Abstract
Context
Evidence is lacking that a dietary pattern high in vegetables, fruit, and fiber and low in total fat can influence breast cancer recurrence or survival.
Objective
To assess whether a major increase in vegetable, fruit, and fiber intake and a decrease in dietary fat intake reduces the risk of recurrent and new primary breast cancer and all-cause mortality among women with previously treated early stage breast cancer.
Design, Setting, and Participants
Multi-institutional randomized controlled trial of dietary change in 3088 women previously treated for early stage breast cancer who were 18 to 70 years old at diagnosis. Women were enrolled between 1995 and 2000 and followed up through June 1, 2006.
Intervention
The intervention group (n=1537) was randomly assigned to receive a telephone counseling program supplemented with cooking classes and newsletters that promoted daily targets of 5 vegetable servings plus 16 oz of vegetable juice; 3 fruit servings; 30 g of fiber; and 15% to 20% of energy intake from fat. The comparison group (n=1551) was provided with print materials describing the "5-A-Day" dietary guidelines.
Main Outcome Measures
Invasive breast cancer event (recurrence or new primary) or death from any cause.
Results
From comparable dietary patterns at baseline, a conservative imputation analysis showed that the intervention group achieved and maintained the following statistically significant differences vs the comparison group through 4 years: servings of vegetables, +65%; fruit, +25%; fiber, +30%, and energy intake from fat, −13%. Plasma carotenoid concentrations validated changes in fruit and vegetable intake. Throughout the study, women in both groups received similar clinical care. Over the mean 7.3-year follow-up, 256 women in the intervention group (16.7%) vs 262 in the comparison group (16.9%) experienced an invasive breast cancer event (adjusted hazard ratio, 0.96; 95% confidence interval, 0.80–1.14; P=.63), and 155 intervention group women (10.1%) vs 160 comparison group women (10.3%) died (adjusted hazard ratio, 0.91; 95% confidence interval, 0.72–1.15; P=.43). No significant interactions were observed between diet group and baseline demographics, characteristics of the original tumor, baseline dietary pattern, or breast cancer treatment.
Conclusion
Among survivors of early stage breast cancer, adoption of a diet that was very high in vegetables, fruit, and fiber and low in fat did not reduce additional breast cancer events or mortality during a 7.3-year follow-up period.
Trial Registration
clinicaltrials.gov Identifier: NCT00003787
Considerable evidence from preclinical studies indicates that plant-derived foods contain anticarcinogens.1 A comprehensive review of the literature found that a diet high in vegetables and fruit probably decreases breast cancer risk and that a diet high in total fat possibly increases risk.2 However, evidence of an association between a diet high in vegetables and fruit and low in total fat and prevention of cancer progression has been mixed in epidemiological studies.3–17 An interim analysis of data from the Women’s Intervention Nutrition Study (WINS), which assessed the effect of a dietary intervention designed to reduce fat intake on relapse-free survival in breast cancer patients,18 found that the intervention was associated with a marginally statistically significant improvement in relapse-free survival. To our knowledge, no other clinical trials investigating dietary change and breast cancer survival have been reported.
The Women’s Healthy Eating and Living (WHEL) Study was a randomized trial assessing whether a dietary pattern very high in vegetables, fruit, and fiber and low in fat reduces the risks of recurrent and new primary breast cancer and all-cause mortality among women with previously treated early stage breast cancer. The study was based on the recommendations of a national committee of experts called to respond to a 1993 challenge grant from a private philanthropist who believed that the role of diet in preventing cancer progression deserved scientific study to enable cancer survivors to make decisions without having “to rely on folklore, rumor and hearsay.”19
METHODS
Details of the study design, eligibility criteria, randomization procedures, and dietary intervention have been reported previously.20,21 In brief, we compared 2 dietary patterns: an intervention group that was intensively counseled to adopt a dietary pattern very high in vegetables, fruit, and fiber and low in fat21 and a comparison group advised to follow the 5-A-Day diet.22,23 The study tested primary hypotheses of whether the intervention dietary pattern was associated with (1) a longer breast cancer event–free interval and (2) increased overall survival among women previously treated for early stage breast cancer. Based on the 6 epidemiological studies that had been published at the time of the trial design, we estimated the likely effect size of this multicomponent diet.20 Following Lachin and Foulkes,24 we determined that a sample size of 3000 would have 82% power to detect a 19% reduction in additional breast cancer events in the intervention group (expected comparison group rate=24%) and a 24% reduction in all cause mortality (expected comparison group rate=15%).
Participants
Participants were enrolled at 7 study sites between 1995 and 2000. Eligibility criteria included diagnosis of a primary operable invasive breast carcinoma categorized using American Joint Committee on Cancer (edition IV) criteria as stage I (≥1 cm), stage II, or stage IIIA within the past 4 years; age at diagnosis between 18 and 70 years; treatment with axillary dissection and total mastectomy or lumpectomy followed by primary breast radiation; no current or planned chemotherapy; no evidence of recurrent disease or new breast cancer since completion of initial local treatment; and no other cancer in the past 10 years. Eligible women were randomly assigned to either the study dietary pattern or the comparison group (FIGURE 1). The institutional review boards at the 7 clinical sites approved the study protocol and consent forms, and all participants provided written informed consent.
Figure 1.
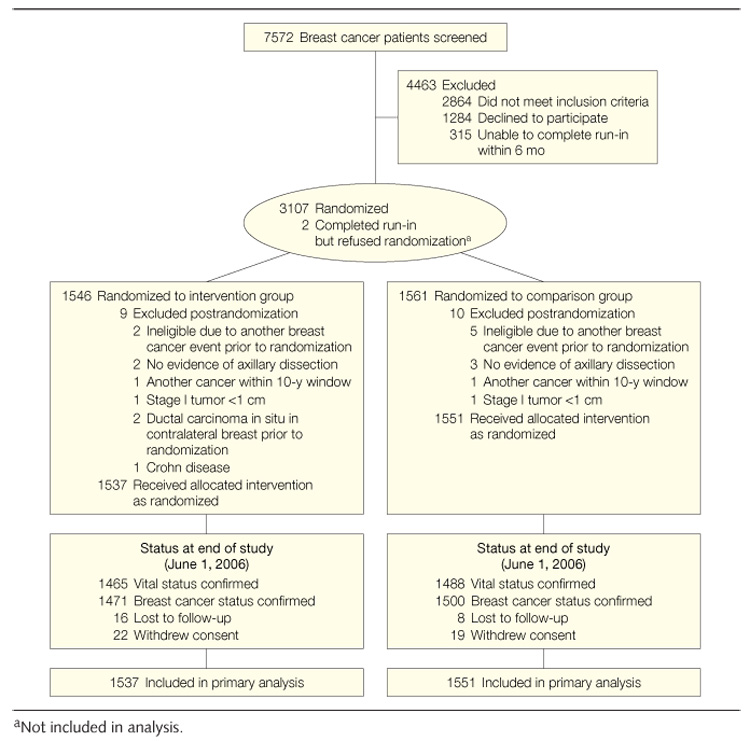
Participant Flow
Dietary Intervention
The intensive intervention was delivered primarily by telephone counseling, supplemented with 12 cooking classes in the first year and monthly newsletters throughout the study. Trained counselors21 followed a computer-assisted protocol that was based on social cognitive theory25 and had 3 phases of decreasing intensity. During the first phase (3–8 calls in 4–6 weeks), counselors focused on building self-efficacy to implement the study targets, which consisted of daily intake of 5 vegetable servings plus 16 oz of vegetable juice, 3 fruit servings, 30 g of fiber, and 15% to 20% of energy intake from fat. Phase 2 (through 5 months) focused on self-monitoring and dealt with barriers to adherence. Phase 3 (through study completion) focused on retaining motivation for the study dietary pattern and preventing setbacks. During the first year of the intervention, participants received an average of 18 counseling calls, attended an average of 4 of 12 offered cooking classes, and received 12 study newsletters. By 4 years, a key point for assessing long-term effect, these participants had received an average of 31 calls and 48 newsletters.
