Abstract
Background
It has been suggested that increasing obesity levels in young women lead to intrauterine environments that, in turn, stimulate increased obesity among their offspring, generating an intergenerational acceleration of obesity levels. If this mechanism is important, the association of maternal body mass index (BMI) with offspring BMI should be stronger than the association of paternal with offspring BMI.
Objective
To compare the relative strengths of association of maternal and paternal BMI with offspring BMI at age 7.5, taking into account the possible effect of non‐paternity.
Methods
We compared strength of association for maternal–offspring and paternal–offspring BMI for 4654 complete parent–offspring trios in the Avon Longitudinal Study of Parents and Children (ALSPAC), using unstandardised and standardised regression analysis. We carried out a sensitivity analysis to investigate the influence of non‐paternity on these associations.
Results
The strength of association between parental BMI and offspring BMI at age 7.5 was similar for both parents. Taking into account correlations between maternal and paternal BMI, performing standardised rather than unstandardised regression and carrying out a sensitivity analysis for non‐paternity emphasised the robustness of the general similarity of the associations. The associations between high parental BMI (top decile) and offspring BMI are also similar for both parents.
Conclusion
Comparison of mother–offspring and father–offspring associations for BMI suggests that intergenerational acceleration mechanisms do not make an important contribution to levels of childhood BMI within the population. Associations at later ages and for different components of body composition now require study.
The increasing prevalence of obesity among children and adults in many countries constitutes a potentially serious threat to the future health of these populations.1,2,3 The importance of a shift in the balance of energy intake to energy expenditure as the proximal determinant of rising obesity levels is generally recognised,1 with changes in social organisation and local, national and international economic forces being seen to underlie this pattern.4 In addition, there is evidence to support a role for prenatal influences on childhood and adulthood obesity.5,6,7 Maternal obesity may lead to greater placental transfer of nutrients during embryonic and fetal development, leading to permanent changes in appetite, metabolism and the neuroendocrine function of offspring.5 Studies in animal models have provided some support for the existence of such mechanisms.8
The consequence of this influence of maternal body composition on offspring body composition, mediated through the intrauterine environment, would be an intergenerational acceleration in obesity levels.1,7 Changes in the balance of energy intake and energy expenditure, leading to an increase in obesity in mothers, would, through such intrauterine processes, generate increases in obesity among offspring. When the female offspring in turn produce their own offspring, the latter will be destined to experience further increases in obesity through the influence of the obesity levels of their mothers on the intrauterine environment they encounter. This feed‐forward mechanism would lead to an intergenerational acceleration of obesity levels, over and above the acute effects of shifts in the balance between energy intake and energy expenditure within populations.
There is clear evidence that mothers with gestational diabetes have offspring with increased obesity levels in childhood and adolescence,9 although the degree to which this persists into adulthood is uncertain.10 The strongest evidence for a specific effect of diabetes during pregnancy on offspring body mass index (BMI) comes from a study of siblings discordant for maternal diabetes during pregnancy.11 At age 22, the sibling exposed to a maternal diabetic environment had, on average, higher BMI than the unexposed sibling. No influence of paternal diabetes was seen on offspring BMI, suggesting that the intrauterine environment, rather than a simple genetic mechanism, was responsible.
Raised BMI will generate a lesser degree of metabolic disturbance than that seen with diabetes, and the influence of this more modest exposure on offspring BMI is not well delineated. One approach to this issue, that would allow estimation of the potential importance of any intergenerational acceleration in obesity levels, is to compare associations between maternal BMI and offspring BMI with those between paternal BMI and offspring BMI. If maternal BMI has an influence on offspring BMI through intrauterine effects, the expectation would be of a stronger correlation of maternal than paternal BMI with offspring BMI. Few studies have approached this issue, and the available data have not been presented in a way that allows formal comparison of the magnitude of associations. Existing studies have yielded conflicting findings, generally from small sample sizes.12,13,14,15,16,17 We have therefore examined this issue in the Avon Longitudinal Study of Parents and Children (ALSPAC), producing directly comparable estimates of maternal and paternal BMI associations with offspring BMI, while taking into account plausible degrees of non‐paternity.
