SUMMARY
Innate immunity depends upon recognition of surface features common to a broad group of pathogens. In gram negative bacteria, lipopolysaccharide recognition by immune cells leads to inflammation. In fungi, the glucose polymer β-glucan has been implicated in fungal recognition. Fungal walls have two kinds of β-glucan: β-1,3-glucan and β-1,6-glucan. The predominance of β-1,3-glucan in the wall and its recognition by macrophages has led to the presumption that β-1,3-glucan is the key immunological determinant for both macrophages and neutrophils. Here we show that in human neutrophils, β-1,6-glucan mediates engulfment, production of reactive oxygen species, and expression of HSPs more efficiently than β-1,3-glucan. Neutrophils rapidly ingest beads coated with β-1,6-glucan, while ignoring those coated with β-1,3-glucan. Complement factors C3b/C3d are deposited on β-1,6-glucan more readily than on β-1,3-glucan, recognized by CR3. β-1,6-glucan is also important for efficient engulfment of Candida albicans. These unique stimulatory effects could have useful medical applications.
INTRODUCTION
The cell walls of fungi evoke a powerful immunostimulatory response, and have been proposed for use as potential anti-infective and anti-tumor drugs (Hong et al., 2003; Hong et al., 2004; Reynolds et al., 1980; Tzianabos et al., 1998). Both pathogenic (Candida albicans) and non-pathogenic (Saccharomyces cerevisiae) fungi share similarities in their cell wall, which is composed of an inner layer of β-glucan (60%) covalently linked to a variety of cell surface mannoproteins (40%) (Klis et al., 2001; Klis et al., 2002; Magnelli et al., 2002). The β-glucan is a polymer of glucose, which is mostly in the β-1,3 linkage, but also contains glucose in the β-1,6 linkage. The β-1,6 glucan is estimated to be between 9% to 20% of the total β-glucan (Kapteyn et al., 1999; Magnelli et al., 2002). β-1,6-glucan has an important role connecting GPI-anchored proteins to the β-1,3-glucan network (Klis et al., 2001; Klis et al., 2002).
Fungal β-glucan is key to immune recognition in macrophages; it stimulates phagocytosis and production of inflammatory cytokines (Brown, 2006; Brown and Gordon, 2001). This non-opsonic recognition of β-glucan is mediated mainly via Dectin-1 with cooperation of TLRs, including TLR2 (Brown, 2006; McGreal et al., 2005). Dectin-1 activity in macrophages is inhibited by both β-1,3-glucans and β-1,6-glucans, but the β-1,3-glucan, laminarin, has a strong effect and the β-1,6-glucan, pustulan, has the weakest (Brown and Gordon, 2001). Oligosaccharide microarray results also show that macrophage Dectin-1 binds specifically to β-1,3-glucans (Palma et al., 2006).
Recent reports have shown that surface mannoproteins can mask the underlying β-glucan from the receptors (Gantner et al., 2005; Wheeler and Fink, 2006). Strains of Saccharomyces cerevisiae and Candida albicans have low reactivity with either Dectin-1 or an antibody to β-glucan unless the mannoprotein layer is disrupted either by heat treatment or mutation (Wheeler and Fink, 2006). There appears to be a conserved genetic network of interacting proteins responsible for the integrity of the cell wall. Once this architecture is perturbed so that the β-glucan is exposed, the fungi interact strongly with Dectin-1 and elicit an enhanced pro-inflammatory response in primary mouse macrophages (Wheeler and Fink, 2006).
Early studies implicated fungal cell wall β-glucan in the stimulation of neutrophils through complement receptor 3 (CR3). CR3 has a lectin domain (Ross et al., 1985; Ross et al., 1987) that binds intact yeast and yeast β-glucan. Ligation of β-glucans to neutrophil CR3 increases their chemotactic capacity towards the complement fragment C5a (Tsikitis et al., 2004), and in-vivo enhances neutrophil migration into the site of injury (LeBlanc et al., 2006). Although these studies provided convincing evidence for the stimulation of neutrophils by β-glucan, the exact chemical composition of the immunostimulant was unclear because the β-glucan used was a mixture of polymers. Yeast cell walls, the source of glucan in these studies, are 80% β-1,3-glucan (Magnelli et al., 2002), which led to the assumption that the β-1,3 polymer was responsible for the immunostimulatory effects. However, these cell wall preparations also contain β-1,6-glucan.
Other sources of glucan mixtures have also been shown to bind and prime neutrophil CR3, including β-glucan from barley (70% β-1,4-glucan, and 30% β-1,3-glucan), and laminarin (β-1,3-glucan with occasional β-1,6 branch points) (Thornton et al., 1996; Vetvicka et al., 1996; Xia et al., 1999). In none of these cases was a glucan of homogeneous composition compared with another for relative efficacy in stimulating immune cells for both phagocytosis and ROS production.
Here we show that β-1,6-glucan, the minor component of the fungal cell wall, causes a much greater stimulation of human neutrophils than β-1,3-glucan under opsonic conditions. β-1,6-glucan is more effective than β-1,3-glucan in eliciting phagocytosis, production of ROS, and elicitation of heat shock proteins by human neutrophils purified from whole blood. Neutrophil recognition of fungi is likely to be mediated by this specific sugar interaction as beads coated with β-1,6-glucan bind the complement factors C3b and its proteolytic fragment C3d better than those coated with β-1,3-glucan. This recognition is inhibited by anti-CR3 blocking antibodies, suggesting that recognition of C3b proteolytic products by CR3 mediate this enhanced phagocytosis and ROS production.
RESULTS
Expression of heat shock proteins in neutrophils is induced by exposed β-glucan
To determine the opsonic response of neutrophils to the yeast form of Candida albicans, we performed microarray experiments using whole genome Affymetrix arrays on RNA extracted from neutrophils that had ingested the fungus. Among the genes induced in neutrophils was a family of heat shock proteins (HSPs) (The 10 kDa HSPE1, 40 kDa DNAJB1 and DNAJB9, 70 kDa HSPA1A and HSPA9B, 90 kDa HSPCA and HSPCB, and 105/110 kDa HSPH1) (Sup. Table 1). These inductions were corroborated by quantitative real time PCR (Fig. 1A). The induction of HSPs was greater if Candida was first heated to unmask the underlying β-glucan (Fig. 1B), suggesting that expression of HSPs is induced by the exposed β-glucan.
Figure 1. β-1,6-glucan stimulates expression of heat shock proteins (HSPs) in neutrophils.
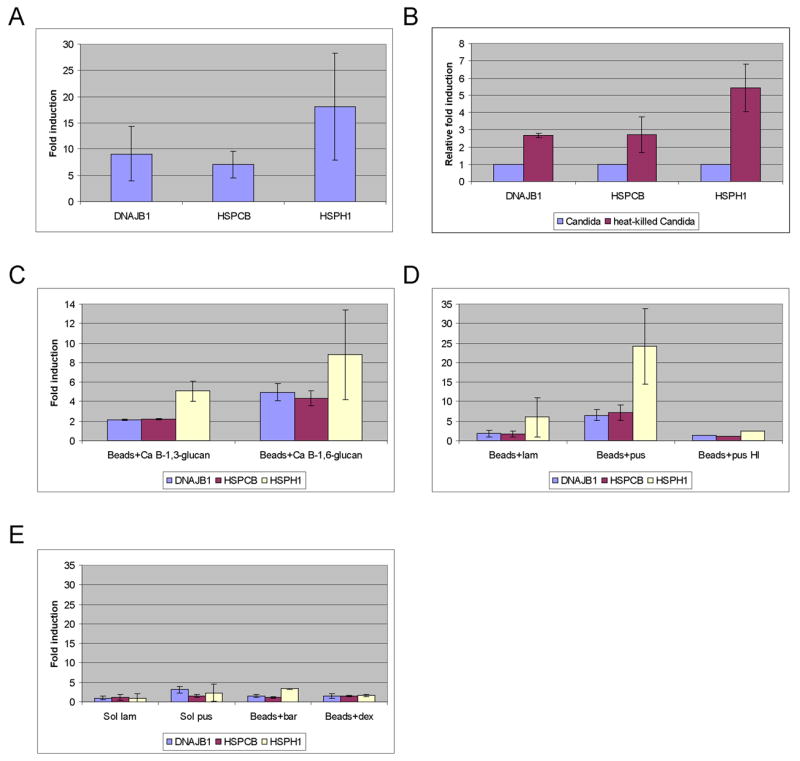
Induction of HSPs was determined by quantitative real-time PCR. The data represent the average of two (A) or at least three experiments with standard deviation. Candida (Ca) or beads were opsonized with pooled human serum and cultured for 2 hours with neutrophils. (A) Candida albicans elicits HSPs in neutrophils. The fold induction represents the ratio of neutrophils+ Candida to neutrophils alone. (B) Heat-killed Candida elicits higher levels of HSPs. Results for heat-killed Candida were normalized to UV-killed Candida. (C–E) β-1,6-glucan stimulates expression of HSPs. Polybead polystyrene 6 μm microspheres (beads) were coated with an equivalent amount of one of the indicated β-glucans. Beads were opsonized with pooled human serum, or with heat inactivated (HI) pooled human serum (D). The fold induction represents the ratio of neutrophils+ glucans- coated beads over neutrophils+ untreated beads. (C) Fungal β-1,6-glucan stimulates expression of HSPs. Beads were coated with β-1,3-glucan purified from Candida albicans (Ca B-1,3-glucan), or β-1,6-glucan purified from Candida albicans (Ca B-1,6-glucan). (D) Standard β-1,6-glucan stimulates expression of HSPs. Beads were coated with laminarin (lam, algal β-1,3-glucan), or pustulan (pus, lichen β-1,6-glucan). (E) Soluble β-1,6-glucan and other glucan- coated beads do not stimulate expression of HSPs. Beads were coated with β-glucan from barley (bar, 30% β-1,3-glucan, and 70% β-1,4-glucan), or dextran (dex, α-1,6-glucan). Neutrophils were cultured with 5 mg/ml of soluble laminarin (sol lam), 5 mg/ml of soluble pustulan (sol pus), or beads. The fold induction represents the ratio of neutrophils+ soluble glucans over neutrophils alone, or neutrophils+ glucans- coated beads over neutrophils+ untreated beads.