Women randomized to the comparison group were provided with print materials (from the US Department of Agriculture26 and the National Cancer Institute22,23) describing a diet with a recommended daily intake of 5 servings of vegetables and fruit, more than 20 g of fiber, and less than 30% total energy intake from fat. The comparison group attended an average of 1 of 4 offered cooking classes in the first year and received 24 newsletters tailored to the comparison group during the first 4 years.
Data Collection
Dietary Assessment
Dietary intake was assessed by sets of 4 prescheduled 24-hour dietary recalls conducted by telephone on random days over a 3-week period, stratified for weekend vs weekdays.20 These dietary recalls were scheduled for all participants at baseline, 1 year, 4 years, and 6 years and on 50% random samples at 6, 24, and 36 months. We report data for participants completing follow-up assessments. We also conducted additional analyses as follows: for participants who did not complete follow-up assessments we imputed estimates by assuming that they changed dietary intake in a manner similar to comparison group respondents, using the expectation maximization algorithm in SAS software, version 9.1 (SAS Institute Inc, Cary, North Carolina).27 As previously described,20 dietary assessors completed a training program and used the multipass software-driven recall protocol of the Nutritional Data System software (NDS-R, 1994–2006, University of Minnesota, Minneapolis). To assess overall adherence, we developed an adherence score.20 A completely adherent participant would score 600 points: 300 points reflected vegetable consumption and the remaining 300 points were divided equally among fiber, fruit, and energy from fat.
Other Data Collection
We collected a detailed family history of cancer at baseline and used the mutation prevalence tables from Myriad Genetic Laboratories (Salt Lake City, Utah)28 to classify families with at least a 10% risk of a BRCA1/2 mutation as high-risk. Clinic visits conducted at baseline, 1 year, 2 or 3 years (randomly determined), 4 years, and 6 years included measured weight and venipuncture. Separated blood samples were stored in cryovials in −80°C freezers for later analysis. Participants were mailed a set of study questionnaires to complete and bring to each clinic visit, including a health status questionnaire with questions on recent physician visits and 2 questionnaires used in the Women’s Health Initiative: the Personal Habits Questionnaire,29 which included a 9-item measure of physical activity validated for our study30 and from which we calculated metabolic equivalent task minutes per week,31 and the Thoughts and Feelings Questionnaire,32 which included measures of psychosocial functioning. Each participant self-reported race/ethnicity as 1 of 8 categories.
Assessment of Study Outcomes
Primary study end points were (1) the combined outcome of invasive breast cancer recurrence or new primary breast cancer and (2) death due to any cause. Recurrences were further classified as local/regional or distant metastasis. Carcinoma in situ was not counted as a study outcome. The breast cancer event–free interval was defined as the time from date of enrollment to development of a new breast cancer event. Follow-up time was censored at the time of the participant’s death, at the last documented staff contact date, or at the study completion date (June 1, 2006).
During semiannual telephone interviews, clinical site staff queried study participants regarding the occurrence of outcome events, any hospitalization, or new or existing medical diagnoses. Any report of a breast cancer event or death triggered a confirmation interview and collection of medical records and/or death certificates. Two independent oncologists reviewed the medical records (pathology reports and physician notes) to confirm reported recurrences. In cases of disagreement, the coordinating center pathologist adjudicated the outcome. Finally, we searched the National Death Index using Social Security number, name, and date of birth.
Statistical Analysis
Participants were randomly assigned to the intervention or comparison group using a random permuted-block design stratified by tumor stage, age, and clinical site. The allocation of participants was conducted by the clinical site coordinator running the study’s randomization computer program, which automatically stamped the assigned study group in the database. An independent data and safety monitoring committee conducted a blind review of potential post-randomization exclusions.20
Baseline group comparisons of demographic, anthropometric, clinical, and dietary measures were conducted with 2-sided t tests, rank-sum tests, or χ² tests. We based the primary analysis of disease-free survival on the intention-to-treat principle using time-to-event methods. A 2-sided log-rank test was performed with P<.041 considered statistically significant (to account for interim analyses undertaken at the request of the data and safety monitoring committee). Both unadjusted and adjusted hazard ratios were computed. We fit a Cox model stratified by stage, age, and clinical site.
The frequencies of antiestrogen therapy use and bilateral oophorectomies differed marginally between study groups at baseline; therefore, these covariates were also included in the model. However, the antiestrogen therapy variable did not satisfy the proportional hazards assumption; hence, the analysis was stratified by this covariate.33 Thus, the final model was stratified by stage of initial tumor, age at randomization, clinical site, and antiestrogen use and was adjusted for oophorectomy status.
The a priori analysis plan20 included fitting a Cox proportional hazards model to evaluate the effect of the intervention on key covariates. These included stage of disease (classified as I, II, or III), age at randomization (<55 years vs ≥55 years), hormone receptor characteristics of initial tumor, body mass index, and years from diagnosis to randomization. Product terms between randomization assignment and indicator variables for covariate categories were included in Cox regression models. Interactions between randomization group and each covariate were formally tested for significance with likelihood ratio tests. The results are presented as hazard ratios and 95% confidence intervals.
In additional analyses, we examined possible interactions between study group and the baseline dietary factors targeted by the intervention (vegetable, fruit, fiber, and fat intakes) to address whether the effects of the dietary intervention might vary by baseline intake level.
Analyses were conducted in the statistics software package R, version 2.3.1 (R Foundation, Vienna, Austria; http://www.r-project.org) or SAS, version 9.1.
RESULTS
Recruitment and Baseline Characteristics
Study staff screened 7572 potential participants and randomized 3107 between March 1995 and November 30, 2000 (Figure 1). There was no difference in postrandomization exclusions by study group (9 vs 10). The final study sample included 1537 women in the dietary intervention group and 1551 in the study comparison group.
The study end date was June 1, 2006. Number and frequency of reported physician visits did not differ significantly between groups at any point during the study. We confirmed vital status on the study end date (Figure 1) for 95% of the intervention group and 96% of the comparison group. Breast cancer status was confirmed for 96% of the intervention group and 97% of the comparison group.
Randomization achieved highly comparable groups (TABLE 1) with regard to demographics (ie, age, minority status, and education), breast cancer characteristics (ie, stage, grade, nodal involvement, hormone receptor status, time from breast cancer diagnosis to randomization, and eligibility for BRCA1/2 testing), and treatment (ie, surgery and radiation). Slight imbalances were observed between groups in bilateral oophorectomy, antiestrogen use, and chemotherapy treatment, all of which favored an intervention effect; however, no between-group difference was observed in the percentage of women who received at least 1 of these therapies (intervention, 93.6%; comparison, 92.3%; P=.12). Fourteen percent of women self-identified as minority race/ethnicity, and these were fairly equally divided among African Americans, Hispanics, and Asian Americans.20
Table 1.