Methods
ALSPAC is a population‐based study investigating environmental and other factors that affect the health and development of children. The study methods are described in detail on the study website (http://www.alspac.bris.ac.uk) and elsewhere.18 In brief, pregnant women living in three health districts in Bristol, England who had an expected date of delivery between the start of April 1991 and end of December 1992 were eligible. A total of 14 541, approximately 85% of those eligible, enrolled in the study, and of these, 13 822 (95%) had a singleton, liveborn child. Ethical approval of the study was obtained from the ALSPAC law and ethics committee and the local research ethics committees.
Detailed information was obtained from the mother and her partner during pregnancy using self‐reported questionnaires. At enrolment, the mother was asked to record her height and pre‐pregnancy weight, from which BMI was calculated (weight/height,2 with weight in kilograms and height in metres). She was also asked whether her partner was the father of her unborn child. Age at delivery was derived from her date of birth. Her partner was asked to record his height and weight, and also his date of birth. The entire cohort of children was invited to a health examination at approximately age 7.5, and 7623 singletons attended. Weight was measured to the nearest 0.1 kg using Seca scales while the child was wearing underwear, and height was measured to the nearest 0.1 cm using a Harpenden stadiometer; BMI values for 7550 children were calculated from these measurements.
BMI values for 78 partners who were not confirmed as being the biological father of the child by the mother were excluded. An additional 225 partners were excluded as their age was not recorded. Hence, there were 7116 children whose BMI was measured as well as the BMI of at least one parent. Of these, BMI values were available for 6815 mothers and 4955 fathers; the BMI of both parents were available for 4654 parent–offspring trios. Parental BMI values were age adjusted, and childrens' BMI values were age and sex adjusted for all analyses. Adjusted values were calculated in two ways. Firstly, the residuals from the linear regression of BMI on age (and gender) were used. In practice, the mean BMI value was added to the residuals, although this constant would only affect the intercept in subsequent regression models with no effect on the estimated coefficients for parental BMI. The effects of parental BMI on offspring BMI were assessed using linear regression. Analyses were also repeated using standardised parental BMI and standardised offspring BMI, to allow comparisons to be made that are not influenced by the greater range in absolute BMI among mothers compared to fathers. Secondly, in the case of offspring BMI, to take account of the non‐normal distribution, adjusted values were calculated by transforming the BMI data using the LMS method.19 These derived data were then rescaled to have the same variance as the regression adjusted data to allow direct comparison between the results.
To examine the potential role of non‐paternity in generating greater associations between maternal and offspring BMI than between paternal and offspring BMI, given the non‐biological relationship between some fathers and their apparent offspring, we conducted a sensitivity analysis modelling the effects of non‐paternity rates of between 1% and 10%,20 using the modified equation given in appendix A. This sensitivity analysis adjusted the variance–covariance matrix used in the regression analysis assuming that the non‐biological father's BMI is unrelated to the child's BMI but is related to maternal BMI to a similar extent as the biological father's BMI. This adjustment allowed corrected estimates for the effects of parental BMI to be calculated. A range of non‐paternity rates of between 1% and 10% was chosen to include the likely but unknown true non‐paternity rate. Analyses were performed using Stata V.8.
Results
The mean BMI for the mothers was 22.9 (standard deviation (SD) 3.7) kg/m2 and 25.1 (SD 3.2) kg/m2 for fathers, using all available data. For offspring the mean BMI was 16.2 (SD 2.0) kg/m2, with no evidence of a sex difference (p = 0.98).
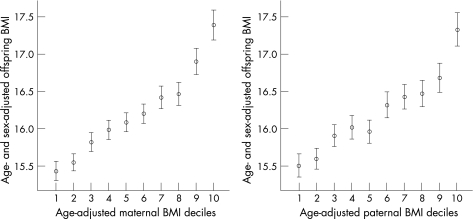
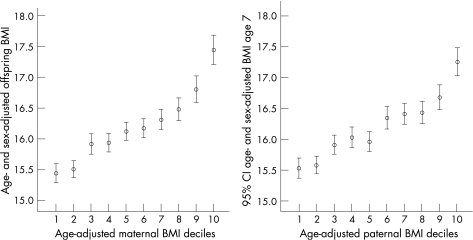
Offspring BMI according to maternal and paternal BMI is presented in fig 1 for all parent–offspring pairs available, and in fig 2 for the 4654 complete parent–offspring trios. As can be seen, the mother–offspring and father–offspring associations are similar. For all parent–offspring pairs the correlation between maternal and offspring BMI was 0.29 and for paternal–offspring pairs the correlation was BMI 0.26 (both p<0.001). For the complete parent–offspring trios the correlations were very similar: maternal–offspring was 0.30 and paternal–offspring was 0.25 (both p<0.001). Within the complete trios the correlation between maternal and paternal BMI was 0.17 (p<0.001).