β-1,6-glucan elicits expression of HSPs in neutrophils
To assay which of the glucan components was responsible for induction of HSPs, we presented neutrophils with various glucan polymers. As unprimed neutrophils respond best to insoluble material (Fossati et al., 2002), we conjugated several sources of β-glucan to 6 μm polystyrene beads, which are similar in size to Candida albicans yeast form cells (5 μm). We utilized β-1,3-glucan and β-1,6-glucan purified from Candida cell walls, as well as non-fungal sources of β-1,3-glucan (laminarin) and β-1,6-glucan (pustulan), both used as standards for these polymers in previous studies (Brown and Gordon, 2001; Palma et al., 2006). The extent of coating was quantified as described in Experimental procedures.
Beads coated with Candida derived β-1,6-glucan were more efficient than beads coated with Candida derived β-1,3-glucan at elicitation of HSPs in neutrophils (Fig. 1C). Similar results were observed with the standards: pustulan (β-1,6-glucan) being more powerful stimulant than laminarin (β-1,3-glucan). Beads coated with pustulan elicited high levels of HSPs in neutrophils (Fig. 1D), whereas beads coated with laminarin elicited only low level of HSPs expression (Fig. 1D), despite efficient coating of the beads. Glucans in the soluble form elicited only low levels of HSPs in neutrophils (Fig. 1E), suggesting that neutrophils respond when the polysaccharides are presented on particles. β-glucan from barley, which is composed of β-1,3-glucan (30%) and β-1,4-glucan (70%) also did not elicit HSPs expression (Fig. 1E). Beads coated with α-1,6-glucan (dextran) did not elicit any response (Fig. 1E), suggesting that the neutrophil response is specific to the β configuration. Elicitation of HSPs required heat-labile serum components, as pustulan-coated beads did not elicit HSPs when opsonized with heat-inactivated (HI) serum (Fig. 1D). We conclude that neutrophils express HSPs following ingestion of opsonized beads coated with β-1,6-glucan.
The induction of HSPs by pustulan is due to β-1,6-glucan
Digestion of pustulan with an endoglucanase specific for the β-1,6-glucan linkage (Lora et al., 1995) confirmed that it consisted of β-1,6-glucan. Pustulan digested with the enzyme resulted in as much as 80% reduction in elicitation of HSPs (Fig. 2A). Analysis of the digestion products of pustulan by column chromatography (see Experimental procedures) revealed the expected peak (Fig. 2 compare B to C), composed of di-and tri-saccharides, as determined by TLC (G2-G3, compare lane 2 and 3, Fig. 2D). These short polymers in the G2-G3 fraction did not coat the beads efficiently (see Experimental procedures).
Figure 2. Elicitation of heat shock proteins (HSPs) by pustulan is due to β-1,6-glucan.
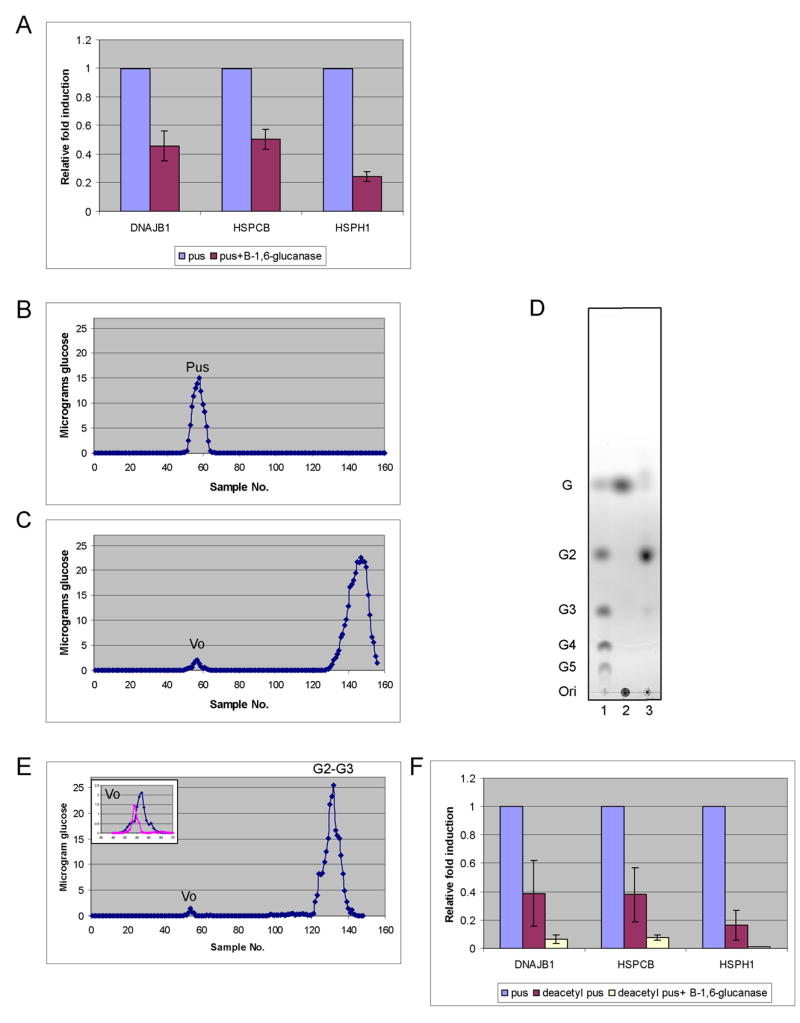
Neutrophils were cultured with opsonized beads for 2 hours at 37 °C in A and F. (A) Endo-β-1,6-glucanase reduces the induction of HSPs by pustulan. Beads were coated with an equivalent amount of pustulan (pus) or endo-β-1,6-glucanase digested pustulan. Induction of HSPs with enzyme treated pustulan is relative to that with untreated. The data represent the average of three experiments with standard deviation. (B) Pustulan chromatographed on Biogel P6 column. (C) Pustulan digested first with endo-β-1,6-glucanase and run on a P6 column generated a large and a small peak. The small peak represents a tiny fraction of the original pustulan that was resistant to enzymatic digestion (Vo). (D) The large peak in C was shown by thin layer chromatography to be the expected degradation products, gentiobiose and gentiotriose. Lane 1 contains standard oligosaccharides (G to G5) as controls. Lane 2 is pustulan spiked with glucose (G). Lane 3 is endo-β-1,6-glucanase digested pus. Ori = origin. (E) Chromatography of deacetylated pustulan. The insert is an overlay of the Vo from C and E. (F) Deacetylation of pustulan followed by digestion with endo-β-1,6-glucanase eliminates induction of HSPs. Induction of HSPs in deacetylated pustulan or deacetylated pustulan digested with endo-β-1,6-glucanase is relative to that with untreated pustulan.
