Abstract
Mutations in the leucine-rich repeat kinase 2 (LRRK2) gene are the leading cause of autosomal dominant Parkinson’s disease (PD). LRRK2, a member of the ROCO protein family, contains both Ras GTPase-like (Roc) and kinase (MAPKKK) domains, as well as other functional motifs. Here, we have identified LRRK2 as the first mammalian ROCO protein that is an authentic and functional GTPase, defined by the ability to bind GTP and undergo intrinsic GTP hydrolysis. Furthermore, the Roc domain is sufficient for this native GTPase activity and binds and hydrolyzes GTP indistinguishably from the Ras-related small GTPase, Rac1. The PD-associated mutation, R1441C, located within the Roc domain, leads to an increase in LRRK2 kinase activity and a decrease in the rate of GTP hydrolysis, compared to the wild-type protein, in an in vitro assay. This finding suggests that the R1441C mutation may help stabilize an activated state of LRRK2. Additionally, LRRK2 mediated phosphorylation is stimulated upon binding of non-hydrolyzable GTP analogs, suggesting that LRRK2 is a MAPKKK activated intramolecularly by its own GTPase. Since GTPases and MAPKKKs are upstream regulators of multiple signal transduction cascades, LRRK2 may play a central role in integrating pathways involved in neuronal cell signaling and the pathogenesis of PD.
Keywords: Ieucine-rich repeat kinase 2, R1441C, Parkinson’s disease, Ras-related small GTP-binding protein, GTPase, MAPKKK
Introduction
Parkinson’s disease (PD)1 is a chronic and progressive neurodegenerative disorder affecting 1–2% of individuals aged 65 years and above. Pathological features of the disease include selective loss of dopaminergic neurons in the substantia nigra and the presence of cytoplasmic proteinaceous inclusions, termed Lewy bodies, in surviving neurons. The main clinical symptoms of PD include tremor, bradykinesia, rigidity and gait instability [1]. While the pathogenesis of PD remains unclear, mutations in several genes have been linked to rare familial forms of the disease including α-synuclein, parkin, DJ-1, UCH-L1 and PINK1 [2]. These genes implicate dysfunction in several signaling pathways as being potentially involved in disease pathogenesis, including those related to protein aggregation, the ubiquitin-proteosome system, mitochondrial function, maintenance of oxidative homeostasis, and protein phosphorylation [3].
Most recently, mutations in the leucine-rich repeat kinase 2 (LRRK2) gene have been shown to be the leading cause of autosomal dominantly inherited PD with pathological features resembling those seen in idiopathic forms of the disease [4–7]. Interestingly, a common LRRK2 mutation, G2019S, is also present in a significant fraction of idiopathic PD [8]. LRRK2, also known as dardarin, is a large 286 kDa protein of unknown function belonging to the ROCO protein family. ROCO family members are characterized by the presence of a Ras GTPase-like domain termed Roc (Ras of complex protein) immediately followed by a COR (C-terminal of Roc) domain [9]. LRRK2 contains additional domains including N-terminal leucine-rich repeats (LRRs), a mitogen-activated protein kinase kinase kinase (MAPKKK) domain and C-terminal WD40 repeats [9, 10]. To date, forty-seven LRRK2 mutations segregate with PD and of these mutations, several are known to be pathogenic: R1441C/G/H in the Roc domain, Y1699C in the COR domain, and G2019S and I2020T in the MAPKKK domain [11–14]. These LRRK2 mutations are the most common cause of Parkinson’s disease identified to date, establishing the study of LRRK2 as a crucial component in understanding and combating the pathogenesis of PD. The reported finding that LRRK2 is a component of Lewy bodies in post-mortem brains of PD patients further highlights the role of LRRK2 in disease pathogenesis [15, 16].
LRRK2 contains a Roc domain that shares sequence homology with all five subfamilies of the Ras-related small GTPase superfamily (Ras, Rho, Rab, Sar/Arf and Ran) including conservation of amino acids involved in GTP-binding and hydrolysis [9]. Ras-related GTPases serve as molecular switches to regulate diverse cellular functions by cycling between GTP-bound (active) and GDP-bound (inactive) conformations. Several guanine nucleotide binding regulatory proteins, specific to each subfamily of GTPases, exist to facilitate GTP-binding and hydrolysis. For example, guanine nucleotide exchange factors (GEFs) facilitate GTP-binding and subsequent effector interaction and downstream signaling, GTPase activating proteins (GAPs) increase the intrinsic rate of GTP hydrolysis to terminate signaling, and GDP-dissociation inhibitors (GDIs) stabilize an inactive GDP-bound pool of protein [17]. While recent studies have demonstrated the ability of LRRK2 to bind to GTP-agarose, studies illustrating GTP hydrolysis activity of this protein or other mammalian ROCO family members are lacking [18, 19]. Therefore, in the absence of GTP hydrolysis data it has been premature to refer to LRRK2 as a GTPase.
Based on phylogenetic analysis, the MAPKKK domain of LRRK2 resembles mixed lineage kinases, which are part of the tyrosine kinase-like branch of the human kinome. These kinases commonly have both Ser/Thr and Tyr kinase activity [20–22]. While neither a biological role nor an endogenous substrate has been assigned to LRRK2, the protein has been shown to confer kinase activity and several pathogenic mutations, located throughout the protein, increase LRRK2 kinase activity [18, 19, 23, 24]. This “gain of function” phenotype is consistent with the autosomal dominant inheritance of LRRK2 mutations in familial PD.
Interestingly, MAPKKKs are known effectors of Ras-related GTPases. For example, a plasma membrane receptor/Ras complex directly activates the MAPKKK, B-Raf, thereby relaying an extracellular signal that activates a cytosolic phosphorylation cascade ultimately resulting in changes in gene expression [25]. The presence of both a Ras GTPase-like (Roc) domain and a MAPKKK domain within LRRK2 raises the exciting possibility of intramolecular signaling in which the activated (GTP-bound) GTPase directly stimulates kinase activity within the same protein. Recently, it was demonstrated that LRRK2 kinase activity is stimulated upon GTP binding [18].
In this study, we have demonstrated that LRRK2 is a protein with dual enzymatic properties, displaying both GTPase and kinase activities. This is the first report, to our knowledge, identifying LRRK2 as an authentic GTPase, which by definition is a protein that can bind GTP and undergo intrinsic GTP hydrolysis. Furthermore, we have demonstrated that the Roc domain of LRRK2 is sufficient for this intrinsic GTPase activity and binds and hydrolyzes GTP indistinguishably from the Ras-related small GTPase, Rac1. This is also the first report, to our knowledge, that the isolated Roc domain functions similarly to other Ras-related small GTPases. Based on in vitro assays, the PD-associated pathogenic mutation, R1441C, found within the Roc domain, leads to an increase in LRRK2 kinase activity as well as a decrease in the rate of GTP hydrolysis compared to the wild-type protein. These results suggest that the R1441C pathogenic mutation may help stabilize an activated state of LRRK2. Additionally, we have confirmed that LRRK2 mediated phosphorylation is stimulated upon GTP binding, suggesting that LRRK2 is a unique kinase activated intramolecularly by its own GTPase. Typically, GTPases and MAPKKKs are upstream regulators of multiple signal transduction cascades, which may indicate that LRRK2 plays a central role in integrating pathways involved in neuronal cell signaling. Perturbation to this signaling, due to LRRK2 mutations, may therefore lead to the pathogenesis of PD.
Materials and methods
Plasmids and cloning
Construction of LRRK2-FLAG constructs: A cDNA clone (TC124162) from a human cDNA library encoding full-length LRRK2 was isolated, sequenced and subcloned into the plasmid pCMV6-XL4 by OriGene Technologies (Rockville, MD). A C-terminal FLAG (DYKDDDDK) epitope tag was added to this cDNA by shuttling from pCMV6-XL4 into the mammalian expression plasmid pCEP4 (Invitrogen) to produce LRRK2(WT)-FLAG:pCEP4. The R1441C and T1348N mutations were introduced into LRRK2(WT)-FLAG:pCEP4 by site-directed mutagenesis using the QuikChange II XL mutagenesis kit (Stratagene), according to the manufacturer’s instructions, to produce LRRK2(R1441C)-FLAG:pCEP4 and LRRK2(T1348N)-FLAG:pCEP4, respectively. The integrity of the cDNA open reading frames was confirmed by DNA sequencing (Genomics Core Facility of Case Western Reserve University, Cleveland, OH).
Construction of GST-Roc constructs: The isolated Roc domain of LRRK2 was constructed by PCR using LRRK2(WT)-FLAG:pCEP4 as a template with the following primers; 5’-GCA GGA TCC ATG CGA ATG AAA CTT ATG ATT GTG GGA-3’ and 5’-TAA CCC GGG TCA GAA ATT AAG GCT CTC-3’. This region corresponds to amino acids 1334–1512 of LRRK2 (GenBank accession no. AY792511). cDNA encoding the Roc domain of LRRK2 and cDNA encoding Rac1, used as a positive control, were subcloned C-terminally into the GST-fusion bacterial expression plasmid pGEX-KT to produce GST-Roc(WT):pGEX-KT and GST-Rac1(WT):pGEX-KT, respectively [26]. The mutations K1347A, R1441C and R1441G were introduced into GST-Roc(WT):pGEX-KT by site-directed mutagenesis using overlapping extension PCR to produce GST-Roc(K1347A):pGEX-KT, GST-Roc(R1441C):pGEX-KT and GST-Roc(R1441G):pGEX-KT, respectively. The integrity of all PCR products was confirmed by DNA sequencing (Davis Sequencing, Davis, CA).