Figure 1 Offspring body mass index (BMI) according to deciles of maternal and paternal BMI (based on 11 parent–offspring pairs available). Values are mean and 95% CIs.
Figure 2 Offspring body mass index (BMI) according to deciles of maternal and paternal BMI (based on complete parent–offspring trios).
As the findings for the complete parent–offspring trios were very similar to those for all parent–offspring pairs, the regression analysis was restricted to complete trios.
Table 1 presents the coefficients for offspring BMI regressed on parental BMI, singularly and simultaneously. In the analysis with both maternal and paternal BMI there was no evidence of an interaction between the two (p for interaction = 0.5). In the case of unstandardised BMI, there was no indication of a difference in effect size between maternal and paternal BMI (p = 0.4), but when standardisation was applied, maternal BMI appeared to have a greater influence (per standard deviation change) than paternal BMI (p = 0.006), although the difference in effect size was small.
Table 1 Unstandardised and standardised regression coefficients for offspring BMI on parental BMI, singularly and simultaneously*.
| Unstandardised age and sex adjusted | Standardised age and sex adjusted | |
|---|---|---|
| Coefficient (95% CI) | Coefficient (95% CI) | |
| Singularly | ||
| Maternal BMI | 0.156 (0.142 to 0.171) | 0.295 (0.267 to 0.322) |
| Paternal BMI | 0.155 (0.137 to 0.172) | 0.250 (0.218 to 0.274) |
| Simultaneously | ||
| Maternal BMI | 0.138 (0.124 to 0.153) | 0.261 (0.233 to 0.288) |
| Paternal BMI | 0.127 (0.110 to 0.144) | 0.202 (0.175 to 0.229) |
*The standardised coefficients analysed z scores for parental body mass index (BMI) and offspring BMI (see the Methods section), whereas the unstandardised regression utilised the actual values of parental and offspring BMI. The top two rows (singularly) report maternal and paternal BMI analysed separately; the bottom two rows (simultaneously) report the results of an analysis in which both maternal and paternal BMI were included in the same model.
Tables 2 and 3 present results of the sensitivity analysis with non‐paternity assumed to be between 0% and 10%. For the regression adjusted BMI, the results for 0% non‐paternity are, of course, the same as those presented in table 1. It can be seen that with increasing rates of non‐paternity, the associations for paternal BMI with offspring BMI approach (and for unstandardised regression coefficients in table 2, overtake) those of maternal BMI and offspring BMI.
Table 2 Unstandardised regression coefficients for offspring BMI (age and sex adjusted by regression) on parental BMI considered simultaneously, assuming various proportions of non‐paternity, with p values for differences in parental effects.
| Rate of non‐paternity | Age adjusted parental BMI* | p Value for difference | |
|---|---|---|---|
| Paternal | Maternal | ||
| 0 | 0.127 | 0.138 | 0.371 |
| 0.01 | 0.129 | 0.138 | 0.456 |
| 0.02 | 0.130 | 0.138 | 0.553 |
| 0.03 | 0.132 | 0.137 | 0.661 |
| 0.04 | 0.134 | 0.137 | 0.779 |
| 0.05 | 0.135 | 0.137 | 0.905 |
| 0.06 | 0.137 | 0.137 | 0.963 |
| 0.07 | 0.139 | 0.136 | 0.830 |
| 0.08 | 0.141 | 0.136 | 0.699 |
| 0.09 | 0.143 | 0.136 | 0.574 |
| 0.10 | 0.145 | 0.136 | 0.458 |
*Assuming that the covariance between the mother's and biological father's body mass index (BMI) is equal to the covariance between the mother's and reported father's BMI.
