Summary
The study aim was to determine whether Prostaglandin E2 (PGE2) in gingival crevicular fluid (GCF) could serve as a risk factor for periodontitis in human immunodeficiency virus-positive (HIV+) patients.
Clinical measurements, including gingival index (GI), plaque index, bleeding index, probing depth (PD), attachment loss (AL) and GCF samples were taken from 2 healthy sites (including sites with gingival recession, GI=0; PD≤ 3 mm; AL≤ 2 mm), 3 gingivitis sites (GI>0; PD≤ 3 mm; AL=0) and 3 periodontitis sites (GI>0; PD≥5 mm; AL≥3 mm) of each of the 30 patients at baseline and 6-month visits. GCF samples were also taken by means of paper strips. GCF PGE2 levels were determined by a sandwich ELISA. The progressing site was defined as a site which had 2 mm or more attachment loss during the 6-month study period.
The mean amounts of PGE2 were significantly higher in gingivitis and periodontitis sites than in healthy sites (p<0.0001). GCF levels of PGE2 were significantly correlated with probing depth, attachment loss, CD4+ cells, viral load, age, and smoking pack-years at baseline and 6-month visits (0.0001<p<0.05). Repeated measures analysis of 19 active sites versus 221 inactive sites indicated that PGE2 levels were significantly higher in active sites than in inactive sites (p<0.0001). It is likely that the compromised immune system contributes to the pathogenesis of periodontitis in HIV+ patients.
It is well known that the activated inflammatory cells produce inflammatory mediators which stimulate the production of PGE2. Longitudinal evaluation of GCF PGE2 with respect to the progression of untreated periodontitis sites in HIV+ subjects will contribute to the understanding of the pathogenesis of periodontitis in HIV+ patients. These data indicate that sites with high GCF levels of PGE2 in HIV+ patients are at significantly greater risk for progression of periodontitis.
Keywords: Prostaglandin E2, gingival crevicular fluid, human immunodeficiency virus, periodontitis, smoking, age, viral load, CD4+ cells
Introduction
Periodontal diseases are common in HIV infected individuals. It is well recognized that the development of periodontal disease depends on the interaction between the resident oral microbiota found in the dentogingival plaque and the host response. The bacteria colonize and invade the periodontal tissue while the host uses a variety of defense mechanisms to maintain a dynamic equilibrium with the resident oral microbial flora. As a result of these interactions between the bacteria and the host, a sequence of host immune mechanisms is activated even at the expense of damaging the periodontal tissues. Most of the tissue damage is caused by the host response to infection. The etiology of periodontal disease in HIV+ patients remains unclear. It is likely that the compromised immune system contributes to the pathogenesis of the lesions. 1,2
Cells from the macrophage/monocyte lineage and CD4+ T lymphocytes are the main targets of human immunodeficiency virus type 1 (HIV-1), the etiologic agent of AIDS, along with HIV-2. CD4+ T cells serve as reservoirs for HIV-1 and some are rapidly destroyed by the virus, whereas macrophages are resistant to the HIV-1-mediated cytopathic effect. 3 These HIV-1-infected macrophages can survive longer periods and function as an important reservoir for HIV-1 infection. 4 It has been suggested that HIV-1 replication could be up-modulated in patients when apoptotic cells are cleared by HIV-1-infected macrophages. Lima et al. 5 reported that the TGF-beta1 released by macrophages upon interaction with apoptotic cells contributes to the impairment of the ability of macrophages to control the growth of the harbored infectious agents. Besides TGF-beta1, PGE2 and platelet activating factor (PAF) are produced by macrophages following uptake of apoptotic cells, and may contribute to the immunosuppressive responses. 6,7 PGE2 is released by many cells, including macrophages, and is active only near the site where it is produced. 8 PGE2 and PAF contribute to increasing viral replication in HIV-infected macrophages exposed to apoptotic cells. 9 It has been reported that PGE2 production is up-modulated in HIV infection. 9
Prostaglandins play significant roles in mucosal inflammation and host responses as inflammatory mediators, and this may play an important role in the pathogenesis of periodontal diseases. It has been reported that an elevated GCF PGE2 level at baseline is a reliable biochemical predictor for future periodontal disease activity in chronic periodontitis patients.10 It has been suggested that patients with periodontitis may possess a hyperinflammatory monocytic response to lipopolysaccharide (LPS) challenge of putative periodontal pathogens. 11-13 Hessle et al. reported that gram-negative bacteria induced strong PGE2 production in human monocytes. 14 This upregulated monocytic response is even more important in immunosuppressed patients, and may contribute to the elevated levels of inflammatory mediators such as PGE2, interleukin-1 beta, and tumor necrosis factor-alpha. 11 Patients with AIDS show elevated levels of circulating PGE2. 15 It has been suggested that PGE2 suppresses both Th1- and Th2-polarized antigen-specific human T-cell responses. 16 There is little information available with respect to the role of prostaglandins in the etiology of periodontitis in HIV+ patients. The aim of this study was to determine whether prostaglandin E2 (PGE2) in gingival crevicular fluid (GCF) could serve as a risk factor for the progression of periodontitis in HIV+ patients.