Expression of recombinant GST-fusion proteins
Recombinant protein expression was induced in BL21 codon plus DES (RIL) Escherichia coli (Stratagene) with 1mM isopropyl β-D-thiogalactoside (IPTG). Bacteria were pelleted at 5,000×g for 10min at 4°C and lysed on ice in lysis buffer (100mM Hepes, pH 7.4; 2.5mM MgCl2; 0.1mM DTT; 100µM GTP; complete protease inhibitors (Sigma)) by sonication. Sonicated lysates were pelleted at 10,000×g for 15min at 4°C and the supernatants filtered through 0.22µm filters resulting in a cleared protein lysate that was stored with 20% glycerol at −80°C. GST-fusion protein expression was verified by resolving on 12.5% Tris-glycine SDS-PAGE, electrophoretically transferring to polyvinylidine difluoride (PVDF) membrane (Millipore), and analyzing by Western blot with anti-GST antibody (Novagen).
Cell culture and transfection
Human embryonic kidney 293T (HEK293T) cells were cultured in DMEM (Invitrogen) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin at 37°C in 5% CO2. Cells were transiently transfected with various pCEP4 constructs in serum free media using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Cells were approximately 80% confluent prior to transfection and harvested 40–48hrs post-transfection.
LRRK2 immunoprecipitation
HEK293T cells transiently transfected with LRRK2 constructs were washed with Dulbecco’s phosphate buffered saline (DPBS), without calcium and magnesium, and then harvested in ice-cold lysis buffer (1% Triton X-100 in DPBS; complete mini EDTA-free protease inhibitor (Roche)). Cells were lysed on ice for 30min followed by centrifugation at 14,000×g for 10min at 4°C to remove cellular debris. Cleared supernatants were incubated overnight at 4°C with either anti-FLAG M2 affinity agarose (Sigma) or anti-LRRK2 antibody p268 (Novus Biologicals) conjugated to tosylactivated Dynabeads (Invitrogen). Beads were pelleted and washed three times with cold wash buffer (0.5% Triton X-100 in DPBS; complete protease inhibitors (Roche)) and once with ice-cold DPBS. Immunoprecipitates were collected and an aliquot was heated at 95°C for 10min in SDS sample buffer (3% SDS; 2mM EDTA; 10% glycerol; 50mM Tris-HCl, pH 6.8), and resolved by Novex 6% Tris-glycine SDS-PAGE. For the detection of LRRK2 by Western blotting, proteins were electrophoretically transferred to PVDF membrane and analyzed with anti-FLAG M2 antibody (Stratagene) to recognize the FLAG epitope or anti-LRRK2 antibody p268 (Novus Biologicals) to recognize a C-terminal epitope of human LRRK2 (residues 2500–2527).
In vitro kinase assay
LRRK2 immunoprecipitates were incubated in kinase assay buffer (20mM Hepes, pH 7.4; 15mM MgCl2; 5mM EGTA; 20mM β-glycerol phosphate) containing 20µM ATP, 1µCi[γ-32P]ATP (4,500Ci/mmol) and 2µg of myelin basic protein (MBP) (Sigma), as a generic substrate, at 30°C for 15min. Kinase reactions were terminated by addition of SDS sample buffer followed by immediate boiling at 95°C for 10min. Proteins were resolved by Novex 6% Tris-glycine SDS-PAGE for LRRK2 detection and 12% Tris-glycine SDS-PAGE for MBP detection and phosphorylation. Proteins were then electrophoretically transferred to PVDF membrane and phosphorylation was detected by autoradiography on Kodak BioMax MS film. PVDF membranes were also subjected to Western blotting with anti-FLAG antibody for LRRK2 detection. Gels were subjected to Coomassie blue staining for MBP detection. To determine the effect of GTP-binding on LRRK2 kinase activity immunoprecipitates were preincubated in the presence of 5µM non-hydrolyzable GTP analogs, guanosine 5’-(β,γ)-imidotriphosphate (Gpp(NH)p) or GTPγS, at 30°C for 15min, followed by the in vitro kinase assay with MBP as described above. Autoradiographs of 32P incorporated protein were quantified by computer-assisted densitometry using UN-SCAN-IT software (Silk Scientific, Inc, Orem, UT).
GTP-binding and hydrolysis of full length LRRK2
For GTP binding experiments, LRRK2 immunoprecipitates were preincubated in the presence of 10mM unlabeled CTP or GTP (Sigma), as competing nucleotides, for 10min at 23°C. Subsequently, immunoprecipitates were incubated with 1µCi [α-32P]GTP (3,000Ci/mmol) for 30min at 23°C followed by addition of 20mM MgCl2 to stabilize and prevent dissociation of already bound nucleotide, as has been demonstrated for other Ras-related GTPases [27, 28]. Binding was terminated by rapid filtration and washing with cold PBS containing 20mM MgCl2, three times, through 0.45µm microcentrifuge filter units (Millipore). Scintillation cocktail was added to the samples and radioactivity associated with LRRK2 immunoprecipitates was quantified in a Beckman LS-100 liquid scintillation counter.
For thin-layer chromatography (TLC) based GTP hydrolysis assays, LRRK2 immunoprecipitates were preincubated in the presence of 100nM [α-32P]GTP (3,000Ci/mmol) in 50mM Tris-HCl, pH 7.5 for 10min at room temperature. The reaction was initiated by addition of MgCl2 to a final concentration of 10mM. At 0, 3, 10, 30 and 60min time points the reaction was terminated by mixing with an equal volume of stop solution containing 0.2% (v/v) SDS, 2mM EDTA, 1mM GDP and 1mM GTP. The mixture was immediately vortexed and stored on dry ice. The frozen samples were heated at 90°C for 5min, cooled down and spotted onto polyethleneimine-cellulose TLC plates (Selecto Scientific). Plates were developed in 0.75M potassium phosphate buffer, pH 3.4, dried and subjected to autoradiography on Kodak Biomax MS film. Autoradiographs of TLC-separated [α-32P]GDP were quantified by computer-assisted densitometry using Quantity One software (Bio-Rad, Hercules, CA).
Infinite dilution and rapid filtration GTP hydrolysis experiments were performed similarly as described previously [27, 28]. Briefly, immunoprecipitated LRRK2 was incubated with 1.5µCi [γ-32P]GTP (4,500Ci/mmol) in assay buffer (100mM Tris-HCl, pH 7.5; 1mM DTT; 2mM EDTA; 10mM CTP; 100mM NaCl) for 20min at 23°C. 10mM unlabeled GTP and 20mM MgCl2 were then added to infinitely dilute the radioactive nucleotide, therefore decreasing the possibility of further binding, and to stabilize and prevent dissociation of the already bound nucleotide, respectively [27, 28]. Equivalent aliquots were removed at 0, 3, 10, 30 and 60min time points. Reactions were diluted in 10ml ice-cold dilution buffer (20mM Tris-HCl, pH 7.5; 100mM NaCl; 10mM MgCl2) and rapidly filtered through nitrocellulose filter discs on a Millipore model 1225 sampling manifold. Nitrocellulose filters were washed in 40ml ice-cold dilution buffer and radioactivity bound to the filters was quantified by liquid scintillation in a Beckman LS-100 liquid scintillation counter. Rate constants of GTP dissociation were calculated by semi-logarithmic plot with the following exponential equation: y=cebx.
GTP-binding and hydrolysis of the Roc domain of LRRK2
Recombinant GST-fusion proteins were purified from bacterial lysates using glutathione agarose (Sigma) according to the manufacturer’s instructions. TLC based GTP hydrolysis experiments were performed as described above with approximately 0.2µg of GST-fusion protein assayed per time point (0, 3, 10 and 30mins). Infinite dilution and rapid filtration GTP binding and hydrolysis experiments were performed similarly as described above. Briefly, for GTP binding experiments, approximately 2µg of purified GST-fusion proteins were incubated with 1.5µCi [α-32P]GTP (4,500Ci/mmol) in the absence or presence of 10mM unlabeled GTP in assay buffer (100mM Tris-HCl, pH 7.5; 1mM DTT; 10mM EDTA; 10mM ATP; 100mM NaCl) for 20min at 23°C. For GTP hydrolysis experiments approximately 2µg of GST-fusion proteins were incubated with 1.5µCi [γ-32P]GTP (4,500Ci/mmol) in assay buffer as described above. In both cases, after 20min incubation, 10mM unlabeled GTP and 20mM MgCl2 were added to infinitely dilute the radioactive nucleotide, therefore decreasing the possibility of further binding, and to stabilize and prevent dissociation of the already bound nucleotide, respectively [27, 28]. For GTP binding experiments the entire reaction was diluted in 10ml ice-cold dilution buffer (20mM Tris-HCl, pH 7.5; 100mM NaCl; 10mM MgCl2) and rapidly filtered through nitrocellulose filter discs on a Millipore model 1225 sampling manifold, while equivalent aliquots were removed at 0, 3, 10 and 30min time points for the GTP hydrolysis experiments. Nitrocellulose filters were washed in 40ml ice-cold dilution buffer and bound radioactivity was quantified by liquid scintillation.