Table 3 Standardised regression coefficients for offspring BMI (age and sex adjusted by regression) on parental BMI considered simultaneously, assuming various proportions of non‐paternity, with p values for differences in parental effects.
| Rate of non‐paternity | Age adjusted parental BMI* | p Value for difference | |
|---|---|---|---|
| Paternal | Maternal | ||
| 0 | 0.202 | 0.261 | 0.006 |
| 0.01 | 0.205 | 0.260 | 0.009 |
| 0.02 | 0.207 | 0.260 | 0.014 |
| 0.03 | 0.210 | 0.259 | 0.020 |
| 0.04 | 0.213 | 0.259 | 0.030 |
| 0.05 | 0.215 | 0.258 | 0.043 |
| 0.06 | 0.218 | 0.258 | 0.062 |
| 0.07 | 0.221 | 0.257 | 0.088 |
| 0.08 | 0.224 | 0.257 | 0.123 |
| 0.09 | 0.227 | 0.256 | 0.169 |
| 0.10 | 0.230 | 0.256 | 0.228 |
*Assuming that the covariance between the mother's and biological father's body mass index (BMI) is equal to the covariance between the mother's and reported father's BMI.
Tables 4 and 5 present the unstandardised and standardised regression coefficients utilising offspring BMI data adjusted by the LMS method, and show even less evidence of a meaningfully greater strength of association with offspring BMI for maternal than paternal BMI.
Table 4 Unstandardised regression coefficients for offspring BMI (age and sex adjusted by the LMS method) on parental BMI considered simultaneously, assuming various proportions of non‐paternity, with p values for differences in parental effects.
| Rate of non‐paternity | Age adjusted parental BMI* | p Value for difference | |
|---|---|---|---|
| Paternal | Maternal | ||
| 0 | 0.130 | 0.128 | 0.895 |
| 0.01 | 0.132 | 0.128 | 0.779 |
| 0.02 | 0.133 | 0.128 | 0.665 |
| 0.03 | 0.135 | 0.128 | 0.556 |
| 0.04 | 0.137 | 0.128 | 0.455 |
| 0.05 | 0.139 | 0.127 | 0.363 |
| 0.06 | 0.140 | 0.127 | 0.283 |
| 0.07 | 0.142 | 0.127 | 0.214 |
| 0.08 | 0.144 | 0.127 | 0.157 |
| 0.09 | 0.146 | 0.126 | 0.111 |
| 0.10 | 0.148 | 0.126 | 0.076 |
*Assuming that the covariance between the mother's and biological father's body mass index (BMI) is equal to the covariance between the mother's and reported father's BMI.
Table 5 Standardised regression coefficients for offspring BMI (age and sex adjusted by the LMS method) on parental BMI considered simultaneously, assuming various proportions of non‐paternity, with p values for differences in parental effects.
| Rate of non‐paternity | Age adjusted parental BMI* | p Value for difference | |
|---|---|---|---|
| Paternal | Maternal | ||
| 0 | 0.207 | 0.242 | 0.096 |
| 0.01 | 0.209 | 0.242 | 0.128 |
| 0.02 | 0.212 | 0.242 | 0.168 |
| 0.03 | 0.215 | 0.241 | 0.218 |
| 0.04 | 0.218 | 0.241 | 0.279 |
| 0.05 | 0.220 | 0.240 | 0.353 |
| 0.06 | 0.223 | 0.240 | 0.440 |
| 0.07 | 0.226 | 0.239 | 0.540 |
| 0.08 | 0.229 | 0.239 | 0.653 |
| 0.09 | 0.232 | 0.238 | 0.777 |
| 0.10 | 0.235 | 0.238 | 0.910 |
*Assuming that the covariance between the mother's and biological father's body mass index (BMI) is equal to the covariance between the mother's and reported father's BMI.
Discussion
Our findings suggest that the association between maternal BMI and offspring BMI is similar to that between paternal BMI and offspring BMI. Furthermore, there is no evidence that at the high end of BMI – in the obesity range – there is any marked difference in the strength of association. This basic conclusion is not changed by analyses utilising unstandardised or standardised regression coefficients, or with or without LMS adjustment of offspring BMI. With respect to a genetic contribution to parent–offspring BMI associations, it would be expected that non‐paternity, where the fathers for whom we have data are not the biological fathers, would generate a greater association for mothers than for fathers. There are no reliable estimates for non‐paternity rates in Britain, with rates between 2% and 15% having been quoted.21 Taking plausible levels of non‐paternity into account emphasised the lack of a substantial difference in the association of offspring BMI with either maternal or paternal BMI. At the very least, the maternal BMI influence on offspring BMI is not meaningfully greater than the paternal BMI influence.
