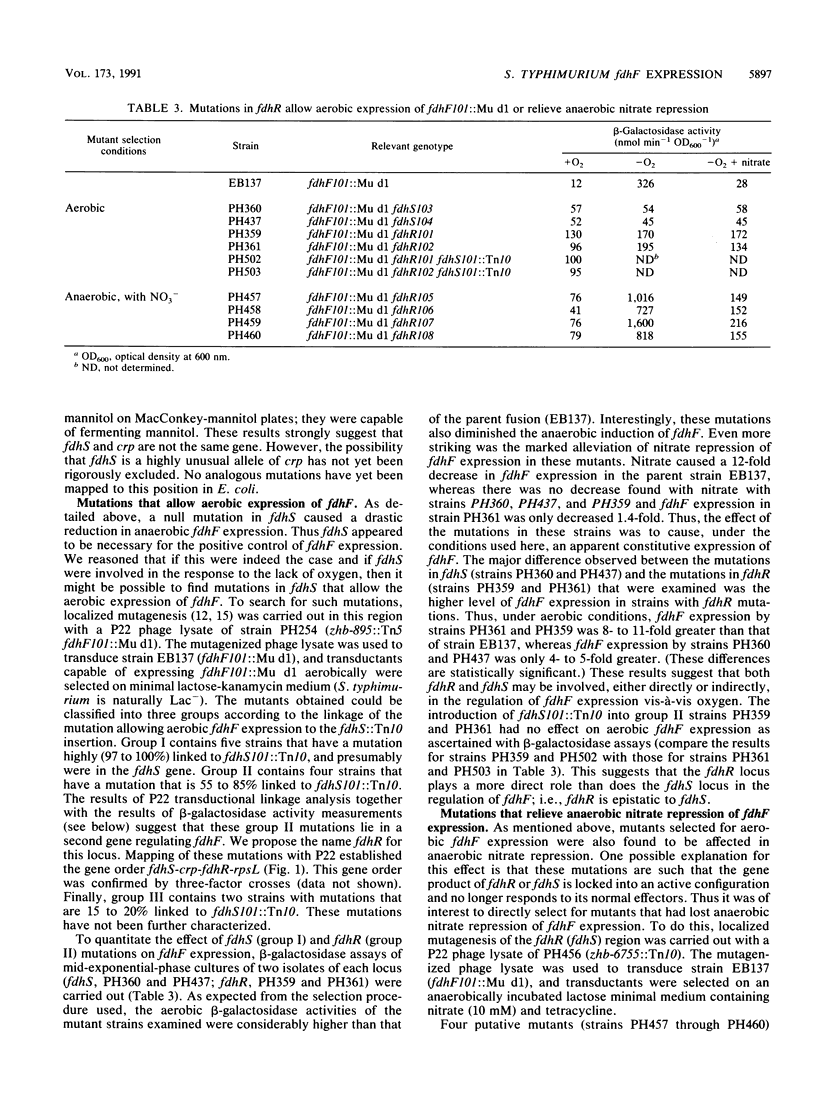
Expression of the fdhF locus of Salmonella typhimurium is shown to be dependent upon ntrA and oxrB. However, the oxrB8 mutation is pleiotropic and also affects the expression of hyd, pepT, and chlC, whereas a mutation in ntrA does not. Insertional inactivation with Tn10 and localized mutagenesis permitted the definition and partial characterization of two new genes, fdhS and fdhR, which appear to be involved in the positive regulation of fdhF expression. Both genes were mapped to the 71- to 72-min region of the S. typhimurium chromosome with the gene order fdhS-crp-fdhR-rpsL. Mutations in fdhS specifically affect fdhF expression without affecting the expression of the other anaerobically induced genes or enzymes that were tested, including hyd, pepT, chlC, nitrite reductase, sulfite reductase, and trimethylamine-N-oxide reductase. Both fdhR and fdhS may be involved in fdhF regulation vis-à-vis oxygen, since localized mutagenesis produced alleles of both genes that permitted the aerobic expression of fdhF. However, fdhR may more directly interact with fdhF, since insertional inactivation of fdhS does not abolish aerobic expression of fdhF in fdhR mutant strains. Taken together, these results suggest that fdhS and fdhR act in concert under anaerobic conditions to activate fdhF transcription.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett E. L., Kwan H. S., Macy J. Anaerobiosis, formate, nitrate, and pyrA are involved in the regulation of formate hydrogenlyase in Salmonella typhimurium. J Bacteriol. 1984 Jun;158(3):972–977. doi: 10.1128/jb.158.3.972-977.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkmann A., Böck A. Characterization of a cis regulatory DNA element necessary for formate induction of the formate dehydrogenase gene (fdhF) of Escherichia coli. Mol Microbiol. 1989 Feb;3(2):187–195. doi: 10.1111/j.1365-2958.1989.tb01807.x. [DOI] [PubMed] [Google Scholar]
- Birkmann A., Hennecke H., Böck A. Construction of chimaeric promoter regions by exchange of the upstream regulatory sequences from fdhF and nif genes. Mol Microbiol. 1989 Jun;3(6):697–703. doi: 10.1111/j.1365-2958.1989.tb00218.x. [DOI] [PubMed] [Google Scholar]
- Birkmann A., Sawers R. G., Böck A. Involvement of the ntrA gene product in the anaerobic metabolism of Escherichia coli. Mol Gen Genet. 1987 Dec;210(3):535–542. doi: 10.1007/BF00327209. [DOI] [PubMed] [Google Scholar]
- Birkmann A., Zinoni F., Sawers G., Böck A. Factors affecting transcriptional regulation of the formate-hydrogen-lyase pathway of Escherichia coli. Arch Microbiol. 1987 Jun;148(1):44–51. doi: 10.1007/BF00429646. [DOI] [PubMed] [Google Scholar]
- Buck M., Woodcock J., Cannon W., Mitchenall L., Drummond M. Positional requirements for the function of nif-specific upstream activator sequences. Mol Gen Genet. 1987 Nov;210(1):140–144. doi: 10.1007/BF00337770. [DOI] [PubMed] [Google Scholar]
- Böhm R., Sauter M., Böck A. Nucleotide sequence and expression of an operon in Escherichia coli coding for formate hydrogenlyase components. Mol Microbiol. 1990 Feb;4(2):231–243. doi: 10.1111/j.1365-2958.1990.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Chippaux M., Pascal M. C., Casse F. Formate hydrogenlyase system in Salmonella typhimurium LT2. Eur J Biochem. 1977 Jan 3;72(1):149–155. doi: 10.1111/j.1432-1033.1977.tb11234.x. [DOI] [PubMed] [Google Scholar]