In addition to the expected digestion products there was a small amount of polysaccharide (~ 5% of the starting material) that was resistant to the enzyme (Fig. 2C, designated as Vo) and elicited expression of the HSPs in the neutrophils. Since pustulan is reported to have a small percentage of O-acetylated β-1,6-glucan (Nishikawa et al., 1970), the small peak resistant to enzymatic digestion could be the acetylated polymer. Consistent with this interpretation, deacetylation of pustulan prior to digestion and fractionation (see Experimental procedures) virtually abolished this minor component (Fig. 2E, insert) and rendered the digested material unable to elicit HSPs (Fig. 2F). The residual activity of pustulan digested with the endo-β-1,6-glucanase is therefore attributable to O-acetylated β-1,6-glucan that was resistant to the enzyme and coated the beads efficiently. Chemically acetylated laminarin (β-1,3-glucan) did not elicit any expression of HSPs (Sup. Fig. 1), suggesting that the stimulation of neutrophils by β-1,6-glucan is not due to acetylation per se. Based on these results we conclude that the activity of pustulan is due to the β-1,6-glucan.
β-1,6-glucan mediates efficient phagocytosis and production of reactive oxygen species by neutrophils
Time-lapse microscopy of neutrophils ingesting beads revealed that beads coated with pustulan (β-1,6-glucan) were more efficiently internalized than beads coated with laminarin (β-1,3-glucan) (Fig. 3A, compare d to b, and compare Sup. movie 2 to 1). Neutrophils readily ingested beads when coated with pustulan. In contrast, most neutrophils ignored the laminarin-coated beads, and only a few of them ingested beads (Fig. 3b and Sup. movie 1, a neutrophil ingesting one bead on the upper left corner, and one neutrophil ingesting two beads on the upper right corner).
Figure 3. β-1,6-glucan stimulates phagocytosis and production of reactive oxygen species (ROS) in neutrophils.
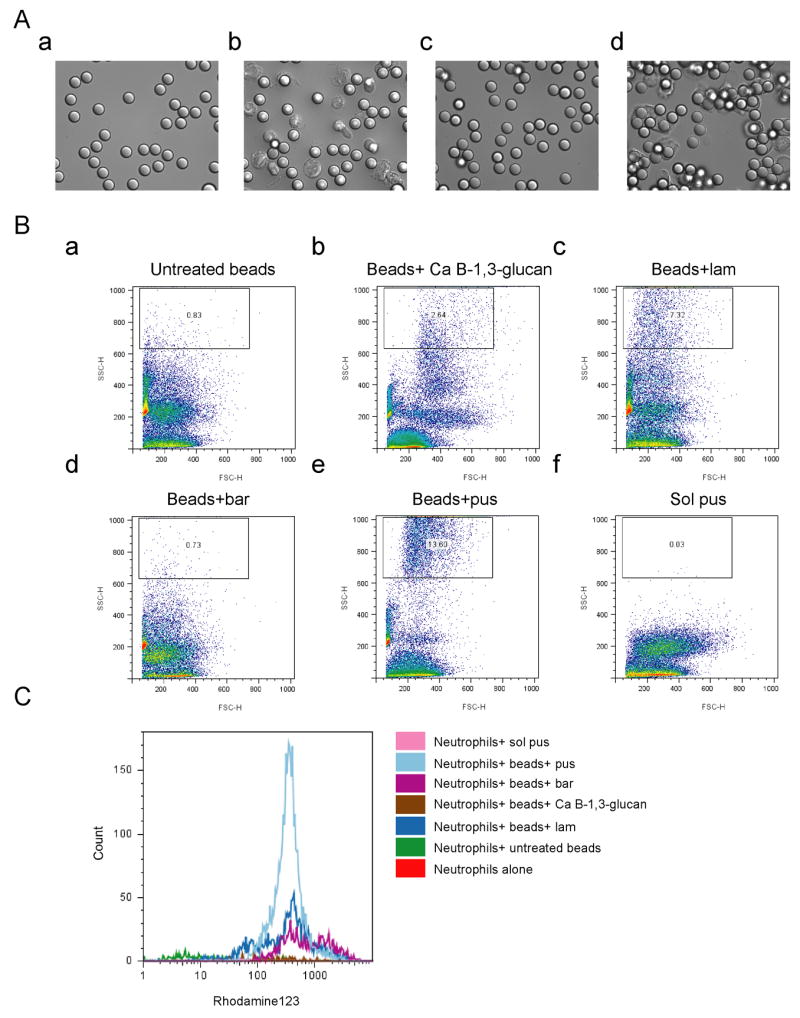
Polybead polystyrene 6 μm microspheres (beads) were coated with an equivalent amount of the indicated β-glucans and then opsonized. (A) β-1,6-glucan stimulates phagocytosis. Phagocytosis was assessed by time-lapse microscopy for beads that were coated with laminarin (a, and b), or pustulan (c and d). The images at a and c were taken at time 0. Images b and d were taken after culturing with neutrophils for 40 minutes. (B) β-1,6-glucan stimulates phagocytosis. Phagocytosis was assessed by Fluorescence Activated Cell Sorting (FACS) by the change in side scatter for neutrophils with (a) untreated beads, (b) beads coated with β-1,3-glucan from Candida (c) beads coated with laminarin (lam, β-1,3-glucan), (d) beads coated with glucan from barley (bar), (e) beads coated with pustulan (pus, β-1,6-glucan) (f) soluble pustulan. (C) β-1,6-glucan stimulates ROS production. ROS production was assayed by FACS using DHR123. β-1,3-glucan shows only a modest stimulation.
Quantification of a population of neutrophils ingesting beads by Fluorescence Activated Cell Sorting (FACS) support the idea that pustulan mediates more efficient internalization of beads than laminarin, β-1,3-glucan from Candida, or β-glucan from barley, although laminarin and β-1,3-glucan from Candida promoted internalization somewhat better than uncoated beads (Fig. 3B, compare panel e to b–d, respectively).
Since killing of pathogens by neutrophils depends on a burst of reactive oxygen species (ROS) (Babior et al., 1973), we tested whether ROS were induced by either of the two glucans. ROS could not be detected in neutrophils alone or in neutrophils presented with beads that had not been treated (Fig. 3C, red and green, respectively). Low levels were detected with beads coated with laminarin, β-glucan from Candida, or β-1,3-glucan from barley (Fig. 3C, blue, brown and purple, respectively). However, beads coated with pustulan stimulated the generation of significant amounts of ROS (Fig. 3C, light blue). No ROS was detected by adding soluble pustulan (Fig. 3C, pink). These data show that β-1,6-glucan evokes a massive production of ROS, whereas the response to β-1,3-glucan is much lower by comparison.
Complement factor C3 proteolytic fragments are efficiently deposited on β-1,6-glucan
As serum is required for phagocytosis and the induction of HSPs by beads coated with β-1,6-glucan (Fig. 1D), we determined whether there was a serum component that was differentially deposited on beads coated with either β-1,6-glucan or β-1,3-glucan. Beads were coated with laminarin or pustulan and opsonized as described in Experimental procedures. Bound proteins were removed from beads and separated by SDS gel electrophoresis (see Experimental procedures). Although a number of serum proteins bound both β-1,3-glucan or β-1,6-glucan, there were two prominent proteins that adhered more avidly to β-1,6-glucan (Fig. 4A). These proteins were extracted from the gel and subjected to analysis by mass spectrometry. The peptides from these bands gave masses that identified both proteins as C3, suggesting that proteolytic fragments of C3 are deposited more avidly on β-1,6-glucan than β-1,3-glucan.
Figure 4. C3 proteolytic fragments are deposited on β-1,6-glucan.