The weight and height data for the offspring were measured, whereas for the mothers and fathers these were self‐reported. Studies relating self‐reported to measured weight and height suggest that reporting is generally accurate, with no evidence of substantial sex differences.22,23,24,25
Few comparable data exist comparing mother–offspring and father–offspring associations for BMI and obesity.12,13,14,15,16,17,26,27,28 Most of the existing studies are of small sample size and/or do not report the associations of offspring characteristics with those of the mothers and fathers in such a way as to be directly comparable. Although in particular individual studies claims have been made that stronger effects are seen for either maternal or paternal BMI or obesity with offspring measures, the overall evidence suggests effect sizes are similar, in line with our formal examination of this issue.
There is clearly an important genetic contribution to BMI and obesity,29,30 and both for genetic and shared environmental reasons it would be expected that parental and offspring BMI would be related. A major environmental contribution to increasing obesity levels is indicated by substantial and rapid increases, for example from 12% in 1991 to 19% in 1999 in the US.31,32 There are several reasons for an apparent discrepancy between high heritability estimates and a clear and major environmental contribution to BMI levels and obesity. Firstly, the statistical models used to generate heritability estimates may be misleading: they can make untenable assumptions about equal similarity of the environment of monozygotic (MZ) and dizygotic (DZ) twins, ignore the environmental influence of intra‐uterine experiences and, most importantly, ignore gene–environment and gene–gene interactions. Indeed, there has been some downshifting of estimates of heritability of obesity made by some authorities, with early claims of 80%33 being reduced to less than a third.34 Secondly, evaluating the contribution of genetic influences is an area where Geoffrey Rose's distinction between the determinants of disease rates for a population and factors influencing who gets a disease within a population is crucial.35 With very general shifts in the population to a higher energy intake/energy expenditure ratio – illustrated, for example, by the consistency of increases in obesity in the US within ethnic, gender, socioeconomic and geographical area of residence subgroups over the 1990s31,32 – the variance between individuals can remain strongly genetically based, while such genetic factors on their own make a minimal contribution to the population burden of obesity.
There are two caveats to our study. First, BMI of offspring was measured at age 7.5 and relative maternal and paternal BMI associations with offspring BMI may differ at greater offspring age. However, similar correlations of maternal and paternal BMI with offspring BMI at ages 7 through to 33 have been reported.17 Second, aspects of body composition other than BMI, such as fat distribution or fat to lean body mass ratio, may show differential associations with maternal and paternal BMI. If seen this would suggest that the intra‐uterine environments provided by mothers with different body composition had a specific effect on aspects of offspring body composition and/or parent of origin (imprinting) effects. As yet data are not available to investigate these issues.
What is already known on this topic
Obesity levels are increasing at all ages in many populations.
The obesity of mothers could influence obesity among their offspring through an intrauterine programming effect.
What this study adds
The association between maternal BMI and offspring childhood BMI is similar to that between paternal BMI and offspring BMI.
If there were a specific maternal effect, through an intrauterine programming influence, it would be anticipated that the maternal–offspring association would be stronger.
Our study suggests that, at a population level, the influence of intrauterine environment effects due to maternal obesity on offspring childhood obesity levels is not strong.
Our study does not provide evidence regarding the relative contribution of genetic or environmental factors to BMI; however, it does suggest that genetic and shared environmental factors influence associations between the BMI of both parents and their offspring to the same extent. However, within the limits of the caveats discussed above, our study argues against an important contribution of maternal BMI and obesity, through an influence of intra‐uterine environment, on offspring BMI and obesity. This also argues against the importance, at a population level, of the intergenerational acceleration hypothesis.1,7,36 While much human and animal evidence provides proof of principal that the maternal metabolic state can influence offspring weight and obesity,6,7,8,10,11,36 our data suggest that this is quantitatively of minor importance within the general population. The source of increasing BMI and obesity levels should be sought in the postnatal environment. A similar conclusion could be drawn from the findings from the 1958 birth cohort, in which the association between birth weight – influenced by the intra‐uterine environment provided by the mother – and later BMI was largely statistically accounted for by maternal or paternal BMI.37 However, further investigation of different components of parental and offspring body composition, and at different offspring ages, is required to establish the generalisability of our findings.