Results
The R1441C PD-associated mutation augments LRRK2 kinase activity
To study the enzymatic properties of LRRK2, we generated C-terminal FLAG-tagged cDNA of the full-length LRRK2 open reading frame in the pCEP4 mammalian expression plasmid. Fig. 1A is a schematic representation of the multi-domain structure of LRRK2. The protein contains several functional domains including N-terminal leucine-rich repeats (LRRs), a Roc (Ras of complex protein) domain, a COR (C-terminal of Roc) domain, a mitogen-activated protein kinase kinase kinase (MAPKKK) domain and C-terminal WD40 repeats [9]. The locations of various amino acid substitutions (K1347A, T1348N, R1441C/G) utilized in this study are also indicated (Fig. 1A).
Fig. 1. The R1441C PD-associated mutation augments LRRK2 kinase activity.
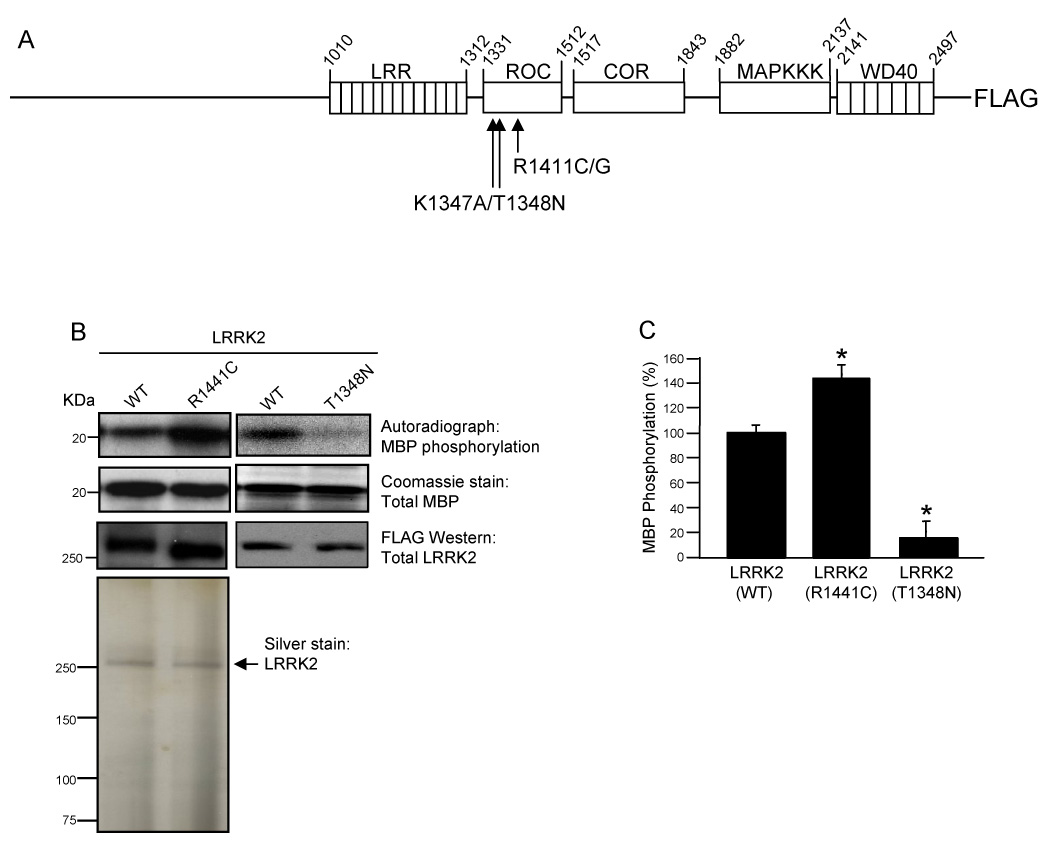
(A) Schematic diagram of LRRK2 multi-domain structure. LRRK2 contains several functional domains including N-terminal leucine-rich repeats (LRRs), a Roc (Ras of complex protein) domain, a COR (C-terminal of Roc) domain, a mitogen-activated protein kinase kinase kinase (MAPKKK) domain and C-terminal WD40 repeats. Full-length wild-type and mutant LRRK2 proteins were cloned in frame into a modified pCEP4 vector containing a C-terminal FLAG epitope tag. Arrows indicate the location of the K1347A, T1348N and R1441C/G mutations, within the Roc domain. (B) Phosphorylation of MBP by LRRK2. Autoradiographs show incorporated 32P in MBP resolved by 12% Tris-glycine SDS-PAGE. MBP levels were determined by Coomassie staining. Immunoprecipitated LRRK2 was resolved by Novex 6% SDS-PAGE and LRRK2 protein levels were examined by anti-FLAG Western blot analysis. The purity of LRRK2 immunoprecipitates was analyzed by silver staining. (C) Quantification of phosphorylation of MBP by LRRK2. Autoradiographs of 32P incorporated MBP were quantified by densitometry and normalized to LRRK2 protein levels. Wild-type LRRK2 phosphorylation activity was set to 100%. Error bars for part C represent standard error of the mean (SEM) for three independent experiments. * indicates p<0.01 compared to wild-type LRRK2, assessed by a two-tailed unpaired Student’s t-test.
The MAPKKK domain of LRRK2 displays similarity to mixed lineage kinases that commonly have both Ser/Thr and Tyr kinase activity [20]. While an endogenous substrate for LRRK2 is unknown, several reports have demonstrated that LRRK2 immunoprecipitated from mammalian cells is a functional kinase, capable of undergoing both auto-phosphorylation and trans-phosphorylation of the generic substrate MBP [23, 24]. Several reports have also indicated that various PD-associated pathogenic mutations, found throughout LRRK2, increase kinase activity [18, 19, 23, 24].
We were particularly interested in the pathogenic R1441C mutation of LRRK2 because it is one of the most common mutations linked to PD and it occurs within the Roc domain, which is likely a potential regulator of the MAPKKK domain. To determine how the R1441C mutation affects LRRK2 kinase activity we employed an in vitro kinase assay. Briefly, FLAG-tagged wild-type and mutant LRRK2 proteins were overexpressed in HEK293T cells and proteins were immunoprecipitated from cell lysates utilizing anti-FLAG M2 affinity agarose. The kinase reaction was initiated by addition of [γ-32P]ATP and MBP (as a generic substrate). The autoradiograph of SDS-PAGE separated proteins demonstrated an increase in MBP phosphorylation by LRRK2(R1441C) relative to wild-type LRRK2 (Fig. 1B). The LRRK2(T1348N) mutant, previously shown to be defective in guanine nucleotide binding and kinase activity, was essentially inactive in regards to MBP phosphorylation (Fig. 1B) [30]. Utilization of the T1348N kinase dead mutant confirmed that the kinase activity displayed by LRRK2 immunoprecipitates is in fact due to LRRK2 and not to other potentially contaminating kinases [30]. The Coomassie stained gels and anti-FLAG Western blots verified that equivalent amounts of MBP and LRRK2 proteins, respectively, were utilized in the kinase assay (Fig. 1B). The silver stained gel of the immunoprecipitates utilized in the assays also demonstrated the apparent purity of LRRK2 preparations (Fig. 1B). The amount of phosphorylated MBP was quantified after normalizing to the amount of LRRK2 in each reaction (Fig. 1C). There is a statistically significant increase in MBP phosphorylation by LRRK2(R1441C) compared to the wild-type protein (Fig. 1C). These results confirm that LRRK2 is a functional kinase and that the R1441C PD-associated pathogenic mutation, found within the Roc domain, increases kinase activity [18, 19, 23]. The mechanism of increased LRRK2 kinase activation by the R1441C mutant, however, is unknown and may be regulated by GTP-binding and/or hydrolysis since this mutation occurs within the GTPase-like or Roc domain. To determine the functional consequence of the R1441C mutant in regards to GTPase activity, it was first necessary to characterize LRRK2 as a functional GTPase.
LRRK2 is a functional GTPase and the R1441C PD-associated mutation has a decreased rate of GTP hydrolysis
Alignment and sequence comparison of ROCO family proteins with members of the Ras-related GTPase superfamily reveal that Roc domains include many conserved motifs necessary for GTPase activity, which is defined as the ability to bind and intrinsically hydrolyze GTP [9]. While LRRK2 has recently been shown to bind GTP-agarose, studies demonstrating intrinsic GTP hydrolysis activity are lacking [18, 19]. Therefore, LRRK2 has yet to be definitively characterized as an authentic and functional GTPase.
To determine the specificity of LRRK2 for binding to guanine nucleotides, we performed [α-32P]GTP binding assays. Briefly, wild-type and mutant LRRK2 were overexpressed in HEK293T cells and the proteins were immunoprecipitated from cell lysates utilizing anti-LRRK2 coupled to Dynabeads. [α-32P]GTP, in the presence of competing nucleotides (unlabeled CTP or GTP), was added to the immunoprecipitates and allowed to bind. Reactions were rapidly filtered, washed and radioactivity associated with LRRK2 immunoprecipitates was quantified in a liquid scintillation counter. Both wild-type and R1441C mutant LRRK2 were able to bind radioactive GTP and this interaction was blocked by an excess of unlabeled GTP, but not CTP, indicating that the binding is specific for guanine nucleotides (Fig. 2A). The T1348N mutant of LRRK2, previously demonstrated to exist in a nucleotide free state, was used as a negative control (Fig. 2A) [30]. These results demonstrate that both wild-type LRRK2 and the PD-associated pathogenic mutant, R1441C, specifically bind GTP.