- Clark D. P. The fermentation pathways of Escherichia coli. FEMS Microbiol Rev. 1989 Sep;5(3):223–234. doi: 10.1016/0168-6445(89)90033-8. [DOI] [PubMed] [Google Scholar]
- Clark M. A., Barrett E. L. The phs gene and hydrogen sulfide production by Salmonella typhimurium. J Bacteriol. 1987 Jun;169(6):2391–2397. doi: 10.1128/jb.169.6.2391-2397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutnick D., Calvo J. M., Klopotowski T., Ames B. N. Compounds which serve as the sole source of carbon or nitrogen for Salmonella typhimurium LT-2. J Bacteriol. 1969 Oct;100(1):215–219. doi: 10.1128/jb.100.1.215-219.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschman J., Wong P. K., Sei K., Keener J., Kustu S. Products of nitrogen regulatory genes ntrA and ntrC of enteric bacteria activate glnA transcription in vitro: evidence that the ntrA product is a sigma factor. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7525–7529. doi: 10.1073/pnas.82.22.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Ames B. N. Localized mutagenesis of any specific small region of the bacterial chromosome. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3158–3162. doi: 10.1073/pnas.68.12.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D. J., Higgins C. F. Two genetically distinct pathways for transcriptional regulation of anaerobic gene expression in Salmonella typhimurium. J Bacteriol. 1986 Oct;168(1):389–397. doi: 10.1128/jb.168.1.389-397.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D. J., Sawers R. G., Rugman P. A., Boxer D. H., Higgins C. F. Effects of anaerobic regulatory mutations and catabolite repression on regulation of hydrogen metabolism and hydrogenase isoenzyme composition in Salmonella typhimurium. J Bacteriol. 1986 Oct;168(1):405–411. doi: 10.1128/jb.168.1.405-411.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz S., Böhm R., Beier A., Böck A. Characterization of divergent NtrA-dependent promoters in the anaerobically expressed gene cluster coding for hydrogenase 3 components of Escherichia coli. Mol Microbiol. 1990 Jan;4(1):13–20. doi: 10.1111/j.1365-2958.1990.tb02010.x. [DOI] [PubMed] [Google Scholar]
- Lutz S., Jacobi A., Schlensog V., Böhm R., Sawers G., Böck A. Molecular characterization of an operon (hyp) necessary for the activity of the three hydrogenase isoenzymes in Escherichia coli. Mol Microbiol. 1991 Jan;5(1):123–135. doi: 10.1111/j.1365-2958.1991.tb01833.x. [DOI] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maupin J. A., Shanmugam K. T. Genetic regulation of formate hydrogenlyase of Escherichia coli: role of the fhlA gene product as a transcriptional activator for a new regulatory gene, fhlB. J Bacteriol. 1990 Sep;172(9):4798–4806. doi: 10.1128/jb.172.9.4798-4806.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecher A., Zinoni F., Jatisatienr C., Wirth R., Hennecke H., Böck A. On the redox control of synthesis of anaerobically induced enzymes in enterobacteriaceae. Arch Microbiol. 1983 Nov;136(2):131–136. doi: 10.1007/BF00404787. [DOI] [PubMed] [Google Scholar]