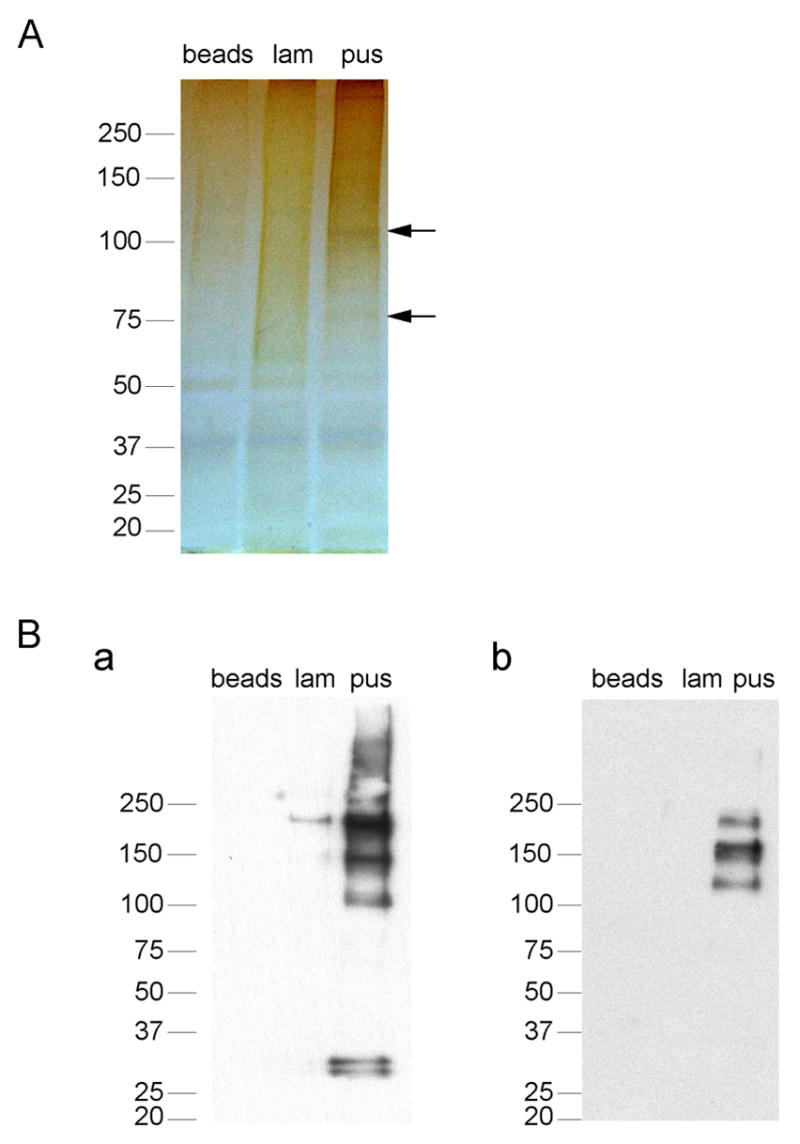
Beads were untreated (Beads), or coated with equivalent amount of laminarin (lam, β-1,3-glucan), or pustulan (pus, β-1,6-glucan). Following opsonization, the beads were suspended in 2% SDS 1M ammonium hydroxide buffer and incubated at 37 °C for 1 hour. The supernatant solution was loaded on 4–20% acrylamide SDS gel. The migration of the molecular weight protein standards is indicated. (A) The gel was incubated with silver stain, and the bands were extracted for analysis by mass spectrometry. (B) Western analysis was performed using monoclonal antibodies directed against (a) the alpha or (b) the beta chains of C3.
Western analysis revealed that indeed β-1,6-glucan was deposited with more C3 (Fig. 4B). Antibodies specific for the alpha chain detected high molecular weight bands, including bands the size of the complete C3 or C3b (105 and 115 kDa, respectively), as well as a lower molecular doublet the size of C3d (31 and 33 kDa, Fig. 4B a). Beads coated with laminarin had low levels of these C3 fragments (Fig. 4B a). Antibodies specific for the C3 beta chain revealed only the high molecular weight C3/C3b (Fig. 4B b). The 75 kDa chain of C3b or iC3b were not detected (Fig. 4B b).
To assess further the differences in C3 deposition on β-1,6-glucan as compared with β-1,3-glucan, serum was preincubated with soluble pustulan or laminarin before using it to opsonize beads coated with pustulan. Preincubation of the serum with soluble pustulan eliminated phagocytosis (Fig. 5A compare c and a) and ROS production (Fig. 5B compare red and blue) by neutrophils, suggesting that serum C3 was titrated out by the soluble β-1,6-glucan. Serum that was preincubated with an equivalent amount of soluble laminarin still mediated phagocytosis (Fig. 5A compare b and a) and ROS production (Fig. 5B compare red and green), suggesting that β-1,3-glucan did not efficiently block the interaction of C3 with beads coated with pustulan.
Figure 5. Preincubation of serum with soluble pustulan (β-1,6-glucan) abolishes stimulation of neutrophils, whereas soluble laminarin (β-1,3-glucan) does not.
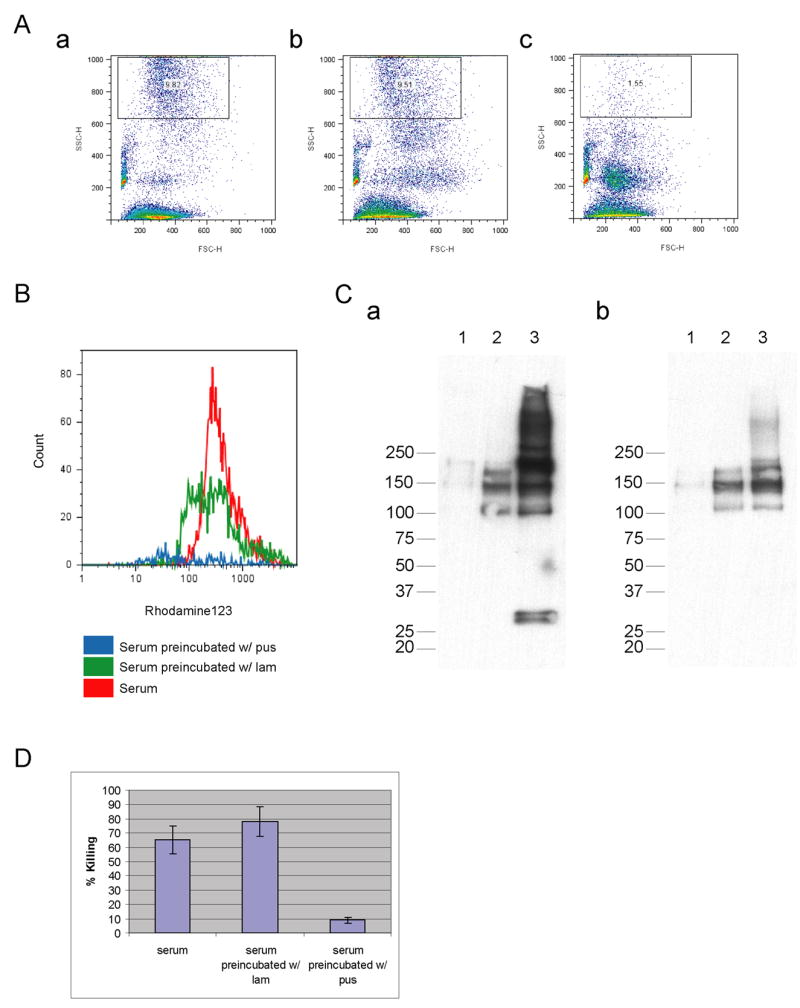
(A) Phagocytosis of pustulan- coated beads was assessed by FACS, by the change in side scatter. Serum was untreated (a), or incubated for 5 minutes at 37 °C with 1 mg (quantified by phenol- sulfuric acid method) of soluble laminarin (lam) (b) or pustulan (pus) (c). (B) Reactive oxygen species production in response to pustulan- coated beads was assayed by FACS using DHR123. Serum was untreated (red), incubated with soluble laminarin (green), or with pustulan (blue). (C) C3 deposition on pustulan- coated beads was eliminated by preincubation of the serum by soluble pustulan but not laminarin. C3 deposition was assayed by Western analysis using monoclonal antibodies directed against the (a) alpha or (b) the beta chains of C3. Serum was preincubated with soluble pustulan (1), laminarin (2), or was untreated (3). The molecular weight protein standard is indicated. (D) Preincubation of serum with soluble pustulan reduces Candida killing. Serum was untreated, or preincubated with soluble pus or lam prior to Opsonization of Candida. Candida viability was assayed using XTT following incubation of 30 minutes with neutrophils.
Western analysis revealed that preincubation with soluble pustulan eliminated most of the C3/C3b and C3d deposition on the pustulan-coated beads (Fig. 5C compare 1 to 3). Preincubation with laminarin eliminated the low molecular weight C3d doublet, but retained the high molecular weight C3/C3b (Fig. 5C compare 2 to 3), suggesting that the remaining C3/C3b was mediating phagocytosis and induction of ROS (Fig. 5A and B). Preincubation of serum with soluble pustulan but not laminarin reduced the killing of Candida albicans by 6-fold (Fig. 5D). This inhibition by soluble β-1,6-glucan is consistent with the idea that complement deposition on β-1,6-glucan is required for efficient killing of the fungus.
CR3 recognizes C3 fragments that are deposited on particulate β-1,6-glucan
Because complement receptor 3 (CR3) is known to mediate phagocytosis of opsonized yeast and the yeast cell wall preparation zymosan, as well as ROS production (Cain et al., 1987; Ross et al., 1985), we surmised that CR3 could mediate phagocytosis of beads coated with β-1,6-glucan. Anti- human CR3 blocking antibodies reduced the extent of phagocytosis (Fig. 6A compare b to a) and ROS production (Fig 6B compare red to green), suggesting that CR3 recognized C3b proteolytic fragments (C3d) that are deposited on particulate β-1,6-glucan.