Fig. 2. LRRK2 is a functional GTPase and the R1441C PD-associated mutation has a decreased rate of GTP hydrolysis.
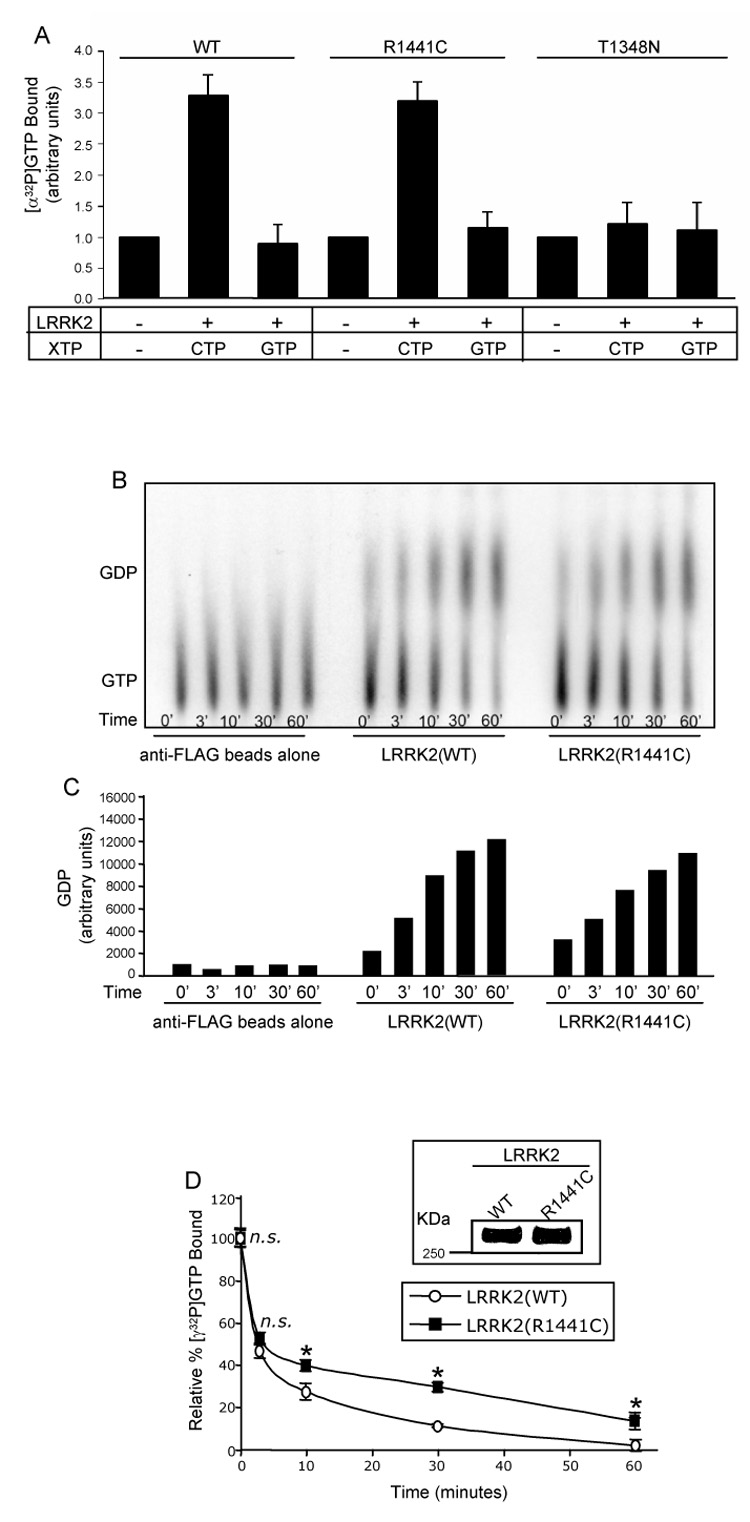
(A) GTP-binding to LRRK2. LRRK2-FLAG immunoprecipitates were assayed for [α-32P]GTP binding in the presence of competing nucleotides (unlabeled CTP or GTP). CPM of [α-32P]GTP bound were normalized to background levels of the negative control (set to 1, arbitrary units) and plotted. Negative control represents immunoprecipitates of anti-LRRK2 antibody coupled to Dynabeads prepared from untransfected cell lysates. Error bars represent SEM for three independent experiments. (B) GTP hydrolysis of LRRK2 analyzed by the TLC method. Immunoprecipitated wild-type and R1441C mutant LRRK2 were assayed for [α-32P]GTP hydrolysis at 0, 3, 10, 30 and 60mins by spotting on TLC plates. The anti-FLAG beads alone negative control represents immunoprecipitates of anti-FLAG beads prepared from untransfected cell lysates. The autoradiograph shown is representative of five independent experiments. (C) Quantification of GTP hydrolysis of LRRK2 analyzed by the TLC method. Amounts of GDP on the representative autoradiograph, shown in Fig. 2B, were quantified by densitometry. (D) GTP hydrolysis of LRRK2 analyzed by the infinite dilution and rapid filtration method. Inset shows anti-FLAG Western blot of immunoprecipitated LRRK2 proteins to demonstrate equivalent amounts of protein utilized in the GTP hydrolysis assay. Immunoprecipitated wild-type and R1441C mutant LRRK2 were assayed for GTP hydrolysis by incubation with [γ-32P]GTP and equivalent aliquots were removed at 0, 3, 10, 30 and 60mins. The relative percent of [γ-32P]GTP bound, after subtracting background binding to FLAG beads alone (negative control), were plotted. CPM of [γ-32P]GTP bound at time zero was set to 100%. Error bars represent SEM for one experiment performed in triplicate. Similar results were observed in two independent experiments performed in triplicate. * indicates p<0.01 and n.s. is non-significant compared to wild-type LRRK2, assessed by a two-tailed unpaired Student’s t-test.
The next step was to determine if LRRK2 undergoes intrinsic GTP hydrolysis, thereby rendering the protein a functional GTPase. We compared the GTP hydrolysis properties of wild-type versus R1441C mutant LRRK2 by two different methods, a TLC based method (Fig. 2B) as well as an infinite dilution and rapid filtration method (Fig. 2D). Briefly, FLAG-tagged wild-type and mutant LRRK2 were overexpressed in HEK293T cells and proteins were immunoprecipitated from cell lysates utilizing anti-FLAG M2 affinity agarose. For the TLC based assay, LRRK2 immunoprecipitates were incubated with [α-32P]GTP in the presence of MgCl2 and equivalent aliquots were removed at various time points, quenched and spotted onto TLC plates. The autoradiograph of a representative TLC plate, shown in Fig. 2B, demonstrates the loss of GTP and simultaneous accumulation of GDP over time as catalyzed by wild-type and R1441C mutant LRRK2, compared to the anti-FLAG beads alone (negative control). The accumulation of GDP over time can also be appreciated by quantification of the representative autoradiograph (Fig. 2C). These results demonstrate that both wild-type and R1441C mutant LRRK2 are authentic GTPases that readily catalyze the hydrolysis of GTP to GDP.
To more carefully and quantitatively compare rates of GTP hydrolysis we utilized an infinite dilution and rapid filtration method in which LRRK2 immunoprecipitates were incubated with [γ-32P]GTP and equivalent aliquots were removed at various time points. GTP hydrolysis was measured as a function of loss of radioactivity (γ-labeled 32P) associated with LRRK2 upon hydrolysis. To ensure that loss of radioactivity was due to hydrolysis, and not due to nucleotide dissociation, an excess of Mg2+ was added at time zero to stabilize and inhibit dissociation of bound GTP [28]. Immunoprecipitated LRRK2 was analyzed by an anti-FLAG Western blot to demonstrate that equivalent amounts of wild-type and R1441C mutant protein were utilized in the assay (Fig. 2D, inset). LRRK2(R1441C) displayed a statistically significant decrease in the rate of GTP hydrolysis at the 10, 30 and 60min time points compared to the wild-type protein, however, total initial binding of [γ-32P]GTP (time zero) was equivalent for both proteins (Fig. 2D). Based on the shape of the curves in Fig. 2D, two rate constants of GTP dissociation were determined by analyzing the GTP hydrolysis data by semi-logarithmic plots, one rate constant for the 0–3min time points and another for the 10–60min time points. For the 0–3min time points rate constants of GTP dissociation were: LRRK2(WT) = 0.23min−1 and LRRK2(R1441C) = 0.19min−1. For the 10–60min time points rate constants of GTP dissociation were: LRRK2(WT) = 0.028min−1 and LRRK2(R1441C) = 0.015min−1, which are significantly different based on a two-tailed Student’s unpaired T-test (p<0.01), and demonstrate the slowed rate of GTP hydrolysis for the R1441C mutant, at least at these later time points. Unfortunately, the limitations of the method make it unreliable to measure the multiple early time points that would be required to calculate a more precise initial rate constant of GTP hydrolysis. Nevertheless, the data shown here demonstrate that both wild-type and R1441C mutant LRRK2 bind GTP and undergo intrinsic GTP hydrolysis, thus establishing LRRK2 as a functional GTPase. The R1441C mutant, however, exhibits a significantly slower rate of GTP hydrolysis compared to the wild-type protein in this in vitro assay.