Baseline Characteristics of WHEL Study Participants by Study Groupa
| Characteristics | Intervention (n = 1537) | Comparison (n = 1551) |
|---|---|---|
| Age at study entry, mean (SD), y | 53.3 (8.9) | 53.0 (9.0) |
| College graduate | 853 (55.5) | 820 (52.9) |
| Race/ethnicity | ||
| White | 1306 (85.0) | 1328 (85.6) |
| African American | 61 (4.0) | 57 (3.7) |
| Hispanic | 87 (5.7) | 78 (5.0) |
| Asian American | 46 (3.0) | 50 (3.2) |
| Mixed/other | 37 (2.4) | 38 (2.5) |
| Cancer stage at diagnosis | ||
| I | 585 (38.1) | 606 (39.1) |
| II | 876 (57.0) | 867 (55.9) |
| IIIA | 76 (4.9) | 78 (5.0) |
| Nodal statusb | ||
| Negative | 879 (57.2) | 896 (57.8) |
| 1–3 positive nodes | 436 (28.4) | 448 (28.9) |
| > positive nodes | 221 (14.4) | 207 (13.4) |
| Hormone receptor statusb | ||
| ER+/PR+ | 955 (62.1) | 948 (61.1) |
| ER+/PR− | 197 (12.8) | 169 (10.9) |
| ER−/PR+ | 52 (3.4) | 77 (5.0) |
| ER−/PR− | 300 (19.5) | 319 (20.6) |
| Initial treatment | ||
| Mastectomy | 812 (52.8) | 801 (51.6) |
| Breast-sparing surgery | 725 (47.2) | 750 (48.4) |
| Radiation | 937 (61.0) | 962 (62.0) |
| Adjuvant chemotherapy | 1095 (71.2) | 1064 (68.6) |
| Ever antiestrogen use | 1067 (69.4) | 1012 (65.3) |
| Tumor grade | ||
| I (well differentiated) | 239 (15.6) | 245 (15.8) |
| II (moderately differentiated) | 620 (40.3) | 620 (40.0) |
| III (poorly differentiated) | 551 (35.9) | 557 (35.9) |
| Unspecified | 127 (8.3) | 129 (8.3) |
| Prior bilateral oophorectomy | 223 (14.6) | 177 (11.4) |
| Time from diagnosis to randomization, mean (SD), mo | 23.6 (12.5) | 23.5 (12.5) |
| Baseline eligibility for BRCA testing | 132 (8.6) | 123 (7.9) |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor.
Data are expressed as No. (%) of participants unless otherwise indicated. There were no significant between-group differences in baseline characteristics based on χ² test for categorical variables or t test for continuous variables, except for ever antiestrogen use (P = .03) and prior bilateral oophorectomy (P = .01).
Numbers do not total 3088 because of missing data.
Dietary Changes Between Groups
In analyses of dietary change, only participants without a study end point at the time of assessment were included. A high proportion of women completed the dietary assessments (TABLE 2). At baseline, no between-group differences were observed in in-takes of vegetables, fruit, or fiber or energy intake from fat, with both groups consuming a daily mean of more than 7 servings of vegetables and fruit. No between-group differences were observed in measured mean body weight or in energy intake.
Table 2.
Dietary Pattern and Body Weight by Groupa
| Baseline | 6 mo | 12 mo | 24 mo | 36 mo | 48 mo | 72 mo | |
|---|---|---|---|---|---|---|---|
| Intervention group | |||||||
| Eligible sample, No. | 1537 | 738 | 1463 | 715 | 676 | 1355 | 1308 |
| Response rate, % | 99.9 | 91.3 | 88.3 | 85.5 | 84.8 | 83.0 | 77.9 |
| Comparison group | |||||||
| Eligible sample, No. | 1551 | 765 | 1484 | 699 | 713 | 1363 | 1313 |
| Response rate, % | 99.8 | 96.9 | 93.0 | 90.6 | 89.5 | 88.6 | 86.2 |
| Total vegetable servings/d | |||||||
| Intervention | 3.9 (0.05) | 8.4 (0.13) | 7.8 (0.09) | 7.1 (0.13) | 6.6 (0.13) | 6.4 (0.09) | 5.8 (0.09) |
| Comparison | 3.8 (0.05) | 3.9 (0.07) | 3.9 (0.05) | 3.7 (0.07) | 3.7 (0.07) | 3.7 (0.05) | 3.6 (0.05) |
| Total fruit servings/d | |||||||
| Intervention | 3.5 (0.05) | 4.4 (0.08) | 4.2 (0.06) | 3.9 (0.08) | 3.8 (0.09) | 3.6 (0.06) | 3.4 (0.07) |
| Comparison | 3.4 (0.05) | 3.6 (0.08) | 3.4 (0.05) | 3.3 (0.08) | 2.9 (0.07) | 2.8 (0.05) | 2.6 (0.05) |
| Fiber, g/d | |||||||
| Intervention | 21.1 (0.21) | 30.9 (0.40) | 29.0 (0.28) | 27.6 (0.41) | 26.1 (0.42) | 25.2 (0.29) | 24.2 (0.30) |
| Comparison | 21.2 (0.20) | 21.4 (0.30) | 21.0 (0.22) | 20.5 (0.30) | 20.0 (0.30) | 19.3 (0.21) | 18.9 (0.24) |
| Energy from fat, % | |||||||
| Intervention | 28.5 (0.18) | 21.2 (0.26) | 22.7 (0.20) | 24.5 (0.29) | 25.4 (0.32) | 27.1 (0.24) | 28.9 (0.25) |
| Comparison | 28.7 (0.18) | 27.8 (0.27) | 28.4 (0.19) | 29.2 (0.30) | 30.6 (0.30) | 31.4 (0.22) | 32.4 (0.22) |
| Adherence scoreb | |||||||
| Intervention | 286 (3) | 574 (7) | 533 (6) | 485 (8) | 461 (8) | 435 (6) | 396 (6) |
| Comparison | 283 (3) | 301 (5) | 292 (4) | 282 (5) | 268 (5) | 262 (3) | 246 (3) |
| Energy intake, kcal/d | |||||||
| Intervention | 1719 (10) | 1619 (14) | 1603 (10) | 1592 (15) | 1523 (15) | 1552 (11) | 1538 (11) |
| Comparison | 1717 (11) | 1615 (15) | 1605 (11) | 1606 (15) | 1601 (16) | 1574 (11) | 1559 (12) |
| Body weight, kg | |||||||
| Intervention | 73.5 (0.42) | NAc | 73.0 (0.45) | 74.2 (0.71) | 73.9 (0.73) | 74.2 (0.51) | 74.1 (0.54) |
| Comparison | 73.3 (0.43) | NAc | 73.8 (0.47) | 74.0 (0.68) | 74.9 (0.74) | 74.1 (0.50) | 73.7 (0.53) |
Data are expressed as mean (SE) unless otherwise indicated.
Adherence score was calculated as 30 points for each vegetable or fruit serving (excluding white potatoes, juice, and iceberg lettuce); 10 points for each ounce of vegetable juice; 5 points for each percentage point of energy from fat below 40% to a maximum of 100 points for 20% energy from fat; and 7.7 points for each gram of fiber per 1000 kcal above 5 g/1000 kcal. Perfect adherence was 600 points.
Data for body weight are not applicable (NA) because it was not measured at 6 months.