Methods
Thirty HIV-infected patients were randomly selected from the database of an ongoing epidemiologic project. The demographic, clinical, and immunologic data related to these 30 HIV+ patients were used from the same database. The HIV+ patients were recruited from the CARE clinic at the University of the Pacific Arthur A. Dugoni School of Dentistry. The inclusion criteria included the following: study subjects were older than 18 years of age, had 2 healthy, 3 gingivitis and 3 periodontitis sites, did not require premedication with antibiotics for a periodontal examination, and had not gone through periodontal therapy within the last six months. The protocol for all procedures was approved by the Institutional Review Board of the California Pacific Medical Center. All study participants signed the committee approved consent. Medical and demographic variables including medical history, age, race, cigarette smoking, alcohol use, oral hygiene practices, dental care utilization, level of education and income were obtained using a structured interview with the subject. Current CD4+ cell count and viral load values (within 2 months of initial study visit) were recorded from the chart review. Clinical measurements, including gingival index (GI), plaque index, bleeding index, probing depth (PD), attachment loss (AL) and GCF samples were taken from 2 healthy sites (including sites with gingival recession, GI=0; PD≤ 3 mm; AL≤ 2 mm), 3 gingivitis sites (GI>0; PD≤ 3 mm; AL=0) and 3 periodontitis sites (GI>0; PD≥5 mm; AL≥3 mm) of each of the 30 patients at baseline and 6-month visits. The plaque index (PI) 17, gingival index (GI) 18, probing depth (PD), attachment loss (AL), and bleeding on probing (BI) were recorded for each experimental site by a calibrated examiner.
GCF sampling was carried out using sterile paper strips. A sterile periopaper (IDE Interstate, Amityville, NY) was gently inserted 1-2mm into the orifice of the gingival crevice and left in place for 30 seconds. The sample volume was measured with a calibrated Periotron 6000 prior to transfer of the strip to a microfuge tube containing 350 μl of elution media (physiologic saline-0.1% Tween 20). The GCF samples were stored in a −80 °C freezer. GCF PGE2 levels were determined by a sandwich ELISA. The same procedures were repeated at the 6-month visit. All study subjects received scaling-polishing and oral hygiene instructions immediately after the completion of their baseline visit. No additional periodontal treatment was performed during the course of the 6-month study period.
Sample sites were classified as:
Healthy sites (including sites with gingival recession): Gingival Index=0, Probing Depth ≤3 mm and Attachment Loss ≤2 mm.
Gingivitis sites = Gingival index>0, Probing Depth ≤3 mm, Attachment Loss = 0 mm.
Periodontitis sites = Gingival index>0, Probing Depth ≥5 mm, Attachment Loss ≥ 3 mm.
A progressing site was defined as a site which had 2 mm or more new attachment loss during the 6-month study period.