The Roc domain is responsible for LRRK2 GTPase activity
Since Roc domains of ROCO family proteins contain conserved motifs necessary for GTP-binding and hydrolysis, we next sought to determine if the isolated Roc domain of LRRK2 is sufficient for GTPase activity [9]. Utilizing this isolated domain enables the careful quantitative analysis of GTP-binding and hydrolysis properties, along with well-characterized Ras-related small GTPases, without the difficulty of working with the full-length, 286 kDa, multi-domain protein.
To determine if the Roc domain is responsible for LRRK2 GTPase activity we isolated and cloned this domain into a bacterial GST-tagging plasmid for GST-fusion recombinant protein expression. We initially attempted to analyze the GTPase properties of the isolated GST-Roc(R1441C) mutant domain, however, this mutant domain exhibited extremely poor protein expression in bacteria, possibly due to inappropriate folding as a result of the introduction of a cysteine residue. However, the ability of the R1441C mutant to bind and hydrolyze GTP, within the physiological context of full-length LRRK2, demonstrates that this mutant retains GTPase properties (Fig. 2). Therefore, for subsequent GTPase studies on the isolated Roc domain of LRRK2, we utilized the R1441G pathogenic mutant instead of the R1441C mutant. We also utilized the GST-Roc(K1347A) mutant as a negative control because this mutant has been demonstrated in the context of full-length LRRK2 to be defective in GTP-binding [18]. We next performed radiolabeled GTP binding and hydrolysis experiments with the isolated GST-Roc proteins. GST and GST-Rac1, a well-characterized Ras-related small GTPase, were also used as negative and positive controls, respectively. Western blot analysis demonstrates that equivalent amounts of these proteins were utilized in the assay (Fig. 3A).
Fig. 3. The Roc domain is responsible for LRRK2 GTPase activity.
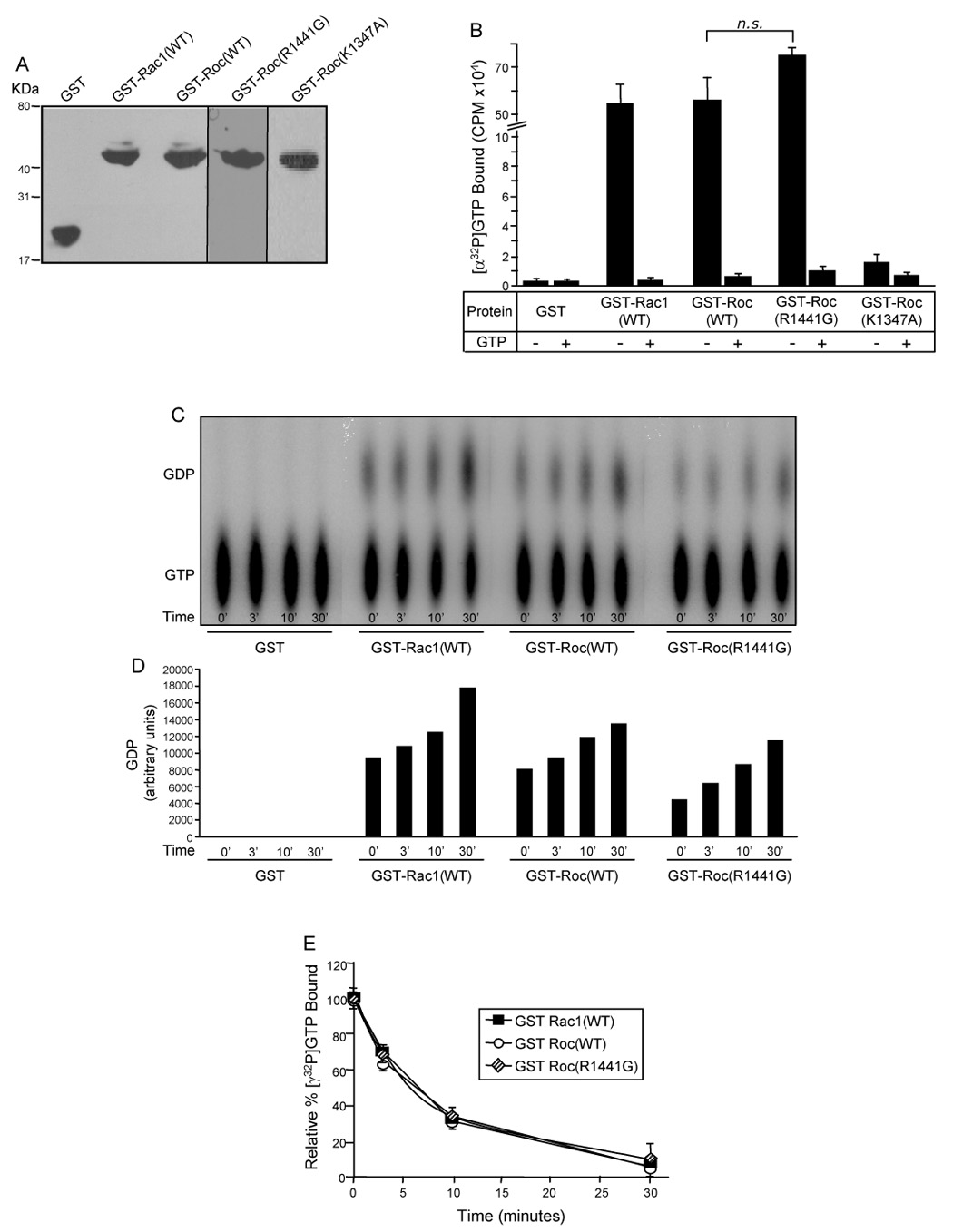
(A) Western blot of GST-fusion proteins. Purified GST fusion proteins were resolved by 12.5% Tris-glycine SDS-PAGE, transferred to PVDF membrane and Western blotted with anti-GST antibody to demonstrate equivalent amounts of protein utilized in the GTP-binding and hydrolysis assays. (B) GTP-binding to the GST-Roc domain of LRRK2. Purified recombinant GST-fusion proteins were assayed for [α-32P]GTP binding in the absence (−) or presence (+) of competing nucleotide (unlabeled GTP). CPM of [α-32P]GTP bound were plotted. (C) GTP hydrolysis of the GST-Roc domain of LRRK2 analyzed by the TLC method. GST-fusion proteins were assayed for [α-32P]GTP hydrolysis at 0, 3, 10 and 30mins by spotting on TLC plates. The autoradiograph shown is representative of three independent experiments. (D) Quantification of GTP hydrolysis of the GST-Roc domain of LRRK2 – TLC method. Amounts of GDP on the representative autoradiograph, shown in Fig. 3C, were quantified by densitometry. (E) GTP hydrolysis of the GST-Roc domain of LRRK2 – infinite dilution and rapid filtration method. GST-fusion proteins were incubated with [γ-32P]GTP and equivalent aliquots were removed at 0, 3, 10 and 30mins. The relative percent of [γ-32P]GTP bound, after subtracting background binding to GST alone (negative control), were plotted. CPM of [γ-32P]GTP bound at time zero was set to 100%. For all experiments GST and GST-Rac1(WT) were used as negative and positive controls, respectively. Error bars represent SEM for one experiment performed in triplicate. Similar results were observed in three independent experiments performed in triplicate. n.s. is non-significant compared to GST-Roc(WT), assessed by a two-tailed unpaired Student’s t-test.
To determine if the isolated Roc domain of LRRK2 retains the ability to specifically bind GTP we performed [α-32P]GTP binding assays. Briefly, [α-32P]GTP was incubated with GST-fusion proteins in the presence or absence of unlabeled GTP. ATP was used in the assay buffer as a competing nucleotide. The reactions were rapidly filtered through nitrocellulose filter discs and the amount of bound radioactivity was quantified in a liquid scintillation counter. Both the wild-type and R1441G isolated GST-Roc domains of LRRK2 bind radioactive GTP and this interaction is specifically competed with an excess of unlabeled GTP (Fig. 3B). Interestingly, the isolated GST-Roc domain of LRRK2 binds GTP to the same extent as equimolar amounts of GST-Rac1 (Fig. 3B). The inability of the K1347A mutant to bind GTP, in the isolated Roc domain, confirms that this is a GTP-binding defective mutation. The inability of GST alone to bind GTP demonstrates that the GTP-binding seen in the presence of the GST-fusion proteins is specific for the Roc and Rac1 proteins (Fig. 3B). These results demonstrate that the isolated Roc domain of LRRK2 is sufficient for binding specifically to GTP, similar to well-characterized Ras-related small GTPases.
Our next step was to determine if the isolated Roc domain undergoes intrinsic GTP hydrolysis. Similar to assays performed with full-length LRRK2, we compared the GTP hydrolysis properties of wild-type versus R1441G mutant Roc domain proteins by two different methods, a TLC based method (Fig. 3C) as well as an infinite dilution and rapid filtration method (Fig. 3E). For the TLC based assay, equivalent amounts of GST-fusion proteins were incubated with [α-32P]GTP in the presence of MgCl2 and equivalent aliquots were removed at various time points, quenched and spotted onto TLC plates. The autoradiograph of a representative TLC plate, shown in Fig. 3C, demonstrates the loss of GTP and simultaneous accumulation of GDP over time as catalyzed by the wild-type and R1441G mutant Roc domains, compared to the GST negative control. The accumulation of GDP over time can also be appreciated by quantification of the representative autoradiograph (Fig. 3D). These results demonstrate that both the wild-type and R1441G mutant Roc domains retain the ability to intrinsically catalyze the hydrolysis of GTP to GDP.