Figure 6. CR3 mediates β-1,6-glucan stimulation of neutrophils.
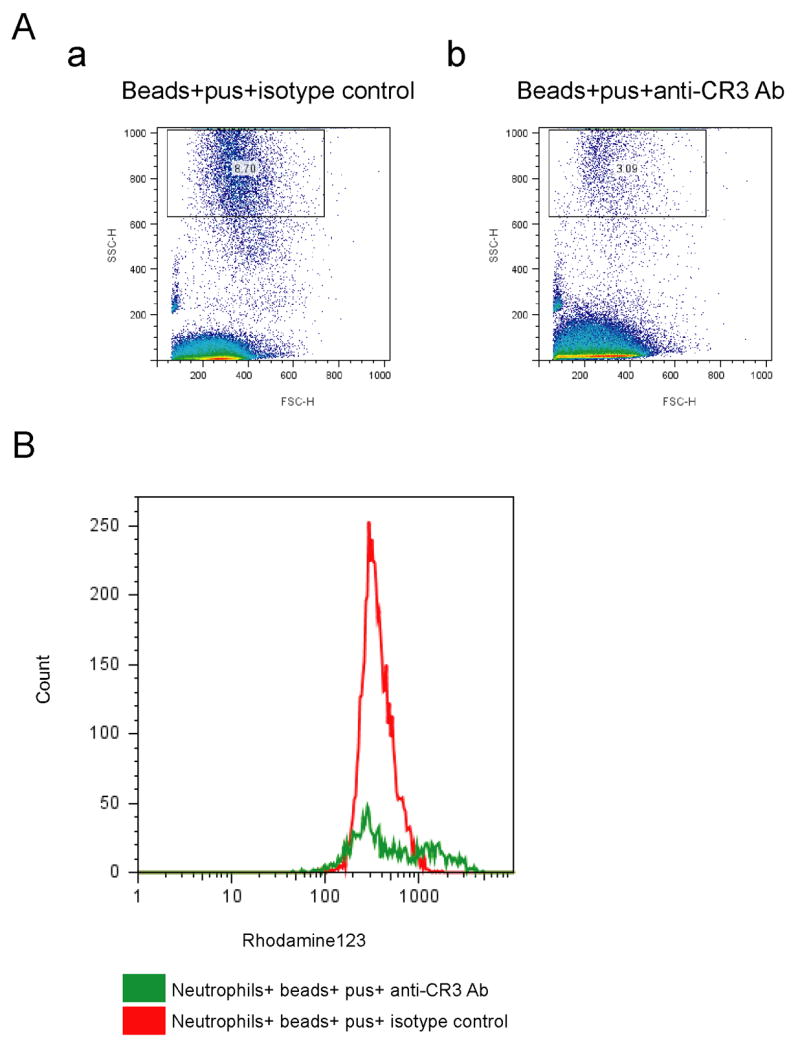
Polybead polystyrene 6 μm microspheres (beads) were coated with the β-1,6-glucan pustulan (pus). Beads were opsonized with pooled human serum and cultured with neutrophils for 15 minutes. Neutrophils were preincubated with CR3 blocking antibodies or IgG isotype control for 30 minutes on ice before culturing with pustulan- coated beads. (A) CR3 blocking antibodies reduce β-1,6-glucan stimulated phagocytosis. Phagocytosis of pustulan- coated beads was assessed by FACS by the change in side scatter for neutrophils preincubated with: (a) isotype control IgG, or (b) anti CR3 blocking antibodies. (B) CR3 blocking antibodies reduce β-1,6-glucan stimulated ROS production. ROS production in response to pustulan- coated beads was assayed by FACS using DHR123. Neutrophils were preincubated with isotype control IgG (green) or anti CR3 blocking Ab (red).
β-1,6-glucan recognition enhances phagocytosis of Candida albicans
β-1,6-glucan also plays an important role in the neutrophil’s recognition of whole fungal cells, which contain other polysaccharides such as chitin and β-1,3-glucan as well as many cell surface proteins. Candida albicans cells were heat killed to expose their masked β-glucan, and one sample was digested with an endo β-1,6-glucanase, and the other was incubated without the enzyme. Both samples were opsonized (see Experimental procedures) and tested for their reactivity. The cells exposed to the enzyme (as compared with those that were not) exhibited a ~50% reduction in phagocytosis (Fig. 7A) and ROS production (Fig. 7B), as well as expression of HSPs (Fig. 7C). As EM93, a feral progenitor of many Saccharomyces cerevisiae laboratory strains, has exposed β-glucan (Rubin-Bejerano and Fink, unpublished), it was possible to test the effect of endo β-1,6-glucanase treatment on recognition without heat killing. Live Saccharomyces treated with enzyme was also less effective than the untreated strain (Sup. Fig. 2), suggesting that in fungi, β-1,6-glucan is an important component of fungal recognition.
Figure 7. β-1,6-glucan is required for efficient phagocytosis of Candida albicans, production of ROS, and expression of HSPs.
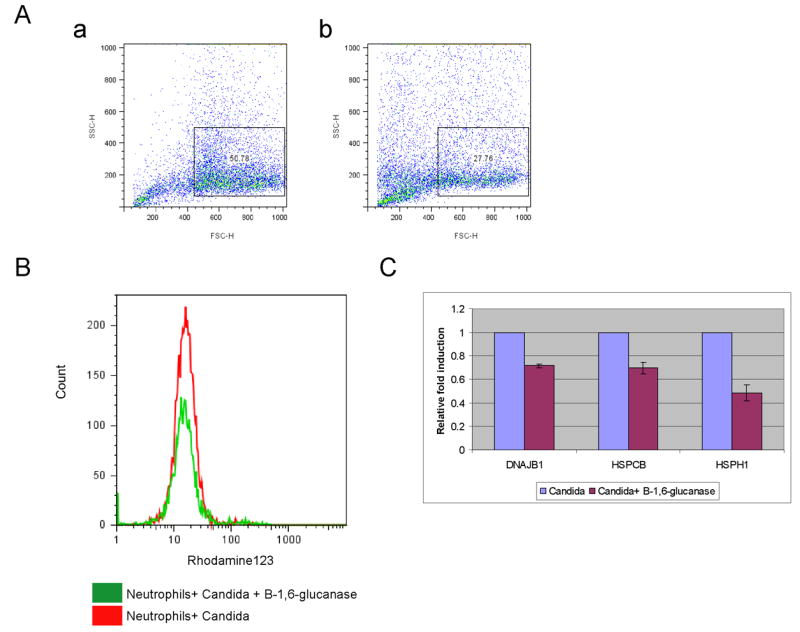
Candida albicans cells were heat killed, digested with an endo-β-1,6-glucanase, and opsonized. (A) β-1,6-glucan is required for efficient phagocytosis. Phagocytosis was assessed by Fluorescence Activated Cell Sorting (FACS) by the change in side scatter. (B) β-1,6-glucan is required for efficient ROS production. ROS production was assayed by FACS using DHR123. (C) β-1,6-glucan is required for induction of HSPs. HSPs induction was determined by quantitative real-time PCR. Results for β-1,6-glucanase digested Candida were normalized to undigested Candida. The data represent the average of two experiments with standard deviation.
DISCUSSION
Our data show that β-1,6-glucan, a minor component of the total β-glucan in the fungal cell wall, is the most active immunostimulant of neutrophils. Under opsonic conditions, human neutrophils recognize particulate β-1,6-glucan, but not β-1,3-glucan. β-1,6-glucan isolated from Candida albicans and pustulan, the standard source of β-1,6-glucan, elicit efficient phagocytosis, heat shock protein (HSPs) expression, and ROS production (the hallmark of the neutrophil response and key to the destruction of pathogens). Laminarin, the standard source of β-1,3-glucan, β-1,3-glucan from Candida, and β-glucan from barley, 30% of which is β-1,3-glucan, do not stimulate these characteristic neutrophil responses in human neutrophils. Moreover, Candida cells digested with an enzyme specific for the β-1,6-glucan linkage show reduced phagocytosis, production of ROS, and expression of HSPs by ~50% (Fig. 7).
Previous studies led to the suggestion that, in addition to an iC3b binding domain, CR3 has a lectin- like domain that binds directly to β-glucans, mediating phagocytosis and ROS production (Ross et al., 1985). However, the nature of the β-glucan polymer responsible for these enhanced interactions remained unresolved because the polymer used in those experiments was a mixture of both β-1,3-glucan and β-1,6-glucan. Our studies suggest that the major stimulatory component of both phagocytosis and ROS production is β-1,6-glucan.