In the comparison group, consumption of vegetables, fruit, or fiber changed only modestly over the 6 years following randomization, while relative energy intake from fat increased 13%, reflecting an identified secular trend (J.P.P., V.A.N., L.N., et al, unpublished data, May 2007). In the intervention group, the dietary pattern changed substantially and a large (P<.001) between-group difference was achieved and maintained for each dietary target across the 6 years of the intervention. From no difference at baseline, the overall adherence score was 91% higher in the intervention group at 6 months and remained 61% higher than the comparison group at 6 years. Details of changes in dietary targets are presented elsewhere (J.P.P., V.A.N., L.N., et al, unpublished data, May 2007). Using the more conservative imputed data approach,27 at 1 year, the intervention group had increased average total vegetable and fruit intake to 12 servings/d. This change in total vegetable and fruit intake reflected a major increase in vegetable intake, averaging 7.8 vegetable servings/d at 1 year and remaining relatively high at 6 servings/d at the 4-year follow-up.
At 4 years, relative differences in mean intake between study groups were +65% for vegetable servings, +25% for fruit servings, +30% for fiber, and −13% for energy intake from fat. All differences were statistically significant at P<.001. Total plasma carotenoid concentration, a biomarker of vegetable and fruit intake, was 73% higher in the intervention group than the comparison group at 1 year and 43% higher at 4 years, differences that were statistically significant (P<.001). In addition, a subsample study identified changes in plasma triacylglycerol and high-density lipoprotein cholesterol concentrations that were specific to the intervention group, supporting self-reported changes in carbohydrate and fat intakes.34 Study groups differed by less than 80 kcal/d in energy intake and by less than 1 kg in body weight at any study point.
Breast Cancer Event-Free Survival
During the study, 518 participants had a breast cancer event (TABLE 3), representing 256 participants (16.7%) in the intervention group and 262 participants (16.9%) in the comparison group. The disease-free survival curves were virtually identical across groups (FIGURE 2). The unadjusted hazard ratio is presented in Figure 2. The hazard ratio after adjustment for antiestrogen use, oophorectomy status, and stratification factors (including tumor stage, clinic site, and age) at baseline was 0.96 (95% confidence interval, 0.80–1.14; P=.63). The likelihood ratio test statistics for group interactions with age, body mass index, physical activity, energy intake, characteristics of the original tumor (including hormone receptor status), and years from diagnosis to study entry were not significant (TABLE 4). Furthermore, hazard ratios for intervention effects within covariate strata were not significant (Table 4).
Table 3.
Study Events
| No. of Events |
||
|---|---|---|
| Study Outcomes | Intervention | Comparison |
| Confirmed breast cancer event | 256 | 262 |
| Local | 35 | 28 |
| Regional | 10 | 10 |
| Distal | 168 | 189 |
| New primary | 43 | 35 |
| Confirmed deaths | 155 | 160 |
| Breast cancer | 127 | 135 |
| Other cancer | 12 | 15 |
| Heart disease | 2 | 5 |
| Other | 14 | 5 |
Figure 2.
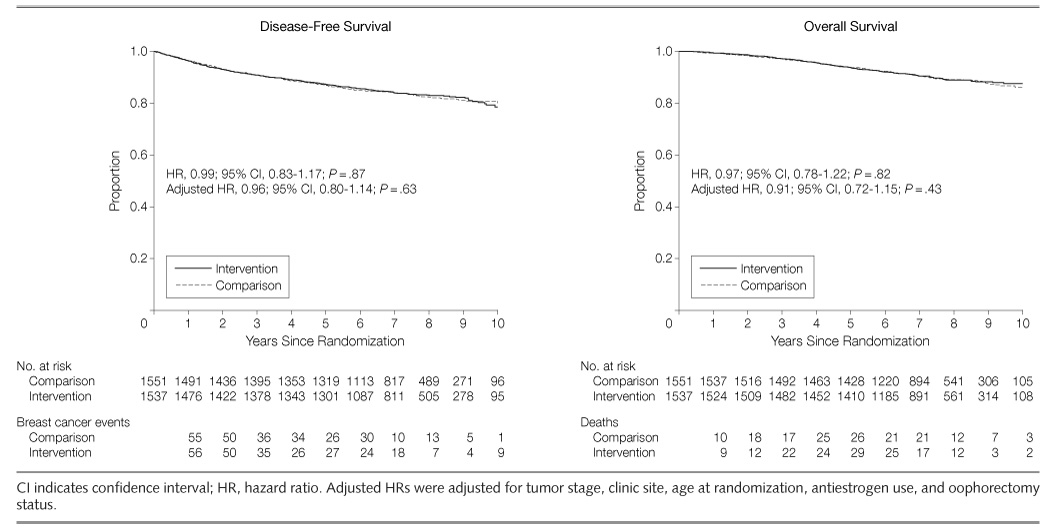
Kaplan-Meier Estimates of Disease-Free Survival and All-Cause Mortality by Diet Group
Table 4.
Intervention Effects on Additional Breast Cancer Events by Baseline Clinical and Demographic Characteristics
| No./Total |
||||
|---|---|---|---|---|
| Intervention | Comparison | HR (95% CI)a | P Valueb | |
| Age at randomization, y | ||||
| <55 | 167/908 | 161/917 | 1.05 (0.84–1.30) | .35 |
| ≥55 | 89/629 | 101/634 | 0.89 (0.67–1.18) | |
| Cancer stage at diagnosis | ||||
| I | 50/585 | 55/606 | 0.94 (0.64–1.38) | |
| II | 178/876 | 182/867 | 0.97 (0.79–1.19) | .74 |
| IIIA | 28/76 | 25/78 | 1.19 (0.69–2.04) | |
| Hormone receptor statusc | ||||
| ER+/PR+ | 140/955 | 145/948 | 0.95 (0.76–1.20) | |
| ER+/PR− | 37/197 | 32/169 | 0.97 (0.60–1.56) | .85 |
| ER−/PR+ | 11/52 | 18/77 | 0.89 (0.42–1.88) | |
| ER−/PR− | 64/300 | 62/319 | 1.14 (0.80–1.61) | |
| Time from diagnosis to randomization, y | ||||
| ≤1 | 59/352 | 65/350 | 0.88 (0.62–1.26) | |
| >1 to 2 | 83/488 | 90/508 | 0.95 (0.71–1.28) | .50 |
| >2 to 3 | 64/375 | 52/372 | 1.26 (0.87–1.82) | |
| >3 to 4 | 50/322 | 55/321 | 0.90 (0.61–1.32) | |
| Tumor differentiation | ||||
| I (well differentiated) | 20/239 | 22/245 | 0.90 (0.49–1.65) | |
| II (moderately differentiated) | 94/620 | 100/620 | 0.93 (0.70–1.24) | .75 |
| III (poorly differentiated) | 121/551 | 114/557 | 1.09 (0.85–1.41) | |
| Unspecified | 21/127 | 26/129 | 0.83 (0.47–1.48) | |
| No. of positive nodesc | ||||
| 0 | 93/879 | 117/896 | 0.80 (0.61–1.06) | |
| 1–3 | 83/436 | 69/448 | 1.25 (0.91–1.72) | .07 |
| 4–6 | 39/116 | 31/115 | 1.29 (0.80–2.06) | |
| ≥7 | 41/105 | 45/92 | 0.75 (0.49–1.15) | |
| Tumor size, cmc | ||||
| 0 to <2 | 82/752 | 88/769 | 0.94 (0.70–1.28) | |
| 2 to <3 | 88/421 | 86/441 | 1.09 (0.81–1.47) | |
| 3 to <4 | 30/174 | 41/160 | 0.62 (0.39–1.00) | .22 |
| 4 to <5 | 21/79 | 20/72 | 0.90 (0.49–1.67) | |
| ≥5 | 35/109 | 27/106 | 1.32 (0.80–2.18) | |
| Body mass indexd | ||||
| <25 | 107/652 | 112/678 | 0.99 (0.76–1.29) | |
| 25 to <30 | 81/475 | 68/480 | 1.22 (0.88–1.69) | .17 |
| ≥30 | 68/410 | 82/393 | 0.79 (0.57–1.09) | |
| Physical activity, MET-min/wkc | ||||
| ≤210 | 68/397 | 66/350 | 0.90 (0.64–1.27) | |
| 211–615 | 62/368 | 71/383 | 0.91 (0.64–1.27) | .56 |
| 616–1290 | 65/375 | 54/374 | 1.22 (0.85–1.74) | |
| >1290 | 49/351 | 60/387 | 0.88 (0.60–1.28) | |
| Energy intake, kcal/dc | ||||
| ≤1430 | 63/377 | 73/396 | 0.89 (0.64–1.25) | |
| 1431–1680 | 59/384 | 64/386 | 0.90 (0.63–1.28) | .69 |
| 1681–1980 | 64/390 | 54/379 | 1.16 (0.81–1.66) | |
| >1980 | 70/383 | 70/386 | 1.04 (0.75–1.45) | |
Abbreviations: CI, confidence interval; ER, estrogen receptor; HR, hazard ratio; MET, metabolic equivalent task; PR, progesterone receptor.