To more carefully and quantitatively compare rates of GTP hydrolysis we utilized an infinite dilution and rapid filtration method in which GST-fusion proteins were incubated with [γ-32P]GTP and equivalent aliquots were removed at various time points. GTP hydrolysis was measured as a function of loss of radioactivity (γ-labeled 32P) associated with recombinant protein upon hydrolysis. As in Fig. 2D, to ensure that loss of radioactivity was due to hydrolysis and not due to nucleotide dissociation, an excess of Mg2+ was added at time zero to stabilize and inhibit dissociation of bound GTP [28]. Similar to the results seen with GTP-binding, both the wild-type and R1441G mutant GST-Roc domains of LRRK2 hydrolyze GTP indistinguishably from equimolar amounts of GST-Rac1 (Fig. 3E). Within the context of full length LRRK2, however, the R1441C mutant displayed a decreased rate of GTP hydrolysis compared to the wild-type protein (Fig. 2D). This difference may be due to structural changes or protein-protein interactions that vary between the R1441C and R1441G mutants or between the GTPase properties of the isolated Roc domain versus those of full length LRRK2. The studies on the isolated Roc domain of LRRK2 demonstrate that this domain is responsible for LRRK2 GTPase activity and that as an isolated entity the Roc domain exhibits GTP-binding and intrinsic GTP hydrolysis properties similar to those seen with Ras-related small GTPases.
LRRK2 kinase activity is stimulated by GTP-binding
Thus far, our results demonstrate that LRRK2 is both a functional kinase and an authentic GTPase. Interestingly, MAPKKKs are known effectors of Ras-related GTPases [25]. The presence of both a functional GTPase domain (Roc) and a MAPKKK domain within LRRK2 raises the exciting possibility of intramolecular regulation.
To determine if the Roc domain of LRRK2 regulates kinase activity of the protein we performed an in vitro kinase assay in the presence or absence of GDP or a non-hydrolyzable GTP analog, Gpp(NH)p. Briefly, FLAG-tagged wild-type and mutant LRRK2 were overexpressed in HEK293T cells and proteins were immunoprecipitated from cell lysates utilizing anti-FLAG M2 affinity agarose. Immunoprecipitates were preincubated in the absence or presence of GDP or Gpp(NH)p and the kinase reaction was initiated by addition of MBP and radioactive ATP. After incubation the reaction was terminated by addition of SDS sample buffer followed by boiling. The autoradiographs of SDS-PAGE separated proteins, shown in Fig. 4, demonstrate increased MBP phosphorylation by both wild-type and R1441C mutant LRRK2 in the presence of Gpp(NH)p compared to GDP. The Coomassie stained gels and anti-FLAG Western blots verified equivalent amounts of MBP and LRRK2 proteins utilized in the assay, respectively (Fig. 4). Based on quantification of the autoradiographs, addition of Gpp(NH)p led to a two-fold increase in LRRK2 kinase activity (Fig. 4). Similar results were also seen using GTPγS (data not shown). These results confirm that LRRK2 undergoes intramolecular regulation in which the activated (GTP-bound) Roc domain stimulates kinase activity of the nearby MAPKKK domain.
Fig. 4. LRRK2 kinase activity is stimulated by GTP-binding.
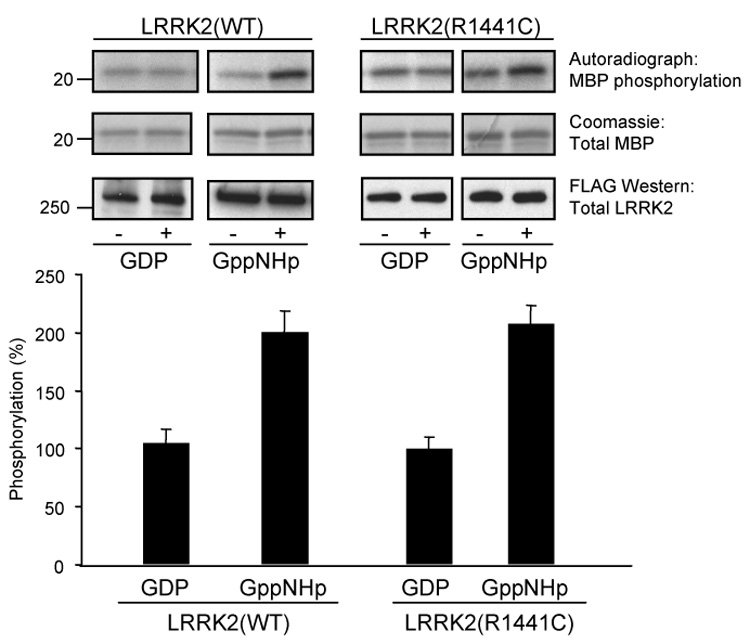
Immunoprecipitated LRRK2 was assayed for phosphorylation of MBP in the absence or presence of 5 µM of either GDP or a non-hydrolyzable GTP analog, Gpp(NH)p. Autoradiographs demonstrate incorporated 32P in MBP resolved by 12% Tris-glycine SDS-PAGE (top panel). MBP levels were determined by Coomassie staining (middle panel) and LRRK2 protein levels were determined by Western blot analysis using anti-FLAG antibody (bottom panel). Autoradiographs of 32P incorporated MBP (top panel) were quantified by densitometry and normalized to LRRK2 protein expression levels (bottom panel). LRRK2 trans-phosphorylation activity in the presence of GDP was set to 100%. Error bars represent SEM for three independent experiments.
Discussion
In this study, we examined the GTPase and kinase activities of LRRK2 and the functional regulation that occurs between these two enzymatic motifs. We have provided strong evidence that LRRK2 is an authentic and functional GTPase, able to bind specifically to GTP and undergo intrinsic GTP hydrolysis (Fig. 2). The Roc domain is responsible for the intrinsic GTPase activity demonstrated by LRRK2 (Fig. 3). The data presented here also indicate that LRRK2 kinase activity is stimulated upon binding of GTP to the Roc domain (Fig. 4). Therefore, LRRK2 is a novel GTP-regulated protein kinase containing not only a kinase effector domain but also a GTPase regulatory domain. This is a unique example of intramolecular regulation occurring between two functional domains within the same polypeptide chain. The possibility of additional intermolecular regulation by other signaling molecules in vivo, however, cannot be excluded.
ROCO family members are a novel class of proteins present throughout various species. Cluster analysis reveals that Roc domains stand out as a separate monophyletic group of the Ras-related superfamily of small GTPases and based on sequence analysis these domains contain conserved residues essential for GTP-binding and hydrolysis [9]. In particular, Ras-related small GTPases possess five loops with defined functions and four out of these five loops are conserved within Roc domains [9, 29]. Based on the high similarity between Roc domains and Ras-related small GTPases it was predicted that this domain would exhibit GTPase activity, however, no experimental evidence of this previously existed in the literature. The data presented here support this prediction and demonstrate that the Roc domain of LRRK2 is responsible for intrinsic LRRK2 GTPase activity and binds and hydrolyzes GTP indistinguishably from the well-characterized Ras-related small GTPase, Rac1 (Fig. 2 and Fig. 3). This is the first demonstration, to our knowledge, that the isolated Roc domain of LRRK2 displays both GTP binding and hydrolysis properties like those of Ras-related small GTPases. The findings detailed in this study are in contrast, however, to a recent report by Ito et.al., in which no GTPase activity was observed for immunoprecipitated LRRK2 [30]. The exact cause for this difference (our positive versus their negative findings) is not clear other than possible discrepancies in experimental conditions. We must point out that we used two different, but well-established methods (TLC based and rapid filtration infinite dilution based assays) for measuring LRRK2 GTPase activity and both methods yielded positive results. Moreover, our results were confirmed with both full-length LRRK2 and the isolated Roc domain. The latter may be critical in determining if factors inhibiting GTP hydrolysis were present in the LRRK2 immunoprecipitates.
While a function for the Roc domain of LRRK2, outside of stimulating kinase activity, is unknown, this domain most closely resembles the Rab family of GTPases, which have been implicated in vesicular trafficking and transport [9, 31]. Recent work has demonstrated that LRRK2 localizes to membranous and vesicular structures within the rat brain, suggesting a potential role in the formation and/or regulation of vesicular structures [32]. Determining if LRRK2 is in fact involved in vesicular related processes and if the Roc domain mediates these events awaits further examination.
Mutations in the LRRK2 gene are the most common susceptibility determinant of PD identified to date. Currently, forty-seven LRRK2 mutations, found throughout the various functional domains of the protein, have been associated with disease and of these, several are clearly pathogenic based on genetic linkage analysis. These mutations account for approximately 6–40% of autosomal-dominantly inherited PD, depending on the ethnicity and geographic locations of patients studied. These mutations also account for a significant fraction of idiopathic disease as well [13, 14, 33, 34]. LRRK2-induced PD displays pathological features similar to those seen in sporadic cases of the disease [4, 5]. Based on the strong dependence of disease penetrance upon age and that PD-associated mutations affect every putative catalytic and protein-protein interaction domain of LRRK2, it is likely that this protein is involved in multiple cellular processes and that it may play a central role in integrating these various pathways within neurons [33, 35].