Appendix A
Equation (13) in Clemons' paper20 should be:
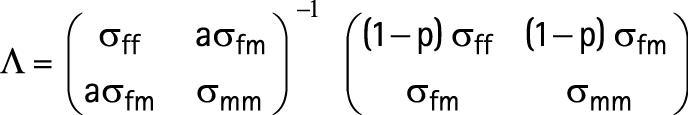 |
where σff is variance of reported father's height,
σmm is variance of mother's height,
σfm is covariance of reported father's and mother's height,
p is probability that the reported father is not the biological father and a is used to indicate the possible covariances between the mother's and biological father's height; we assumed it to be equal to the covariance between the mother's and the reported father's height, and used a = 1.
The observed regression coefficients were multiplied by Λ−1 to obtain the modified coefficients given in table 2.
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. This publication is the work of the authors and GDS will serve as guarantor for the contents of this paper.
Abbreviations
ALSPAC - Avon Longitudinal Study of Parents and Children
BMI - body mass index
Footnotes
The UK Medical Research Council, the Wellcome Trust and the University of Bristol provide core support for ALSPAC.
Competing interests: None.
References
- 1.Ebbeling C B, Pawlak D B, Ludwig D S. Childhood obesity: public‐health crisis, common sense cure. Lancet 2002360473–482. [DOI] [PubMed] [Google Scholar]
- 2.Flegal K M, Troiano R P. Changes in the distribution of body mass index of adults and children in the US population. Int J Obes 200024807–818. [DOI] [PubMed] [Google Scholar]
- 3.Popkin B M. An overview on the nutrition transition and its health implications: the Bellagio meeting. Public Health Nutr 20025(Suppl)93–103. [DOI] [PubMed] [Google Scholar]
- 4.Critser G.Fat land: how Americans became the fattest people in the world. Harmondsworth: Penguin Books, 2004
- 5.Whitaker R C, Dietz W H. Role of the prenatal environment in the development of obesity. J Pediatr 1998132768–776. [DOI] [PubMed] [Google Scholar]
- 6.Oken E, Gillman M W. Fetal origins of obesity. Obes Res 200311496–506. [DOI] [PubMed] [Google Scholar]
- 7.Levin B E. The obesity epidemic: metabolic imprinting on genetically susceptible neural circuits. Obes Res 20008342–347. [DOI] [PubMed] [Google Scholar]
- 8.Levin B E, Govek E. Gestational obesity accentuates obesity in obesity‐prone progeny. Am J Physiol 1998275R1374–R1379. [DOI] [PubMed] [Google Scholar]
- 9.Pettitt D J, Baird H R, Aleck K A.et al Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med 1983308242–245. [DOI] [PubMed] [Google Scholar]
- 10.Dabelea D, Pettitt D J. Intrauterine diabetic environment confers risks for type 2 diabetes mellitus and obesity in the offspring, in addition to genetic susceptibility. J Pediatr Endocrinol Metab 2001141085–1091. [DOI] [PubMed] [Google Scholar]
- 11.Dabelea D, Hanson R L, Lindsay R S.et al Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity. Diabetes 2000492208–2211. [DOI] [PubMed] [Google Scholar]
- 12.Garn S M, Clark D C. Trends in fatness and the origins of obesity. Pediatrics 197657443–456. [PubMed] [Google Scholar]
- 13.Esposito‐Del Puente A, Scalti L, De Filippo E.et al Familial and environmental influences on body composition and body fat distribution in childhood in southern Italy. Int J Obes 199418596–601. [PubMed] [Google Scholar]
- 14.Keiller S M, Colley J R T, Carpenter R G. Obesity in school children and their parents. Ann Hum Biol 19796443–455. [DOI] [PubMed] [Google Scholar]
- 15.Ayatollahi S M T. Obesity in school children and their parents in southern Iran. Int J Obes 199216845–850. [PubMed] [Google Scholar]
- 16.Sorensen T I A, Holst C, Stunkard A J.et al Correlations of body mass index of adult adoptees and their biological and adoptive relatives. Int J Obes 199216227–236. [PubMed] [Google Scholar]