Hazard ratios of group effect were derived from Cox model stratified by covariate categories.
P values based on likelihood ratio test for group X covariate interaction.
Numbers do not total 3088 because of missing data.
Calculated as weight in kilograms divided by height in meters squared.
Overall Mortality
There were 315 deaths reported within the study period, with 155 (10.1%) in the intervention group and 160 (10.3%) in the comparison group (Figure 2). More than 80% of all deaths were due to breast cancer (Table 3). The treatment-associated hazard ratio was 0.91 (95% confidence interval, 0.72–1.15; P=.43) after adjusting for antiestrogen use, bilateral oophorectomy, and stratification factors. The likelihood ratio test statistics for the group interactions with age, body mass index, physical activity, energy intake, characteristics of the original tumor (including hormone receptor status), and years from diagnosis to study entry were not statistically significant, nor were significant effects of the intervention on mortality observed for any subgroups of women classified by major covariates (TABLE 5).
Table 5.
Intervention Effects on All-Cause Mortality by Baseline Demographic and Clinical Characteristics
| No./Total |
||||
|---|---|---|---|---|
| Intervention | Comparison | HR (95% CI)a | P Valueb | |
| Age at randomization, y | ||||
| <55 | 84/908 | 85/917 | 0.99 (0.74–1.34) | .85 |
| ≥55 | 71/629 | 75/634 | 0.95 (0.69–1.32) | |
| Cancer stage at diagnosis | ||||
| I | 33/585 | 32/606 | 1.07 (0.66–1.73) | |
| II | 102/876 | 107/867 | 0.94 (0.71–1.23) | .90 |
| IIIA | 20/76 | 21/78 | 0.96 (0.52–1.77) | |
| Hormone receptor statusc | ||||
| ER+/PR+ | 79/955 | 84/948 | 0.92 (0.68–1.26) | |
| ER+/PR− | 25/197 | 20/169 | 1.03 (0.57–1.85) | .88 |
| ER−/PR+ | 7/52 | 10/77 | 1.08 (0.41–2.83) | |
| ER−/PR− | 44/300 | 42/319 | 1.13 (0.74–1.73) | |
| Time from diagnosis to randomization, y | ||||
| ≤1 | 46/352 | 47/350 | 0.97 (0.64–1.45) | |
| >1 to 2 | 51/488 | 58/508 | 0.90 (0.62–1.32) | .72 |
| >2 to 3 | 34/375 | 28/372 | 1.28 (0.77–2.11) | |
| >3 to 4 | 24/322 | 27/321 | 0.86 (0.50–1.49) | |
| Tumor differentiation | ||||
| I (well differentiated) | 13/239 | 7/245 | 1.80 (0.72–4.52) | |
| II (moderately differentiated) | 58/620 | 64/620 | 0.90 (0.63–1.28) | .32 |
| III (poorly differentiated) | 75/551 | 74/557 | 1.04 (0.75–1.43) | |
| Unspecified | 9/127 | 15/129 | 0.62 (0.27–1.42) | |
| No. of positive nodesc | ||||
| 0 | 58/879 | 67/896 | 0.89 (0.62–1.26) | |
| 1–3 | 48/436 | 39/448 | 1.27 (0.83–1.94) | .20 |
| 4–6 | 21/116 | 19/115 | 1.09 (0.59–2.03) | |
| ≥7 | 28/105 | 35/92 | 0.64 (0.39–1.05) | |
| Tumor size, cmc | ||||
| 0 to <2 | 42/752 | 51/769 | 0.83 (0.55–1.26) | |
| 2 to <3 | 59/421 | 50/441 | 1.27 (0.87–1.85) | |
| 3 to <4 | 17/174 | 28/160 | 0.53 (0.29–0.96) | .13 |
| 4 to <5 | 13/79 | 12/72 | 0.94 (0.43–2.06) | |
| ≥5 | 23/109 | 19/106 | 1.21 (0.66–2.22) | |
| Body mass indexd | ||||
| <25 | 57/652 | 61/678 | 0.97 (0.67–1.38) | |
| 25 to <30 | 48/475 | 44/480 | 1.11 (0.74–1.67) | .70 |
| ≥30 | 50/410 | 55/393 | 0.86 (0.59–1.27) | |
| Physical activity, MET-min/wkc | ||||
| ≤210 | 43/397 | 47/350 | 0.80 (0.53–1.22) | |
| 211–615 | 45/368 | 45/383 | 1.04 (0.69–1.58) | .81 |
| 616–1290 | 36/375 | 35/374 | 1.02 (0.64–1.63) | |
| >1290 | 25/351 | 27/387 | 1.01 (0.58–1.73) | |
| Energy intake, kcal/dc | ||||
| ≤1430 | 44/377 | 48/396 | 0.95 (0.63–1.43) | |
| 1431–1680 | 32/384 | 40/386 | 0.77 (0.49–1.23) | .62 |
| 1681–1980 | 34/390 | 31/379 | 1.08 (0.66–1.75) | |
| >1980 | 45/383 | 40/386 | 1.17 (0.77–1.79) | |
Abbreviations: CI, confidence interval; ER, estrogen receptor; HR, hazard ratio; MET, metabolic equivalent task; PR, progesterone receptor.
Hazard ratios of group effect were derived from Cox model stratified by covariate categories.
P values based on likelihood ratio test for group X covariate interaction.
Numbers do not total 3088 because of missing data.
Calculated as weight in kilograms divided by height in meters squared.
Group Effects by Baseline Quartiles of Dietary Intake
Within each quartile of the targeted dietary components, the intervention group achieved significant change from baseline (TABLE 6). However, there was no evidence of a consistent pattern of an intervention effect for either breast cancer events or mortality according to any baseline diet subgroup, and the findings that are statistically significant in 2 of the 40 compared strata are what one might expect due to chance.