In this study, we focused on the pathogenic R1441C mutation of LRRK2, which is found within the Roc domain. This mutation was initially identified in several families and is thought to have an estimated prevalence of 3–8% [3–5, 36]. It seems that the R1441 residue is critical for LRRK2 function because two additional mutations at this residue (R1441G and R1441H) have also been found to associate with disease [4, 5, 36, 37]. All three substitutions at the R1441 position of LRRK2 are predicted to disrupt what may be a critical residue involved in secondary structure hydrogen bond formation, thereby potentially altering protein conformation and function [38]. Based on our in vitro results, the R1441C mutant of LRRK2 exhibits a statistically significant decrease in the rate of GTP hydrolysis compared to the wild-type protein, suggesting that this pathogenic mutation may help stabilize an activated state of LRRK2 (Fig. 2D). However, total initial binding of [γ-32P]GTP (time zero) was equivalent for both wild-type and mutant LRRK2. This differs from recently published work by West et. al., which states that the R1441C mutant of LRRK2 has a statistically significant increase in GTP binding compared to the wild-type protein [19]. The aforementioned study, however, was performed using GTP-agarose and statistically analyzed with a one-tailed unpaired Student’s t-test while our studies were performed utilizing radiolabeled GTP in a rapid filtration infinite dilution assay and statistically analyzed with a two-tailed unpaired Student’s t-test. It is important to note, though, that the decreased rate of GTP hydrolysis seen with the R1441C mutant of LRRK2 is based on an in vitro assay and that within the cell there are likely several factors that can regulate GTP-binding and hydrolysis properties, potentially altering the effect this mutation has on LRRK2 GTPase activity. For example, local subcellular concentrations of GTP and GDP as well as potential guanine nucleotide binding regulatory proteins (putative GEFs, GAPs and GDIs), will likely exist within the cell to control LRRK2 GTPase activity.
The decrease in the rate of GTP hydrolysis by the R1441C mutant is consistent with the increased kinase activity associated with this mutant (Fig. 1). Due to the decreased rate of GTP hydrolysis, the R1441C mutant may be slower to inactivate and, therefore, can stimulate kinase activity to a greater extent than the wild-type protein, at least in cell-free assay systems. Our studies also demonstrate the ability of non-hydrolyzable GTP analogs to increase LRRK2 kinase activity in vitro, further supporting the role of GTP binding in promoting kinase activation (Fig. 4).
To date, several pathogenic mutations of LRRK2, including those outside of the MAPKKK domain, are associated with increased kinase activity, in vitro, and there is growing evidence that augmented kinase activity is the mechanism of LRRK2-induced neuronal cell death [18, 19, 23, 24]. Two groups have recently shown that GTP-binding is essential for kinase activity, further demonstrating the importance of studying the GTPase properties of LRRK2 [19, 30]. Mutations within LRRK2 may potentially perturb protein conformation or protein-protein interaction with accessory proteins necessary for kinase activity. It is important to note, however, that the increase in kinase activity seen with PD-associated mutations of LRRK2 has to be interpreted with caution until these observations are confirmed with physiologically relevant substrates. Identifying a physiological substrate of LRRK2 and resolving if pathogenic mutations affect phosphorylation of this substrate will ultimately help determine if augmented kinase activity is an important determinant in the mechanism by which LRRK2 induces PD.
The results presented here are consistent with a model in which LRRK2 cycles between an active and an inactive conformation to potentially integrate several signaling pathways crucial for neuronal cell signaling (Fig. 5). GTP-binding to the Roc domain likely activates the protein, which in turn stimulates LRRK2 kinase activity. Putative GEFs may exist to facilitate activation of the GTPase moiety of LRRK2. Once GTP-bound, LRRK2 is poised to undergo auto-phosphorylation and potentially phosphorylate other substrates to initiate neuronal cell signaling cascades. Once GTP is hydrolyzed to GDP, potentially aided by putative GAPs, LRRK2 returns to its inactive state in which kinase activity is terminated. PD-associated mutations in LRRK2 may lead to aberrant signaling pathways and subsequent neurodegeneration by causing augmented unregulated kinase activity [18, 39, 40]. Identifying LRRK2 interacting proteins and physiological substrates as well as establishing how mutations lead to disease will likely provide crucial insights into the pathways involved in PD pathogenesis. Such discoveries will enable the formulation of novel pharmacological interventions for this devastating disease.
Fig. 5. Proposed model for the GTP-dependent activation of LRRK2 kinase activity.
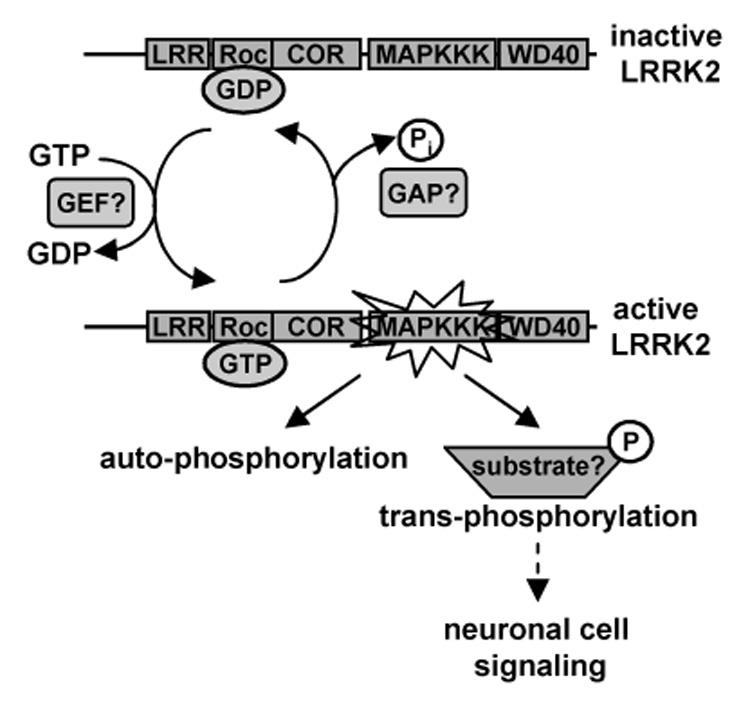
LRRK2 is likely to cycle between an inactive (GDP-bound) and active (GTP-bound) conformation within the cell. GTP-binding to the Roc domain likely activates LRRK2 by stimulating kinase activity. This exchange of GDP for GTP may potentially be facilitated by a putative GEF. In the active conformation, LRRK2 is poised to undergo auto-phosphorylation and potentially phosphorylate other cellular substrates to initiate neuronal cell signaling cascades. Once GTP is hydrolyzed to GDP, potentially aided by a putative GAP, LRRK2 returns to its inactive state in which kinase activity is terminated.
Acknowledgements
*This work was supported in part by the National Institutes of Health (grant 1R21AG028797), National Parkinson Foundation (Mega Research Grants Program), National Science Foundation (Advance Institutional Transformation Grant SBE-0245054) and Johnson & Johnson Corporate Office of Science and Technology (Focused Funding Program).