PGE2 determinations
GCF PGE2 was quantified using a sandwich ELISA (R&D Systems, Minneapolis, MN). This assay is based on the competitive binding technique in which PGE2 present in a sample competes with a fixed amount of horseradish peroxidase-labeled PGE2 for sites on a mouse monoclonal antibody. The standards and samples were incubated in the 96-well microplate precoated with a goat anti-mouse polyclonal antibody. During the incubation, the mouse monoclonal antibody becomes bound to the goat anti-mouse antibody coated onto the microplate. Following a wash to remove excess conjugate and unbound sample, a substrate solution (tetramethylbenzidine) was added to the wells to determine the bound enzyme activity. The reaction was terminated by addition of stop solution (sulfuric acid). Immediately following color development, the absorbance was read at 450-570 nm. The intensity of the color is inversely proportional to the concentration of PGE2 in the sample. The concentration of PGE2 in GCF samples was determined by interpolation from a standard curve. The minimum detectable dose (MDD) of PGE2 ranged from 18.2-36.8 pg/ml. The mean MDD was 27.5 pg/ml. The MDD was determined by subtracting two standard deviations from the mean optical density value of twenty zero standard replicates and calculating the corresponding concentration.
Statistical Analysis
GCF levels of PGE2 were subjected to square root transformation to render variances more homogenous and to reduce skewness. The SAS statistical package was used to analyze the data. The associations between the demographic variables, clinical measurements, and PGE2 values were determined by Pearson's correlation coefficients. Analysis of variance (ANOVA) for repeated measures and paired t-test were used to perform within patient comparisons of healthy, gingivitis and periodontitis sites. Three mean PGE2 values of the study sites were calculated for each subject (the mean of three periodontitis sites, mean of three gingivitis sites and mean of two healthy sites). GCF PGE2 values of active and inactive sites were compared with t-tests. A Bonferroni correction was applied. A p-value of <0.05 was considered indicative of a true difference. No adjustment for multiple testing was done. History of smoking (including current smokers) was reported as pack-years (number of packs of cigarettes smoked per day multiplied by number of years smoked). Smoking pack-years, CD4+ cell counts and viral load values were analyzed as continuous variables.
Results
The descriptive statistics of the study population are shown in Table 1. A total of 30 HIV+ male subjects were enrolled in this 6-month longitudinal study. The mean age of the study population was 42.4 years (range 24-64). The mean clinical measurements, mean PGE2 and GCF values of healthy, gingivitis and periodontitis sites are given in Table 2. For the periodontitis sites, mean (±) standard deviation (SD) probing depths and attachment loss measurements at baseline were 5.74±0.65 mm, and 4.13±1.09 mm, respectively. For the healthy sites, mean (±) SD probing depths and attachment loss measurements at baseline were 1.94±0.63 mm and 0.73±0.71 mm, respectively. The errors associated with measurement of pocket depth and determination of attachment loss were 0.36±0.47 mm and 0.39±0.51 mm, respectively. At baseline, 42 of 90 periodontitis sites (46.6%) showed bleeding upon probing. All baseline clinical parameters for periodontitis sites increased significantly at the 6-month visit (0.0001<p<0.05).
Table 1.
Descriptive statistics at baseline and 6-month visits (Mean and SD of the entire subject group (N=30)
| Variables | Baseline | 6-month |
|---|---|---|
| Age | 42.41±13.37 | 42.90±13.38 |
| Pack-years | 5.90±5.45 | 6.28±5.59 |
| CD4+ cells (per μl) | 316±118 | 359±171 |
| Viral Load (per ml) | 13822±20428 | 14897±21795 |
| Probing Depth (mm) | 3.44±0.28 | 3.69±0.42 |
| Attachment Loss (mm) | 1.95±0.41 | 2.20±0.55 |
| PGE2 (ng/site) | 2.52±1.09 | 2.67±1.11 |
Table 2.