The glucans used in our experiments differ in polymer length. Therefore, to compare the neutrophil response to β-1,3-glucan and β-1,6-glucan we used beads that had the same level of sugar coating, as quantified by the phenol-sulfuric acid method (see Experimental procedures). Soluble β-glucan polymers of different length have different effects on neutrophils. Large soluble β-glucans crosslink membrane CR3, triggering phagocytosis and ROS production (Ross et al., 1987; Vetvicka et al., 1996), whereas shorter soluble β-glucans do not (Thornton et al., 1996). However, particulate β-glucans of any size crosslink CR3 (Ross et al., 1987; Vetvicka et al., 1996). Because we tested the response of neutrophils to particulate β-glucans, the shorter polymer of β-1,3-glucan, laminarin, and the long β-glucans from Candida and barley were all expected to stimulate neutrophils.
Mass spectrometry revealed that C3 proteolytic fragments are more readily deposited on β-1,6-glucan than β-1,3-glucan (Fig. 4). Western analysis revealed that C3/C3b and fragments corresponding to the molecular weight of C3d are deposited on β-1,6-glucan (Fig. 4B). Since C3d, the proteolytic product of iC3b, is the least labile of the C3 proteolytic fragments (Slaney et al., 2006), we cannot rule out the possibility that other C3 fragments, such as iC3b are also present on the β-1,6-glucan coated beads, but are less stable. Our experiments using preincubation of serum with either β-1,3-glucan (laminarin) or β-1,6-glucan (pustulan) support the idea that deposition of C3/C3b and C3d is more efficient on β-1,6-glucan than β-1,3-glucan (Fig. 5). β-1,6-glucan but not β-1,3-glucan from Candida albicans is a chemoattractant for neutrophils (Sato et al., 2006). This is consistent with the idea that complement activation and deposition on β-1,6-glucan is generating anaphylatoxins, leading to neutrophil recruitment.
These data are consistent with a model in which a component(s) of complement binds to β-1,6-glucan on the beads and that this complex is recognized by a receptor on the surface of neutrophils. The major receptor that binds C3d is CR2, which is predominantly expressed on B cells and is absent from neutrophils (Ross, 1980). However, C3d enhances a humoral response in CD21/35−/− (CR1 CR2) knockout mice (Haas et al., 2004), supporting the idea that receptors other than CR2 can also bind C3d. Anti-CR3 antibodies eliminate most of the neutrophil response (Fig. 6), suggesting that recognition of β-1,6-glucan is mediated by CR3. Since C3d binds CR3 on monocytes (Gaither et al., 1987), it is possible that in addition to binding iC3b (Ross et al., 1985), CR3 on neutrophils binds C3d on β-1,6-glucan coated beads.
Since C3 is common to all pathways of complement activation (Janeway et al., 2001), its deposition on β-1,6-glucan could be mediated by antibodies through the classical pathway, or through the alternative or the mannan-binding lectin pathways. Further work is underway to determine if C3 binds β-1,6-glucan directly and is spontaneously cleaved, or if C3 binding to β-1,6-glucan is mediated by IgM or IgG antibodies.
The induction of HSPs by β-1,6-glucan could represent an important signal by which neutrophils alert the immune system to an attack by pathogens. Neutrophils that phagocytose Escherichia coli or Staphylococcus aureus undergo apoptosis and induce HSP60 and HSP70 proteins (Zheng et al., 2004). Different HSPs from bacterial or mammalian origin also induce cytokine production in immune cells (Prohaszka and Fust, 2004). Recently, Fradin et. al also showed that neutrophils express HSPs following phagocytosis of Candida (Fradin et al., 2007). Expression of HSPs is regulated by the transcriptional activator HSF1, which is also known to repress the expression of proinflamatory cytokines, including TNFα and IL1B (Nagarsekar et al., 2005). Taken together, HSP induction in neutrophils might be part of a non-inflammatory signaling to other immune cells.
Previous experiments have shown that murine macrophages recognize β-1,3-glucan in zymosan or yeast and respond by elicitation of pro-inflamatory cytokines such as TNFα (Brown and Gordon, 2001; Wheeler and Fink, 2006). This recognition is mediated mainly through the pattern-recognition receptor, Dectin-1 (Brown and Gordon, 2001). The β-1,3-glucan, laminarin, inhibits the function of Dectin-1 (Brown and Gordon, 2001; Wheeler and Fink, 2006), and directly binds Dectin-1 (Palma et al., 2006), whereas the β-1,6-glucan, pustulan, does not bind Dectin-1 effectively (Palma et al., 2006). Most experiments have been done under non-opsonic conditions (Brown and Gordon, 2001; Wheeler and Fink, 2006). However, recently, Taylor et al. showed that Dectin-1 is also required for TNFα elicitation in macrophages under opsonic conditions (Taylor et al., 2007). It is possible that under opsonic conditions macrophages would also respond to β-1,6-glucan, by a mechanism that is independent of Dectin-1.
The systems used to study the immune response to fungal infection attempt to reconstitute in-vitro some aspect of the fungal recognition system using a purified fungal cell wall component and a specific immune cell, or receptor. Although these simplified systems permit clear conclusions, the fungal cell wall is a complex structure composed not only of glucans but mannoproteins and other compounds, which, in-vivo, are presented in the context of the other wall components to a complex mixture of immune cells. For example, β-glucans are clearly determinants, but they are masked from host receptors by a layer of mannoproteins (Gantner et al., 2005; Wheeler and Fink, 2006). Therefore, immune recognition of β-glucan was previously studied using zymosan, an insoluble extract of yeast cell walls, or heat-killed yeast (Brown and Gordon, 2001; Saijo et al., 2007; Taylor et al., 2007; Wheeler and Fink, 2006), both of which have their β-glucan completely exposed. These considerations suggest that there is likely to be a system capable of unmasking the β-glucan so that it can be recognized by the appropriate receptors in the intact organism.
Many investigators have noted that the immunological response of mice can be quite different from that of humans (Eisenhauer and Lehrer, 1992; Mestas and Hughes, 2004; Zollner et al., 1997), and humans could respond quite differently from each other. In our study neutrophils were obtained from >10 human donors of quite different genetic backgrounds. Despite this diversity, in every case, the neutrophils were stimulated by β-1,6-glucan and not β-1,3-glucan. The differential effects of β-1,6-glucan and β-1,3-glucan on distinct elements of the innate immune system offers the clinical potential of directed stimulation of neutrophils.
EXPERIMENTAL PROCEDURES
Preparation of Candida
The Candida albicans strain was the commonly used laboratory strain CAF2-1. Cells were grown on standard media (YPD), as described (Sherman, 1991).
We used overnight cultures in all experiments (about 3×108 cells/ml), because we found the Candida population to be more homogenous (contain >99% yeast form cells) than in mid logarithmic phase.
In order to test how neutrophils recognize Candida, and to avoid any alteration of the fungus by media or neutrophils or manipulation of neutrophils by the fungus, we inactivated the Candida cells by UV, which kills the cells but does not alter the fungal cell wall structure (Wheeler and Fink, 2006). When indicated, Candida was heat killed as described (Wheeler and Fink, 2006).
Opsonization of Candida and beads
A pool of fresh human serum was generated from ten healthy volunteers, and was used in all experiments. Candida cells or beads were pre-opsonized in 50% serum in Phosphate-Buffered Saline without calcium chloride and without magnesium chloride (PBS) (Gibco) for 15 minutes at 37 °C on a mixer. Cells or beads were then incubated on ice for 5 minutes, washed twice with 0.04 mg/ml of the protease inhibitor AEBSF (Sigma) in PBS, and then washed twice with PBS without AEBSF. Cells or beads were recounted after this treatment.
Coincubation of neutrophils with Candida or beads
Neutrophils were mixed with Candida or beads at ratio of 1:5 neutrophil: target. Neutrophils were cultured with opsonized Candida or beads or alone in RPMI1640 at 37 °C for 2 hours. For RNA extraction, the neutrophils were frozen in TRI reagent (MRC) at −80 °C.
Microarray procedure
Total RNA was prepared following the TRI reagent protocol, except that for RNA precipitation it was incubated with isopropanol overnight at 4°C. First and second strand synthesis, in-vitro transcription, hybridization, and scanning were done as described before (Rubin-Bejerano et al., 2003). The microarrays were GeneChip Human Genome U133A 2.0 array (Affymetrix).
Microarray analysis
Data sets were normalized and floored to 20. Ratios of expression from neutrophils cultured with Candida divided by that from the neutrophils alone control were calculated. Induced and repressed genes were defined as those with an expression ratio greater than two standard deviations from the mean for a given experiment. Only genes that were consistently induced or repressed in two experimental duplicates were considered as induced or repressed.