- 17.Lake J K, Power C, Cole T J. Child to adult body mass index in the 1958 British birth cohort: associations with parental obesity. Arch Dis Child 199777376–381. [DOI] [PubMed] [Google Scholar]
- 18.Golding J, Pembrey M, Jones R, ALSPAC study team ALSPAC‐the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol 20011574–87. [DOI] [PubMed] [Google Scholar]
- 19.Cole T J. The LMS method for constructing normalised growth standards. Eur J Clin Nutr 19904445–60. [PubMed] [Google Scholar]
- 20.Clemons T. A look at the inheritance of height using regression toward the mean. Hum Biol 200072447–454. [PubMed] [Google Scholar]
- 21.Macintyre S, Sooman A. Non‐paternity and prenatal genetic screening. Lancet 1991338869–871. [DOI] [PubMed] [Google Scholar]
- 22.Rowland M L. Self‐reported weight and height. Am J Clin Nutr 1990521125–1133. [DOI] [PubMed] [Google Scholar]
- 23.Stevens J, Keil J E, Waid L R.et al Accuracy of current, 4‐year, and 28‐year self‐reported body weight in an elderly population. Am J Epidemiol 19901321156–1163. [DOI] [PubMed] [Google Scholar]
- 24.Hill A, Roberts J. Body mass index: a comparison between self‐reported and measured height and weight. J Public Health Med 199820206–210. [DOI] [PubMed] [Google Scholar]
- 25.Ziebland S, Thorogood M, Fuller A.et al Desire for the body normal: body image and discrepancies between self reported and measured height and weight in a British population. J Epidemiol Community Health 199650105–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenland K J, Liu K, Dyer A R.et al Body mass index in young adults: associations with parental body size and education in the CARDIA study. Am J Public Health 199686480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duran‐Tauleria E, Rona R J, Chinn S. Factors associated with weight for height and skinfold thickness in British children. J Epidemiol Community Health 199549466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawlor D A, Davey Smith G, O'Callaghan M.et al Epidemiologic evidence for the fetal overnutrition hypothesis: findings from the Mater‐University Study of Pregnancy and its outcomes. Am J Epidemiol 2007165418–424. [DOI] [PubMed] [Google Scholar]
- 29.Maes H H M, Neale M C, Eaves L J. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet 199727325–351. [DOI] [PubMed] [Google Scholar]
- 30.Stunkard A J, Sørensen T I A, Hanis C.et al An adoption study of human obesity. N Engl J Med 1986314193–198. [DOI] [PubMed] [Google Scholar]
- 31.Mokdad A H, Serdula M K, Dietz W H.et al The spread of the obesity epidemic in the United States, 1991–1998. JAMA 19992821519–1522. [DOI] [PubMed] [Google Scholar]
- 32.Mokdad A H, Serdula M K, Dietz W H.et al The continuing epidemic of obesity in the United States. JAMA 20002841650–1651. [DOI] [PubMed] [Google Scholar]
- 33.Stunkard A J, Sorensen T I A, Hanis C.et al An adoption study of human obesity. N Engl J Med 1986314193–198. [DOI] [PubMed] [Google Scholar]
- 34.Bouchard C. Genetics of obesity in humans: current issues. In: Chanwick D, Cardew G, eds. The origins and consequences of obesity. Ciba Foundation Symposium 201. Chichester: Wiley, 1996108–117. [DOI] [PubMed]
- 35.Rose G. Sick individuals and sick populations [Reprinted in Int J Epidemiol 2001;30:427–32]. Int J Epidemiol 19851432–38. [DOI] [PubMed] [Google Scholar]
- 36.Vadlamudi S, Kalhan S C, Patel M S. Persistence of metabolic consequences in the progeny of rats fed a HC formula in their early postnatal life. Am J Physiol 1995269E731–E738. [DOI] [PubMed] [Google Scholar]
- 37.Parsons T J, Power C, Manor O. Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. BMJ 20013231331–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]