Mean and SD of clinical parameters, and PGE2 in healthy, gingivitis and periodontitis sites (N=30 subjects) at Baseline and 6-month visits
| Healthy sites (n=60) | Gingivitis sites (n=90) | Periodontitis sites (n=90) | ||||
|---|---|---|---|---|---|---|
| Variables | Baseline | 6-month | Baseline | 6-month | Baseline | 6-month |
| Plaque index (PI) | 0.39±0.37 | 0.76±0.34 | 1.12±0.16 | 1.26±0.29 | 1.81±0.43 | 2.15±0.41 |
| Gingival index (GI) | 0 | 0 | 1.08±0.14 | 1.15±0.22 | 1.48±0.43 | 1.57±0.39 |
| Bleeding index (BOP) | 0 | 0 | 0.26±0.33 | 0.42±0.27 | 0.54±0.16 | 0.63±0.18 |
| P. depth (mm) (PD) | 1.94±0.63 | 2.09±0.64 | 2.64±0.45 | 2.85±0.57 | 5.74±0.65 | 6.13±0.82 |
| A. loss (mm) (AL) | 0.73±0.71 | 0.79±0.78 | 0.99±0.41 | 1.18±0.47 | 4.13±1.09 | 4.59±1.16 |
| PGE2 (ng/site) | 0.98±0.76 | 0.91±0.68 | 2.69±1.01 | 2.72±1.04 | 3.89±1.16 | 4.41±1.22 |
| GCF volume (μl/30 s) | 0.19±0.07 | 0.21±0.08 | 0.38±0.11 | 0.41±0.13 | 0.58±0.21 | 0.73±0.24 |
The GCF PGE2 values and GCF volume were expressed as absolute amounts (Table 2). The mean PGE2 and GCF volume values of periodontitis sites at baseline and 6-month were significantly higher than those of gingivitis and healthy sites (0.0001<p<0.01) (Fig. 1). There were significant differences between gingivitis and healthy sites with respect to the PGE2 (Fig. 1) and GCF volume values (0.0001<p<0.01). It was also noted that the mean baseline PGE2 and GCF volume values of periodontitis sites increased significantly at 6-month visits (0.0001<p<0.01) (Table 2) (Fig. 1). The correlation values between clinical measurements, age, smoking pack-years, CD4+ cells, viral load, and PGE2 values at baseline and 6-month visits are shown in Table 3. PGE2 was correlated positively with age, smoking pack-years, viral load, probing depth and attachment loss at baseline and 6-month visits (0.001<p<0.05). There was a negative correlation between CD4+ cells and PGE2, age, smoking pack- years, viral load, probing depth, attachment loss at baseline (r:−0.84 ; r:−0.83 and r:−0.86 ; r:−0.78 ; r:−0.65 ; r:−0.86 respectively) and 6-month visits (r:−0.82 ; r:−0.87 and r:−0.84 ; r:−0.79 ; r:−0.49 ; r:−0.76 respectively) (0.0001<p<0.001). Viral load was correlated positively with age, smoking pack-years, probing depth, attachment loss and PGE2 at baseline and 6-month visits (0.0001<p<0.001). Smoking pack-years was positively correlated with age (r:0.92 and 0.93), viral load (r:0.87 and 0.88), probing depth (r:0.54 and 0.47), attachment loss (r:0.87 and 0.83), and PGE2 (r:0.90) at baseline and 6-month visits (0.0001<p<0.01). Age was significantly associated with pack-years (r:0.92 and 0.93), CD4+ cells (r:−0.83 and −0.87), viral load (r:0.79 and 0.80), probing depth (r:0.66 and 0.56), attachment loss (r:0.86 and 0.81), and PGE2 (r:0.57) at baseline and 6-month visits (0.0001<p<0.01).
Fig. 1.