Footnotes
Abbreviations PD, Parkinson’s disease; LRRK2, leucine-rich repeat kinase 2; GEF, guanine nucleotide exchange factor; GAP, GTPase activating protein; GDI, guanine nucleotide dissociation inhibitor; GST, glutathione S-transferase; IPTG, isopropyl β-D-thiogalactoside; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; PVDF, polyvinylidine difluoride; HEK293T, human embryonic kidney 293T cell line; DPBS, Dulbecco’s phosphate buffered saline; MBP, myelin basic protein; Gpp(NH)p, guanosine 5’-(β.γ)-imidotriphosphate; PBS, phosphate buffered saline; TLC, thin-layer chromatography; CPM, counts per minute; SEM, standard error of the mean.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tanner CM, B-S Y. Epidemiology of Parkinson's disease. Adv Neurol. 1999;80:153–159. [PubMed] [Google Scholar]
- 2.Moore DJ, W A, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 3.Cookson MR. The biochemistry of Parkinson's disease. Annu Rev Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- 4.Paisan-Ruiz C, J S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Zimprich A, B S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Goldwurm S, DF A, Simons EJ, Rohe CF, Zini M, Canesi M, Tesei S, Zecchinelli A, Antonini A, Mariani C, Meucci N, Sacilotto G, Sironi F, Salani G, Ferreira J, Chien HF, Fabrizio E, Vanacore N, Dalla Libera A, Stocchi F, Diroma C, Lamberti P, Sampaio C, Meco G, Barbosa E, Bertoli-Avella AM, Breedveld GJ, Oostra BA, Pezzoli G, Bonifati V. The G6055A (G2019S) mutation in LRRK2 is frequent in both early and late onset Parkinson's disease and originates from a common ancestor. J Med Genet. 2005;42:e65. doi: 10.1136/jmg.2005.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez D, PR C, Crawley A, Malkani R, Werner J, Gwinn-Hardy K, Dickson D, Wavrant Devrieze F, Hardy J, Singleton A. The dardarin G 2019 S mutation is a common cause of Parkinson's disease but not other neurodegenerative diseases. Neurosci Lett. 2005;389:137–139. doi: 10.1016/j.neulet.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 8.Lesage S, Janin S, Lohmann E, Leutenegger AL, Leclere L, Viallet F, Pollak P, Durif F, Thobois S, Layet V, Vidailhet M, Agid Y, Durr A, Brice A. LRRK2 Exon 41 Mutations in Sporadic Parkinson Disease in Europeans. Arch Neurol. 2007;64:425–430. doi: 10.1001/archneur.64.3.425. [DOI] [PubMed] [Google Scholar]
- 9.Bosgraaf L, VH P. Roc, a Ras/GTPase domain in complex proteins. Biochim Biophys Acta. 2003;1643:5–10. doi: 10.1016/j.bbamcr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Guo L, W W, Chen SG. Leucine-rich repeat kinase 2: relevance to Parkinson's disease. Int J Biochem Cell Biol. 2006;38:1469–1475. doi: 10.1016/j.biocel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Berg D, S K, Leitner P, Zimprich A, Lichtner P, Belcredi P, Brussel T, Schulte C, Maass S, Nagele T. Type and frequency of mutations in the LRRK2 gene in familial and sporadic Parkinson's disease. Brain. 2005;128:3000–3011. doi: 10.1093/brain/awh666. [DOI] [PubMed] [Google Scholar]
- 12.Khan NL, J S, Lynch JM, Pavese N, Abou-Sleiman P, Holton JL, Healy DG, Gilks WP, Sweeney MG, Ganguly M, Gibbons V, Gandhi S, Vaughan J, Eunson LH, Katzenschlager R, Gayton J, Lennox G, Revesz T, Nicholl D, Bhatia KP, Quinn N, Brooks D, Lees AJ, Davis MB, Piccini P, Singleton AB, Wood NW. Mutations in the gene LRRK2 encoding dardarin (PARK8) cause familial Parkinson's disease: clinical, pathological, olfactory and functional imaging and genetic data. Brain. 2005;128:2786–2796. doi: 10.1093/brain/awh667. [DOI] [PubMed] [Google Scholar]
- 13.Mata IF, K J, Taylor JP, Lincoln S, Aasly J, Lynch T, Hulihan MM, Cobb SA, Wu RM, Lu CS, Lahoz C, Wszolek ZK, Farrer MJ. Lrrk2 pathogenic substitutions in Parkinson's disease. Neurogenetics. 2005;6:171–177. doi: 10.1007/s10048-005-0005-1. [DOI] [PubMed] [Google Scholar]
- 14.Farrer M, S J, Mata IF, Lincoln S, Kachergus J, Hulihan M, Strain KJ, Maraganore DM. LRRK2 mutations in Parkinson disease. Neurology. 2005;65:738–740. doi: 10.1212/01.wnl.0000169023.51764.b0. [DOI] [PubMed] [Google Scholar]
- 15.Zhu X, Siedlak SL, Smith MA, Perry G, Chen SG. LRRK2 protein is a component of lewy bodies. Ann Neurol. 2006;59:388–393. doi: 10.1002/ana.20928. [DOI] [PubMed] [Google Scholar]
- 16.Zhu X, Babar A, Siedlak SL, Yang Q, Ito G, Iwatsubo T, Smith MA, Perry G, Chen SG. LRRK2 in Parkinson's disease and dementia with Lewy bodies. Mol Neurodegener. 2006;1:17. doi: 10.1186/1750-1326-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takai Y, S T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 18.Smith WW, Pei Z, Jiang H, Dawson VL, Dawson TM, Ross CA. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci. 2006;9:1231–1233. doi: 10.1038/nn1776. [DOI] [PubMed] [Google Scholar]
- 19.West AB, Moore DJ, Choi C, Andrabi SA, Li X, Dikeman D, Biskup S, Zhang Z, Lim KL, Dawson VL, Dawson TM. Parkinson's disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet. 2007;16:223–232. doi: 10.1093/hmg/ddl471. [DOI] [PubMed] [Google Scholar]
- 20.Manning G, W D, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 21.Meylan E, T J. The RIP kinases: crucial integrators of cellular stress. Trends Biochem Sci. 2005;30:151–159. doi: 10.1016/j.tibs.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Xu Z, Maroney AC, Dobrzanski P, Kukekov NV, Greene LA. The MLK family mediates c-Jun N-terminal kinase activation in neuronal apoptosis. Mol Cell Biol. 2001;21:4713–4724. doi: 10.1128/MCB.21.14.4713-4724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gloeckner CJ, Kinkl N, Schumacher A, Braun RJ, O'Neill E, Meitinger T, Kolch W, Prokisch H, Ueffing M. The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum Mol Genet. 2006;15:223–232. doi: 10.1093/hmg/ddi439. [DOI] [PubMed] [Google Scholar]
- 25.Chong H, V H, Guan KL. Mechanisms of regulating the Raf kinase family. Cell Signal. 2003;15:463–469. doi: 10.1016/s0898-6568(02)00139-0. [DOI] [PubMed] [Google Scholar]
- 26.Smith DB, J K. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 27.Gibson RM, Wilson-Delfosse AL. RhoGDI-binding-defective mutant of Cdc42Hs targets to membranes and activates filopodia formation but does not cycle with the cytosol of mammalian cells. Biochem J. 2001;359:285–294. doi: 10.1042/0264-6021:3590285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hart MJ, Shinjo K, Hall A, Evans T, Cerione RA. Identification of the human platelet GTPase activating protein for the CDC42Hs protein. J Biol Chem. 1991;266:20840–20848. [PubMed] [Google Scholar]
- 29.Paduch M, J F, Otlewski J. Structure of small G proteins and their regulators. Acta Biochim Pol. 2001;48:829–850. [PubMed] [Google Scholar]
- 30.Ito G, Okai T, Fujino G, Takeda K, Ichijo H, Katada T, Iwatsubo T. GTP Binding Is Essential to the Protein Kinase Activity of LRRK2, a Causative Gene Product for Familial Parkinson's Disease. Biochemistry. 2007;46:1380–1388. doi: 10.1021/bi061960m. [DOI] [PubMed] [Google Scholar]
- 31.Stenmark H, G D. Intracellular trafficking and turnover of phosphatidylinositol 3-phosphate. Semin Cell Dev Biol. 2001;12:193–199. doi: 10.1006/scdb.2000.0236. [DOI] [PubMed] [Google Scholar]
- 32.Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu SW, Savitt JM, Waldvogel HJ, Faull RL, Emson PC, Torp R, Ottersen OP, Dawson TM, Dawson VL. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- 33.Taylor JP, M I, Farrer MJ. LRRK2: a common pathway for parkinsonism, pathogenesis and prevention. Trends Mol Med. 2006;12:76–82. doi: 10.1016/j.molmed.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Zabetian CP, S A, Mosley AD, Roberts JW, Leis BC, Yearout D, Raskind WH, Griffith A. A clinic-based study of the LRRK2 gene in Parkinson disease yields new mutations. Neurology. 2005;65:741–744. doi: 10.1212/01.wnl.0000172630.22804.73. [DOI] [PubMed] [Google Scholar]
- 35.Kachergus J, M I, Hulihan M, Taylor JP, Lincoln S, Aasly J, Gibson JM, Ross OA, Lynch T, Wiley J, Payami H, Nutt J, Maraganore DM, Czyzewski K, Styczynska M, Wszolek ZK, Farrer MJ, Toft M. Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations. Am J Hum Genet. 2005;76:672–680. doi: 10.1086/429256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Fonzo A, T C, De Mari M, Chien HF, Ferreira J, Rohe CF, Riboldazzi G, Antonini A, Albani G, Mauro A, Marconi R, Abbruzzese G, Lopiano L, Fincati E, Guidi M, Marini P, Stocchi F, Onofrj M, Toni V, Tinazzi M, Fabbrini G, Lamberti P, Vanacore N, Meco G, Leitner P, Uitti RJ, Wszolek ZK, Gasser T, Simons EJ, Breedveld GJ, Goldwurm S, Pezzoli G, Sampaio C, Barbosa E, Martignoni E, Oostra BA, Bonifati V. Italian Parkinson's Genetics Network, Comprehensive analysis of the LRRK2 gene in sixty families with Parkinson's disease. Eur J Hum Genet. 2006;14:322–331. doi: 10.1038/sj.ejhg.5201539. [DOI] [PubMed] [Google Scholar]
- 37.Brice A. Genetics of Parkinson's disease: LRRK2 on the rise. Brain. 2005;128:2760–2762. doi: 10.1093/brain/awh676. [DOI] [PubMed] [Google Scholar]
- 38.Tan EK, S L, Chua E, Wong MC, Pavanni R, Bonnard C, Kolatkar P, Liu JJ. Analysis of 14 LRRK2 mutations in Parkinson's plus syndromes and late-onset Parkinson's disease. Mov Disord. 2006;21:997–1001. doi: 10.1002/mds.20875. [DOI] [PubMed] [Google Scholar]
- 39.Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A, van der Brug MP, Beilina A, Blackinton J, Thomas KJ, Ahmad R, Miller DW, Kesavapany S, Singleton A, Lees A, Harvey RJ, Harvey K, Cookson MR. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006;23:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Smith WW, Pei Z, Jiang H, Moore DJ, Liang Y, West AB, Dawson VL, Dawson TM, Ross CA. Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration. Proc Natl Acad Sci U S A. 2005;102:18676–18681. doi: 10.1073/pnas.0508052102. [DOI] [PMC free article] [PubMed] [Google Scholar]