Table 6.
Intervention Effects on Additional Breast Cancer Events and All-Cause Mortality by Baseline Quartiles of Dietary Intake
| Breast Cancer Eventsc |
Deathsc |
||||
|---|---|---|---|---|---|
| Variables by Baseline Quartilea | No. of Participants at Baseline (Difference, %)b | No. | HR (95% CI)d | No. | HR (95% CI)d |
| Vegetables and fruit, servings/d | |||||
| ≤4.94 | 772 (+60) | 152 | 0.97 (0.71–1.34) | 88 | 0.75 (0.49–1.14) |
| 4.95–6.74 | 771 (+52) | 122 | 1.23 (0.86–1.75) | 76 | 1.25 (0.80–1.97) |
| 6.75–8.92 | 768 (+53) | 144 | 0.93 (0.67–1.30) | 83 | 1.28 (0.83–1.98) |
| >8.92 | 770 (+54) | 99 | 0.83 (0.56–1.24) | 67 | 0.76 (0.47–1.23) |
| Vegetables, servings/d | |||||
| ≤2.55 | 772 (+82) | 162 | 1.13 (0.83–1.54) | 101 | 1.05 (0.71–1.55) |
| 2.56–3.54 | 770 (+75) | 127 | 0.98 (0.70–1.39) | 67 | 0.85 (0.52–1.38) |
| 3.55–4.80 | 769 (+74) | 118 | 0.85 (0.59–1.22) | 76 | 0.85 (0.54–1.33) |
| >4.80 | 770 (+68) | 110 | 0.97 (0.67–1.40) | 70 | 1.19 (0.74–1.90) |
| Fruit, servings/d | |||||
| ≤1.76 | 771 (+40) | 139 | 1.02 (0.73–1.42) | 85 | 0.75 (0.49–1.15) |
| 1.77–2.93 | 771 (+21) | 129 | 1.02 (0.72–1.44) | 77 | 1.02 (0.65–1.60) |
| 2.94–4.38 | 772 (+27) | 126 | 1.11 (0.78–1.58) | 77 | 1.60 (1.02–2.51) |
| >4.38 | 767 (+27) | 123 | 0.81 (0.57–1.16) | 75 | 0.76 (0.48–1.19) |
| Fiber, g/d | |||||
| ≤15.6 | 771 (+40) | 135 | 0.97 (0.70–1.36) | 83 | 0.69 (0.45–1.07) |
| 15.7–19.9 | 772 (+33) | 132 | 1.07 (0.76–1.51) | 83 | 1.12 (0.72–1.72) |
| 20.0–25.2 | 769 (+28) | 131 | 1.07 (0.76–1.51) | 87 | 1.36 (0.89–2.08) |
| >25.2 | 769 (+28) | 119 | 0.83 (0.58–1.20) | 61 | 0.80 (0.48–1.34) |
| Fat, % of energy per d | |||||
| ≤23.8 | 782 (−17) | 106 | 0.72 (0.49–1.06) | 59 | 0.73 (0.44–1.22) |
| 23.9–28.6 | 768 (−13) | 149 | 1.27 (0.92–1.76) | 91 | 1.61 (1.06–2.45) |
| 28.7–33.4 | 766 (−15) | 122 | 0.86 (0.60–1.23) | 78 | 0.73 (0.46–1.14) |
| >33.4 | 765 (−10) | 140 | 1.13 (0.81–1.58) | 86 | 0.98 (0.64–1.49) |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Seven participants (4 in comparison group and 3 in intervention group) were missing baseline dietary data.
Percentage relative difference in mean dietary intake ([intervention − control]/control) between intervention and comparison groups at 4 years.
Likelihood ratio test for group X diet quartile interactions was not significant at P < .05 level for any diet component (except energy from fat and mortality; P = .04).
Hazard ratios were derived from Cox model stratified by quartiles of dietary intake.
COMMENT
In this randomized trial of a dietary intervention to achieve a substantial change to a diet very high in vegetables, fruit, and fiber and low in fat, the risk of developing additional breast cancer events and survival were not altered in women previously treated for early stage disease. No significant benefit in recurrence was observed overall among population subgroups characterized by demographic characteristics, baseline diet, or initial tumor types. Although breast cancer mortality rates in the United States declined during the study period,35 the similarity in survival patterns between the WHEL Study groups suggests that continued follow-up would not alter the study results.
It is unlikely that our results were materially affected by bias in assessing the main study end points. Follow-up of participants was nearly complete and did not differ between study groups, and we considered only reports of recurrence and new primary tumors that were validated by medical record review. We observed large and sustained between group differences in vegetable and fruit intake, as assessed by self-report and plasma carotenoid concentrations, a biomarker of vegetable and fruit intake. We also observed significant differences in fiber and fat intake. Although weight has been associated with health outcomes, we observed less than a 1-kg difference in average weight between WHEL Study groups at any time point. While psychosocial factors have been linked with health outcomes, we found no between-group differences for depression, social support, or quality of life during year 1, when the intervention was most intense.36 Therefore, we believe that our investigation provides an adequate test of whether the study dietary pattern (very high in vegetables, fruit, and fiber and low in fat) provided an added benefit over the dietary pattern of the comparison group women.
Many WHEL Study participants had likely changed their dietary pattern since receiving a diagnosis of breast cancer37; 75% were consuming at least 5 servings of vegetables and fruit a day at randomization, an intake that is considerably higher than that observed in other populations of breast cancer survivors.38,39 However, we observed little evidence of recurrence benefit in the quartile of the intervention group that was consuming less than 5 daily servings of vegetables and fruit at baseline, despite a major change in dietary pattern that was specific to the intervention group. Previously, in longitudinal analyses of the comparison group only, we observed a possible threshold effect on recurrence for low levels of baseline plasma carotenoid concentration13 and also that a baseline combination of 5 fruits and vegetables a day and physical activity equivalent to walking at a moderate pace for 30 minutes, 6 d/wk, was associated with lower mortality.14 However, in this analysis of data from the randomized trial, a major increase over the 5-a-day dietary pattern was not associated with reduced breast cancer events or mortality.
We suggest caution in applying our findings to groups of women other than those represented in our study, which was confined to women who had already completed initial therapy for breast cancer and excluded women with diagnoses after age 70 years and those with stage 1 tumors smaller than 1 cm. Also, only 14% of our study population was self-identified as from African American, Hispanic, and Asian American racial/ethnic groups.
Our finding that reducing dietary fat intake did not benefit breast cancer outcomes appears at odds with the interim analyses from the Women’s Intervention Nutrition Study (WINS), which concluded that reducing dietary fat intake was marginally associated with longer relapse-free survival of breast cancer patients, an effect most noted in the subgroup with estrogen-negative tumors. However, differential follow-up between intervention and comparison groups may have influenced the WINS finding.40 Furthermore, the reduced body weight observed only in the intervention group might partially account for the improved relapse-free survival in WINS.41 In addition, although WINS reported an 8% to 9% between-group difference in energy intake from fat maintained through 5 years, they reported a higher rate of missing dietary intake data in the intervention group. At the 3-year point, data were available for 67% of the intervention group vs 74% of the comparison group. At 5 years, data were available for 39% of the intervention group and 44% of the comparison group. If even moderate proportions of nonresponders increased their fat intake, the between-group effect could well be less than the absolute 4% difference that we observed. Finally, it is important to note that the women enrolled in WINS differed from those in the WHEL trial regarding prognosis following the original diagnosis, age, and treatment regimen.