Quantitative Real Time- PCR (RT-PCR)
Total RNA was prepared following the TRI reagent protocol. cDNA was created using High Capacity cDNA Archive Kit (Applied Biosystems). Quantitative RT-PCR was done using TaqMan® Gene Expression Assays (Applied Biosystems) and 7500 Real Time PCR system (Applied Biosystems), following the manufacturer protocol. The following TaqMan® Gene Expression Assays were used: ACTB (Hs99999903_m1), DNAJB1 (Hs00428680_m1), HSPCB (Hs00607336_gH), and HSPH1 (Hs00198379_m1). Fold induction was calculated as the ratio of expression from neutrophils cultured with Candida over a neutrophil alone control, or neutrophils cultured with carbohydrate-coated beads over neutrophils cultured with untreated beads.
Carbohydrates
β-1,6-glucan was extracted from Candida albicans as described (Roemer et al., 1994). β-1,3-glucan was extracted from Candida albicans by digesting β-glucan (Roemer et al., 1994) with Chitinase (Sigma) and endo β-1,6-glucanase (Biomarin Pharmaceutical, Inc).
Laminarin (Sigma) is the standard for β-1,3-glucan, and pustulan (Calbiochem) is the standard for β-1,6-glucan (Hellerqvist et al., 1968; Lindberg and McPherson, 1954). Dextran (Fluka) is an α-1,6-glucan. Pustulan was cleaned as described (Lindberg and McPherson, 1954). Glucan from barley (Sigma) is composed of β-1,3-glucan (30%), and β-1,4-glucan (40%). When indicated, pustulan was specifically digested using an endo β-1,6-glucanase (Lora et al., 1995), a kind gift from Dr. Nick Zecherle (Biomarin Pharmaceutical, Inc). The reaction products were analyzed by gel filtration and thin-layer chromatography.
Gel filtration chromatography
20mg Pustulan was applied to a BioGel P6 column (1.5×120 cm, BioRad, 200–400 mesh). The column was equilibrated in 0.1 M acetic acid and run at a constant flow rate of 15 ml/h, 1.5 ml fractions were collected and carbohydrate was measured by the phenol-sulfuric acid method (Duboius et al., 1956). Fractions containing the peaks were dried twice for complete elimination of acetic acid and suspended in water at 10–20 mg/ml. Cytochrome C was used as a marker of the exclusion volume and glucose as a marker of the inclusion volume.
Thin-layer chromatography (TLC)
Samples (5 μl) were ascended twice on 20 cm silica gel 60 plates (Merck, 0.25 mm). The solvent system was n-butanol/ethanol/water (5:3:2). The samples and standards were visualized by heating the plates at 80 °C after spraying with phenol-sulfuric acid. Standard gentiooligosaccharides were prepared from partial acid hydrolysate of pustulan as described (Magnelli et al., 2002).
Deacetylation of pustulan
Pustulan (3 ml, 15 mg/ml) was adjusted to 0.1 M NaOH, incubated for 1 hour at 37 °C and dialyzed against water.
O-acetylation of laminarin
Laminarin (50mg) was dried, and resuspended in 1.5 ml of acetic anhydride (Mallindcrodt). A few crystals of 4-dimethylaminopyridine (Avocado Research Chemist, Ltd) were added as catalyst. The reaction was allowed to proceed at room temperature for 20 minutes and stopped with 2 volumes of water. The sample was dialyzed against water.
Preparation of glucan-coated beads
Polybead polystyrene 6 μm microspheres (Polysciences, Inc.) (beads) were coated with polysaccharides as described before (Schlesinger et al., 1994). Pustulan tends to solidify at room temperature. We solubilized it in boiling water, and let it cool down to room temperature before applying to beads. We detected coating of the beads by the phenol -sulfuric acid method, which measures carbohydrates (Duboius et al., 1956). Only beads with 6–15 μg glucose per 1 ml (2×108 beads) were included in the analysis. We note that short polysaccharides did not coat the beads efficiently by this method. Moreover, by this method, acetylated glucans were coating the beads better than unacetylated glucans. When not acetylated, more of the glucan was added to the beads to achieve similar levels of coating.
Time-lapse microscopy
Beads coated with an equivalent amount of pustulan or laminarin were opsonized and cultured with neutrophils for 40 minutes at 37 °C. Images were taken every 10 seconds using a Nikon Eclipse TE-300, with a 100x DIC objective.
Reactive oxygen species (ROS) assay
ROS production was assayed using DHR123 (Molecular Probes), which becomes fluorescent (rhodamine123) when oxidized (Conrads et al., 1999). 5×106 neutrophils were cultured with indicated beads at ratio of 1:5 in volume of 1 ml for 15 minutes at 37 °C. 1 μl of DHR123 (D-23806) was added to 200 μl of the culture. Following incubation at room temperature for 30 minutes the cells were assayed by Fluorescence Activated Cell Sorting (FACS). In Fig. 3, 5, and 6, an overlay of the signal from highly phagocytic cells is presented.
Identification of serum proteins binding β-1,6-glucan
Beads were coated with equivalent amounts of β-1,3-glucan and β-1,6-glucan, and opsonized. Beads were suspended in 2% SDS 1M ammonium hydroxide buffer, and incubated at 37 °C for 1 hour. The supernatant representing proteins extracted from beads was loaded on 4–20% acrylamide SDS gel. The gel was stained with silver stain, and bands were cut for analysis by mass spec.
Western analysis of C3
C3 deposition was assayed using monoclonal antibodies directed against the alpha or the beta chain of C3b (RDI Reasearch Diagnostics).
Killing assay
Viability of Candida albicans was determined using XTT as described before (Meshulam et al., 1995).
Preincubation experiments
Serum was preincubated with equivalent amount of soluble laminarin or pustulan for 5 minutes at 37 °C. The serum was then used to opsonize pustulan-coated beads as described above.
CR3 blocking Ab
Neutrophils were preincubated with anti human Mac-1 or isotype control IgG (Bender Med Systems) for 30 minutes on ice before adding to opsonized pustulan-coated beads.
Accesion Numbers
Microarray data were loaded into ArrayExpress with accession number E-MEXP-914.