- Putnam S. L., Koch A. L. Complications in the simplest cellular enzyme assay: lysis of Escherichia coli for the assay of beta-galactosidase. Anal Biochem. 1975 Feb;63(2):350–360. doi: 10.1016/0003-2697(75)90357-7. [DOI] [PubMed] [Google Scholar]
- Reitzer L. J., Magasanik B. Transcription of glnA in E. coli is stimulated by activator bound to sites far from the promoter. Cell. 1986 Jun 20;45(6):785–792. doi: 10.1016/0092-8674(86)90553-2. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, edition VII. Microbiol Rev. 1988 Dec;52(4):485–532. doi: 10.1128/mr.52.4.485-532.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar P., Lee J. H., Shanmugam K. T. Cloning of hydrogenase genes and fine structure analysis of an operon essential for H2 metabolism in Escherichia coli. J Bacteriol. 1985 Apr;162(1):353–360. doi: 10.1128/jb.162.1.353-360.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar P., Lee J. H., Shanmugam K. T. Gene-product relationships of fhlA and fdv genes of Escherichia coli. J Bacteriol. 1988 Dec;170(12):5440–5445. doi: 10.1128/jb.170.12.5440-5445.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar P., Shanmugam K. T. Biochemical and genetic analysis of hydrogen metabolism in Escherichia coli: the hydB gene. J Bacteriol. 1988 Dec;170(12):5433–5439. doi: 10.1128/jb.170.12.5433-5439.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlensog V., Birkmann A., Böck A. Mutations in trans which affect the anaerobic expression of a formate dehydrogenase (fdhF) structural gene. Arch Microbiol. 1989;152(1):83–89. doi: 10.1007/BF00447016. [DOI] [PubMed] [Google Scholar]
- Schlensog V., Böck A. Identification and sequence analysis of the gene encoding the transcriptional activator of the formate hydrogenlyase system of Escherichia coli. Mol Microbiol. 1990 Aug;4(8):1319–1327. doi: 10.1111/j.1365-2958.1990.tb00711.x. [DOI] [PubMed] [Google Scholar]
- Stewart V. Nitrate respiration in relation to facultative metabolism in enterobacteria. Microbiol Rev. 1988 Jun;52(2):190–232. doi: 10.1128/mr.52.2.190-232.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker K., Reijnders W. N., Oltmann L. F., Stouthamer A. H. Initial cloning and sequencing of hydHG, an operon homologous to ntrBC and regulating the labile hydrogenase activity in Escherichia coli K-12. J Bacteriol. 1989 Aug;171(8):4448–4456. doi: 10.1128/jb.171.8.4448-4456.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Lenk J. B., Gamble B. L., Miller C. G. Oxygen regulation in Salmonella typhimurium. J Bacteriol. 1985 Feb;161(2):673–680. doi: 10.1128/jb.161.2.673-680.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. F., Mandrand-Berthelot M. A. Regulation of the fdhF gene encoding the selenopolypeptide for benzyl viologen-linked formate dehydrogenase in Escherichia coli. Mol Gen Genet. 1987 Aug;209(1):129–134. doi: 10.1007/BF00329847. [DOI] [PubMed] [Google Scholar]
- Zinoni F., Beier A., Pecher A., Wirth R., Böck A. Regulation of the synthesis of hydrogenase (formate hydrogen-lyase linked) of E. coli. Arch Microbiol. 1984 Nov;139(4):299–304. doi: 10.1007/BF00408370. [DOI] [PubMed] [Google Scholar]