Although the WHEL Study’s intervention diet focused mainly on increasing vegetable, fruit, and fiber intake, there was a significant between-group difference in fat intake. However, this difference may not have been sufficient to test the dietary fat hypothesis adequately. Unlike the changes observed for intakes of vegetables, fruit and fiber, the smallest dietary fat change was made by participants in the quartile that was furthest from the study target at baseline. Nonetheless, our analyses did not suggest an effect across quartiles of fat intake at baseline, nor did our results indicate an intervention effect in subgroups defined by hormone receptor status, as was seen in WINS.
The absence of an observed effect on breast cancer events or all-cause mortality over a 7.3-year follow-up period in this study does not rule out the possibility of improved longer-term survivorship within this cohort. We did not explore the possibility that increased exercise and weight loss might benefit breast cancer survivors. Finally, our study did not address whether consuming the high–vegetable/fruit/fiber and low-fat diet of our study intervention early in life would alter risk of primary breast cancer.
In conclusion, during a mean 7.3-year follow-up, we found no evidence that adoption of a dietary pattern very high in vegetables, fruit, and fiber and low in fat vs a 5-a-day fruit and vegetable diet prevents breast cancer recurrence or death among women with previously treated early stage breast cancer.
Acknowledgments
Funding/Support: The WHEL Study was initiated with the support of the Walton Family Foundation and continued with funding from National Cancer Institute grant CA 69375. Some of the data were collected from general clinical research centers (National Institutes of Health grants M01-RR00070, M01-RR00079, and M01-RR00827).
Role of the Sponsor: The funding sponsors had no role in the design, protocol development, or conduct of the trial or in data collection, data analysis, or manuscript preparation.
Additional Contributions: We thank the WHEL Study's data and safety monitoring committee (Brian Henderson, MD, Ross Prentice, PhD, Marion Nestle, MPH, PhD, and Charles Loprinzi, MD) and Sharon Ross, PhD (National Cancer Institute project officer) for their assistance with review of the article. We also acknowledge Kaylene Grove, BS, BA, Christine Hayes, MA, and Hollie Ward, BA, Cancer Prevention and Control Program, UCSD, for their administrative support and assistance with manuscript preparation. Finally, we are especially grateful to our dietary counseling team and WHEL Study participants for their sustained commitment and dedication to this long-term trial.
Footnotes
Financial Disclosures: None reported.
WHEL Study Investigators: Research Team by Clinical Site: WHEL Study Coordinating Center: University of California, San Diego (UCSD), Cancer Prevention and Control Program, Moores UCSD Cancer Center, San Diego (John P. Pierce, PhD, Susan Faerber, BA, Barbara A. Parker, MD, Loki Natarajan, PhD, Cheryl L. Rock, PhD, RD, Vicky A. Newman, MS, RD, Shirley W. Flatt, MS, Sheila Kealey, MPH, Linda Wasserman, MD, PhD, Wayne A. Bardwell, PhD, Lisa Madlensky, PhD); WHEL Study Dietary Counselors: Sheila K. Fisher, Joyce Bertaux, Leslie Barbier, Sharon Bonner, Prudy Galagan, Carrie Gonzales, Kaylene Grove, Pam Herskovitz, Susie Newmiller, Lita Simmons, Susan Wancewicz; WHEL Study Dietary Assessors: Andrea Jackson, Lita Simmons, Denice Murillo, Sophie Levy, Nichole Brumley; Laboratory Analysis: Dennis Heath, MS, Mila Pruitt; Clinical Sites: Center for Health Research–Portland, Portland, Oregon (Njeri Karanja, PhD, Mark U. Rarick, MD, Lucy Fulton, DTR, RD); Kaiser Permanente Northern California, Oakland (Bette J. Caan, DrPH, Lou Fehrenbacher, MD, Sarah Josef, RD); Stanford Prevention Research Center, Stanford University, Stanford, California (Marcia L. Stefanick, PhD, Robert Carlson, MD, Charlene Kranz, RD, Gwen D'Antoni, RD, Natalie Ledesma, MS, RD, Monique Schloetter, MS, RD); University of Arizona, Tucson and Phoenix (Cynthia Thomson, PhD, RD, James Warneke, MD, Cheryl Ritenbaugh, PhD, MPH, Tina Green, MS, RD, Emily Nardi, MPH, RD); University of California, Davis (Ellen B. Gold, PhD, Sidney Scudder, MD, Stephanie Burns, Linda Bresnick); University of California, San Diego, Moores UCSD Cancer Center, San Diego (Kathryn A. Hollenbach, PhD, Vicky Jones, MD, Michelle McKinney, Diana Wiggins, RD); University of Texas M. D. Anderson Cancer Center, Houston (Lovell A. Jones, PhD, Richard Hajek, PhD, Richard Theriault, DO, Taylor Tran, RD, LD).
References
- 1.Steinmetz KA, Potter JD. Vegetables, fruit, and cancer, II: mechanisms. Cancer Causes Control. 1991;2(6):427–442. doi: 10.1007/BF00054304. [DOI] [PubMed] [Google Scholar]
- 2.World Cancer Research Fund. Food, Nutrition and the Prevention of Cancer: A Global Perspective. Washington, DC: World Cancer Research Fund, American Institute for Cancer Research; 1997. [Google Scholar]
- 3.Ingram D. Diet and subsequent survival in women with breast cancer. Br J Cancer. 1994;69(3):592–595. doi: 10.1038/bjc.1994.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain M, Miller AB. To T. Premorbid diet and the prognosis of women with breast cancer. J Natl Cancer Inst. 1994;86(18):1390–1397. doi: 10.1093/jnci/86.18.1390. [DOI] [PubMed] [Google Scholar]
- 5.Holm LE, Nordevang E, Hjalmar ML, Lidbrink E, Callmer E, Nilsson B. Treatment failure and dietary habits in women with breast cancer. J Natl Cancer Inst. 1993;85(1):32–36. doi: 10.1093/jnci/85.1.32. [DOI] [PubMed] [Google Scholar]
- 6.Rohan TE, Hiller JE, McMichael AJ. Dietary factors and survival from breast cancer. Nutr Cancer. 1993;20(2):167–177. doi: 10.1080/01635589309514283. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S, Folsom AR, Sellers TA, Kushi LH, Potter JD. Better breast cancer survival for postmenopausal women who are less overweight and eat less fat: the Iowa Women's Health Study. Cancer. 1995;76(2):275–283. doi: 10.1002/1097-0142(19950715)76:2<275::aid-cncr2820760218>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Nomura AM, Marchand LL, Kolonel LN, Hankin JH. The effect of dietary fat on breast cancer survival among Caucasian and Japanese women in Hawaii. Breast Cancer Res Treat. 1991;18 suppl 1:S135–S141. doi: 10.1007/BF02633546. [DOI] [PubMed] [Google Scholar]
- 9.Gregorio DI, Emrich LJ, Graham S, Marshall JR, Nemoto T. Dietary fat consumption and survival among women with breast cancer. J Natl Cancer Inst. 1985;75(1):37–41. [PubMed] [Google Scholar]