Supplementary Material
Acknowledgments
We thank Phillips Robbins, Hidde Ploegh, and Iain Fraser for many valuable discussions, Glenn Brown for advice on opsonization, Glenn Paradis for technical assistance with FACS, Eric Spooner for mass spectrometry, Nicki Watson for advice on time-lapse microscopy, and members of the Fink lab for their many valuable contributions. This work was conducted utilizing the W.M. Keck Foundation Biological Imaging Facility at WI. This work was supported by National Institute of Health RO1 Grants GM 35010, and GM 40266 (to G.R.F.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babior BM, Kipnes RS, Curnutte JT. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- Cain JA, Newman SL, Ross GD. Role of complement receptor type three and serum opsonins in the neutrophil response to yeast. Complement. 1987;4:75–86. doi: 10.1159/000463011. [DOI] [PubMed] [Google Scholar]
- Conrads G, Herrler A, Moonen I, Lampert F, Schnitzler N. Flow cytometry to monitor phagocytosis and oxidative burst of anaerobic periodontopathogenic bacteria by human polymorphonuclear leukocytes. J Periodontal Res. 1999;34:136–144. doi: 10.1111/j.1600-0765.1999.tb02234.x. [DOI] [PubMed] [Google Scholar]
- Duboius M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Anal Biochem. 1956;28:350–356. [Google Scholar]
- Eisenhauer PB, Lehrer RI. Mouse neutrophils lack defensins. Infect Immun. 1992;60:3446–3447. doi: 10.1128/iai.60.8.3446-3447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati G, Bucknall RC, Edwards SW. Insoluble and soluble immune complexes activate neutrophils by distinct activation mechanisms: changes in functional responses induced by priming with cytokines. Ann Rheum Dis. 2002;61:13–19. doi: 10.1136/ard.61.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin C, Mavor AL, Weindl G, Schaller M, Hanke K, Kaufmann SH, Mollenkopf H, Hube B. The Early Transcriptional Response of Human Granulocytes to Infection with Candida albicans Is Not Essential for Killing but Reflects Cellular Communications. Infect Immun. 2007;75:1493–1501. doi: 10.1128/IAI.01651-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaither TA, Vargas I, Inada S, Frank MM. The complement fragment C3d facilitates phagocytosis by monocytes. Immunology. 1987;62:405–411. [PMC free article] [PubMed] [Google Scholar]
- Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. Embo J. 2005;24:1277–1286. doi: 10.1038/sj.emboj.7600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas KM, Toapanta FR, Oliver JA, Poe JC, Weis JH, Karp DR, Bower JF, Ross TM, Tedder TF. Cutting edge: C3d functions as a molecular adjuvant in the absence of CD21/35 expression. J Immunol. 2004;172:5833–5837. doi: 10.4049/jimmunol.172.10.5833. [DOI] [PubMed] [Google Scholar]
- Hellerqvist C, Lindberg GB, Samuelsson K. Methylation analysis of pustulan. Acta Chem Scand. 1968;22:2736–2737. [Google Scholar]
- Hong F, Hansen RD, Yan J, Allendorf DJ, Baran JT, Ostroff GR, Ross GD. Beta-glucan functions as an adjuvant for monoclonal antibody immunotherapy by recruiting tumoricidal granulocytes as killer cells. Cancer Res. 2003;63:9023–9031. [PubMed] [Google Scholar]
- Hong F, Yan J, Baran JT, Allendorf DJ, Hansen RD, Ostroff GR, Xing PX, Cheung NK, Ross GD. Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J Immunol. 2004;173:797–806. doi: 10.4049/jimmunol.173.2.797. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Travers P, Walport M, Shlomchik M. Immunobiology. 5. Garland Publishing; New York, N.Y: 2001. Innate immunity. [Google Scholar]
- Kapteyn JC, Van Den Ende H, Klis FM. The contribution of cell wall proteins to the organization of the yeast cell wall. Biochim Biophys Acta. 1999;1426:373–383. doi: 10.1016/s0304-4165(98)00137-8. [DOI] [PubMed] [Google Scholar]
- Klis FM, de Groot P, Hellingwerf K. Molecular organization of the cell wall of Candida albicans. Med Mycol. 2001;39(Suppl 1):1–8. [PubMed] [Google Scholar]
- Klis FM, Mol P, Hellingwerf K, Brul S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol Rev. 2002;26:239–256. doi: 10.1111/j.1574-6976.2002.tb00613.x. [DOI] [PubMed] [Google Scholar]
- LeBlanc BW, Albina JE, Reichner JS. The effect of PGG-beta-glucan on neutrophil chemotaxis in vivo. J Leukoc Biol. 2006;79:667–675. doi: 10.1189/jlb.0305150. [DOI] [PubMed] [Google Scholar]
- Lindberg B, McPherson J. Studies on the chemistry of lichens. VI. The structure of pustulan. Acta Chem Scand. 1954;8:985–988. [Google Scholar]
- Lora JM, De la Cruz J, Llobell A, Benitez T, Pintor-Toro JA. Molecular characterization and heterologous expression of an endo-beta-1,6-glucanase gene from the mycoparasitic fungus Trichoderma harzianum. Mol Gen Genet. 1995;247:639–645. doi: 10.1007/BF00290356. [DOI] [PubMed] [Google Scholar]
- Magnelli P, Cipollo JF, Abeijon C. A refined method for the determination of Saccharomyces cerevisiae cell wall composition and beta-1,6-glucan fine structure. Anal Biochem. 2002;301:136–150. doi: 10.1006/abio.2001.5473. [DOI] [PubMed] [Google Scholar]
- McGreal EP, Miller JL, Gordon S. Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr Opin Immunol. 2005;17:18–24. doi: 10.1016/j.coi.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshulam T, Levitz SM, Christin L, Diamond RD. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanil ide (XTT) J Infect Dis. 1995;172:1153–1156. doi: 10.1093/infdis/172.4.1153. [DOI] [PubMed] [Google Scholar]
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Nagarsekar A, Hasday JD, Singh IS. CXC chemokines: A new family of heat-shock proteins? Immunol Invest. 2005;34:381–398. doi: 10.1081/imm-200067648. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Tanaka M, Shibata S, Fukuoka F. Polysaccharides of lichens and fungi. IV. Antitumour active O-acetylated pustulan-type glucans from the lichens of Umbilicaria species. Chem Pharm Bull (Tokyo) 1970;18:1431–1434. doi: 10.1248/cpb.18.1431. [DOI] [PubMed] [Google Scholar]
- Palma AS, Feizi T, Zhang Y, Stoll MS, Lawson AM, Diaz-Rodriguez E, Campanero-Rhodes MA, Costa J, Gordon S, Brown GD, Chai W. Ligands for the beta-glucan receptor, Dectin-1, assigned using “designer” microarrays of oligosaccharide probes (neoglycolipids) generated from glucan polysaccharides. J Biol Chem. 2006;281:5771–5779. doi: 10.1074/jbc.M511461200. [DOI] [PubMed] [Google Scholar]
- Prohaszka Z, Fust G. Immunological aspects of heat-shock proteins-the optimum stress of life. Mol Immunol. 2004;41:29–44. doi: 10.1016/j.molimm.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Reynolds JA, Kastello MD, Harrington DG, Crabbs CL, Peters CJ, Jemski JV, Scott GH, Di Luzio NR. Glucan-induced enhancement of host resistance to selected infectious diseases. Infect Immun. 1980;30:51–57. doi: 10.1128/iai.30.1.51-57.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer T, Paravicini G, Payton MA, Bussey H. Characterization of the yeast (1-->6)-beta-glucan biosynthetic components, Kre6p and Skn1p, and genetic interactions between the PKC1 pathway and extracellular matrix assembly. J Cell Biol. 1994;127:567–579. doi: 10.1083/jcb.127.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross GD. Analysis of the different types of leukocyte membrane complement receptors and their interaction with the complement system. J Immunol Methods. 1980;37:197–211. doi: 10.1016/0022-1759(80)90307-5. [DOI] [PubMed] [Google Scholar]
- Ross GD, Cain JA, Lachmann PJ. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985;134:3307–3315. [PubMed] [Google Scholar]
- Ross GD, Cain JA, Myones BL, Newman SL, Lachmann PJ. Specificity of membrane complement receptor type three (CR3) for beta-glucans. Complement. 1987;4:61–74. doi: 10.1159/000463010. [DOI] [PubMed] [Google Scholar]
- Rubin-Bejerano I, Fraser I, Grisafi P, Fink GR. Phagocytosis by neutrophils induces an amino acid deprivation response in Saccharomyces cerevisiae and Candida albicans. Proc Natl Acad Sci U S A. 2003;100:11007–11012. doi: 10.1073/pnas.1834481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- Sato T, Iwabuchi K, Nagaoka I, Adachi Y, Ohno N, Tamura H, Seyama K, Fukuchi Y, Nakayama H, Yoshizaki F, et al. Induction of human neutrophil chemotaxis by Candida albicans-derived beta-1,6-long glycoside side-chain-branched beta-glucan. J Leukoc Biol. 2006;80:204–211. doi: 10.1189/jlb.0106069. [DOI] [PubMed] [Google Scholar]
- Schlesinger LS, Hull SR, Kaufman TM. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol. 1994;152:4070–4079. [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Slaney JM, Gallagher A, Aduse-Opoku J, Pell K, Curtis MA. Mechanisms of resistance of Porphyromonas gingivalis to killing by serum complement. Infect Immun. 2006;74:5352–5361. doi: 10.1128/IAI.00304-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton BP, Vetvicka V, Pitman M, Goldman RC, Ross GD. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18) J Immunol. 1996;156:1235–1246. [PubMed] [Google Scholar]
- Tsikitis VL, Albina JE, Reichner JS. Beta-glucan affects leukocyte navigation in a complex chemotactic gradient. Surgery. 2004;136:384–389. doi: 10.1016/j.surg.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Tzianabos AO, Gibson FC, 3rd, Cisneros RL, Kasper DL. Protection against experimental intraabdominal sepsis by two polysaccharide immunomodulators. J Infect Dis. 1998;178:200–206. doi: 10.1086/515594. [DOI] [PubMed] [Google Scholar]
- Vetvicka V, Thornton BP, Ross GD. Soluble beta-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J Clin Invest. 1996;98:50–61. doi: 10.1172/JCI118777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RT, Fink GR. A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog. 2006;2:e35. doi: 10.1371/journal.ppat.0020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Vetvicka V, Yan J, Hanikyrova M, Mayadas T, Ross GD. The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J Immunol. 1999;162:2281–2290. [PubMed] [Google Scholar]
- Zheng L, He M, Long M, Blomgran R, Stendahl O. Pathogen-induced apoptotic neutrophils express heat shock proteins and elicit activation of human macrophages. J Immunol. 2004;173:6319–6326. doi: 10.4049/jimmunol.173.10.6319. [DOI] [PubMed] [Google Scholar]
- Zollner O, Lenter MC, Blanks JE, Borges E, Steegmaier M, Zerwes HG, Vestweber D. L-selectin from human, but not from mouse neutrophils binds directly to E-selectin. J Cell Biol. 1997;136:707–716. doi: 10.1083/jcb.136.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