- 10.Rock CL, Demark-Wahnefried W. Nutrition and survival after the diagnosis of breast cancer: a review of the evidence. J Clin Oncol. 2002;20(15):3302–3316. doi: 10.1200/JCO.2002.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mai V, Kant AK, Flood A, Lacey JV, Jr, Schairer C, Schatzkin A. Diet quality and subsequent cancer incidence and mortality in a prospective cohort of women. Int J Epidemiol. 2005;34(1):54–60. doi: 10.1093/ije/dyh388. [DOI] [PubMed] [Google Scholar]
- 12.Kroenke CH, Fung TT, Hu FB, Holmes MD. Dietary patterns and survival after breast cancer diagnosis. J Clin Oncol. 2005;23(36):9295–9303. doi: 10.1200/JCO.2005.02.0198. [DOI] [PubMed] [Google Scholar]
- 13.Rock CL, Flatt SW, Natarajan L, et al. Plasma carotenoids and recurrence-free survival in women with a history of breast cancer. J Clin Oncol. 2005;23(27):6631–6638. doi: 10.1200/JCO.2005.19.505. [DOI] [PubMed] [Google Scholar]
- 14.Pierce JP, Stefanick ML, Flatt SW, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol. 2007;25(17):2345–2351. doi: 10.1200/JCO.2006.08.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEligot AJ, Largent J, Ziogas A, Peel D, Anton-Culver H. Dietary fat, fiber, vegetable, and micronutrients are associated with overall survival in postmenopausal women diagnosed with breast cancer. Nutr Cancer. 2006;55(2):132–140. doi: 10.1207/s15327914nc5502_3. [DOI] [PubMed] [Google Scholar]
- 16.Davies AA, Davey Smith G, Harbord R, et al. Nutritional interventions and outcome in patients with cancer or preinvasive lesions: systematic review. J Natl Cancer Inst. 2006;98(14):961–973. doi: 10.1093/jnci/djj263. [DOI] [PubMed] [Google Scholar]
- 17.Harashima E, Nakagawa Y, Urata G, Tsuji K, Shirataka M, Matsumura Y. Time-lag estimate between dietary intake and breast cancer mortality in Japan. Asia Pac J Clin Nutr. 2007;16(1):193–198. [PubMed] [Google Scholar]
- 18.Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study. J Natl Cancer Inst. 2006;98(24):1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 19.Clark C. Study will put folklore on diet to test. San Diego Union-Tribune. 1998 March 11;:A1, A17. [Google Scholar]
- 20.Pierce JP, Faerber S, Wright FA, et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: the Women's Healthy Eating and Living (WHEL) Study. Control Clin Trials. 2002;23(6):728–756. doi: 10.1016/s0197-2456(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 21.Pierce JP, Newman VA, Flatt SW, et al. Telephone counseling intervention increases intakes of micronutrient- and phytochemical-rich vegetables, fruit and fiber in breast cancer survivors. J Nutr. 2004;134(2):452–458. doi: 10.1093/jn/134.2.452. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute. Action Guide for Healthy Eating. Bethesda, MD: National Cancer Institute; 1995. [Google Scholar]
- 23.National Cancer Institute. Eat 5 Fruits and Vegetables a Day. Washington, DC: National Cancer Institute; 1995. [Google Scholar]
- 24.Lachin JM, Foulkes MA. Evaluation of sample size and power for analyses of survival with allowance for nonuniform patient entry, losses to follow-up, noncompliance, and stratification. Biometrics. 1986;42(3):507–519. [PubMed] [Google Scholar]
- 25.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- 26.US Department of Agriculture/US Department of Health and Human Services. Dietary Guidelines for Americans: Home Health and Garden Bulletin No. 232. Washington, DC: US Dept of Health and Human Services; 1995. [Google Scholar]
- 27.Little RJA, Rubin DB. Statistical Analysis With Missing Data. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 28.Myriad Genetic Laboratories. [Accessed June 4, 2007];BRCA risk calculator and mutation prevalence tables. http://www.myriadtests.com/provider/brca-mutation-prevalence.htm.
- 29.Women's Health Initiative. [Accessed June 4, 2007];WHI Personal Habits Questionnaire. http://www.whiscience.org/data/forms/F34v2.pdf.
- 30.Johnson-Kozlow M, Rock CL, Gilpin EA, Hollenbach KA, Pierce JP. Validation of the WHI Brief Physical Activity Questionnaire among women diagnosed with breast cancer. Am J Health Behav. 2007;31(2):193–202. doi: 10.5555/ajhb.2007.31.2.193. [DOI] [PubMed] [Google Scholar]
- 31.Hong S, Bardwell WA, Natarajan L, et al. Correlates of physical activity level in breast cancer survivors participating in the Women's Healthy Eating and Living (WHEL) Study. Breast Cancer Res Treat. 2007;101(2):225–232. doi: 10.1007/s10549-006-9284-y. [DOI] [PubMed] [Google Scholar]
- 32.Women's Health Initiative. [Accessed June 4, 2007];WHI Thoughts and Feelings Questionnaire. http://www.whiscience.org/data/dd_byset/f37_dd.pdf.
- 33.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model, Statistics for Biology and Health. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 34.Rock CL, Flatt SW, Thomson CA, et al. Plasma triacylglycerol and HDL cholesterol concentrations confirm self-reported changes in carbohydrate and fat intakes in women in a diet intervention trial. J Nutr. 2004;134(2):342–347. doi: 10.1093/jn/134.2.342. [DOI] [PubMed] [Google Scholar]
- 35.Jatoi I, Chen BE, Anderson WF, Rosenberg PS. Breast cancer mortality trends in the United States according to estrogen receptor status and age at diagnosis. J Clin Oncol. 2007;25(13):1683–1690. doi: 10.1200/JCO.2006.09.2106. [DOI] [PubMed] [Google Scholar]
- 36.Bardwell W, Major J, Pierce JP. Quality of life change in breast cancer survivors participating in the Women's Healthy Eating and Living (WHEL) Study. Ann Behav Med. 2003;25 suppl:52. [Google Scholar]
- 37.Thomson CA, Flatt SW, Rock CL, Ritenbaugh C, Newman V, Pierce JP. Increased fruit, vegetable and fiber intake and lower fat intake reported among women previously treated for invasive breast cancer. J Am Diet Assoc. 2002;102(6):801–808. doi: 10.1016/s0002-8223(02)90180-x. [DOI] [PubMed] [Google Scholar]
- 38.Caan B, Sternfeld B, Gunderson E, Coates A, Quesenberry C, Slattery ML. Life After Cancer Epidemiology (LACE) study: a cohort of early stage breast cancer survivors (United States) Cancer Causes Control. 2005;16(5):545–556. doi: 10.1007/s10552-004-8340-3. [DOI] [PubMed] [Google Scholar]
- 39.Wayne SJ, Lopez ST, Butler LM, Baumgartner KB, Baumgartner RN, Ballard-Barbash R. Changes in dietary intake after diagnosis of breast cancer. J Am Diet Assoc. 2004;104(10):1561–1568. doi: 10.1016/j.jada.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 40.Pierce JP, Natarajan L, Marshall J, Messer K. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study [letter] J Natl Cancer Inst. 2007;99(11):900. doi: 10.1093/jnci/djk206. [DOI] [PubMed] [Google Scholar]
- 41.Thiébaut AC, Schatzkin A, Ballard-Barbash R, Kipnis V. Dietary fat and breast cancer: contributions from a survival trial. J Natl Cancer Inst. 2006;98(24):1753–1755. doi: 10.1093/jnci/djj504. [DOI] [PubMed] [Google Scholar]