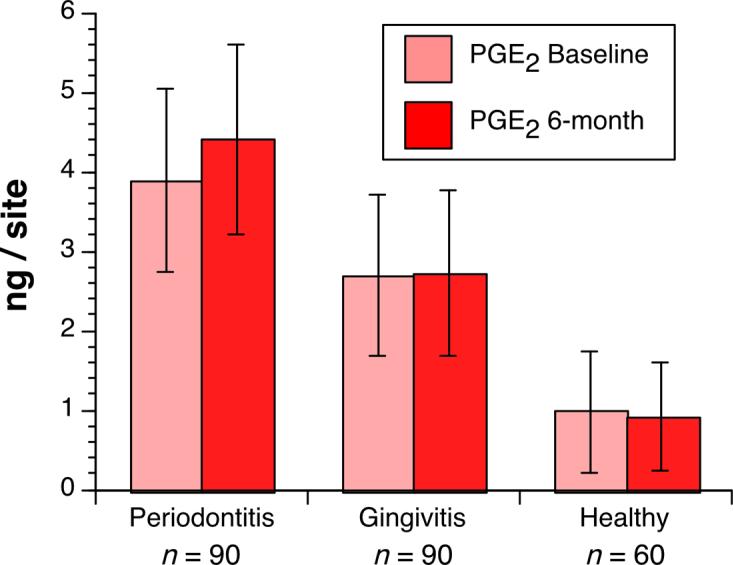
Mean values of gingival crevicular fluid prostaglandin E2 (PGE2) with 95% confidence intervals at periodontitis, gingivitis and healthy sites at baseline and 6-month visits.
Table 3.
Pearson's correlation coefficient (r) values between dependent and independent variables.
|
Parameters |
Age | Pack-years | CD4+ cells |
Viral Load | P. depth | A. loss | PGE2 |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| Baseline | 1.0 | 0.92 | −0.83 | 0.79 | 0.66 | 0.86 | 0.87 |
| 6-month | 1.0 | 0.93 | −0.87 | 0.80 | 0.56 | 0.81 | 0.88 |
| Pack-years | |||||||
| Baseline | 0.92 | 1.0 | −0.86 | 0.87 | 0.54 | 0.87 | 0.90 |
| 6-month | 0.93 | 1.0 | −0.84 | 0.88 | 0.47 | 0.83 | 0.90 |
| CD4+ cells | |||||||
| Baseline | −0.83 | −0.86 | 1.0 | −0.78 | −0.65 | −0.86 | −0.84 |
| 6-month | −0.87 | −0.84 | 1.0 | −0.79 | −0.49 | −0.76 | −0.82 |
| Viral Load | |||||||
| Baseline | 0.79 | 0.87 | −0.78 | 1.0 | 0.39 | 0.78 | 0.79 |
| 6-month | 0.80 | 0.88 | −0.79 | 1.0 | 0.37 | 0.71 | 0.78 |
| P. depth | |||||||
| Baseline | 0.66 | 0.54 | −0.65 | 0.39 | 1.0 | 0.70 | 0.61 |
| 6-month | 0.56 | 0.47 | −0.49 | 0.37 | 1.0 | 0.79 | 0.58 |
| A. loss | |||||||
| Baseline | 0.86 | 0.87 | −0.86 | 0.78 | 0.70 | 1.0 | 0.85 |
| 6-month | 0.81 | 0.83 | −0.76 | 0.71 | 0.79 | 1.0 | 0.86 |
| PGE2 | |||||||
| Baseline | 0.87 | 0.90 | −0.84 | 0.79 | 0.61 | 0.85 | 1.0 |
| 6-month | 0.87 | 0.90 | −0.82 | 0.78 | 0.58 | 0.86 | 1.0 |
The dependent and independent variables were correlated at a significance level of 0.0001<p<0.05
The repeated measures analysis of variance demonstrated a significant effect when periodontitis, gingivitis and healthy sites were compared for their GCF PGE2 values measured at baseline and 6-month visits (Table 4). There were significant differences in PGE2 values between healthy, gingivitis, and periodontitis Sites at baseline and 6-month visits (p<0.0001).
Table 4.
Repeated Measures Analysis at baseline and 6-month visits for PGE2 at healthy (H), Gingivitis (G) and Periodontitis (P) sites
| Parameters | H. Site Vs G. Site | H. Site Vs P. Site | G. Site Vs P. Site |
|---|---|---|---|
| PGE2 | |||
| Observed t value baseline (CI interval) | −19.96 (−1.22,−0.98) | −9.42 (−4.56,−2.81) | −7.16 (−3.39,−1.78) |
| Observed t value 6-month (CI interval) | −24.71 (−1.24,−1.03) | −8.70 (−5.40,−3.20) | −6.31 (−4.27,−2.04) |
There were significant differences in PGE2 values between Healthy, Gingivitis, and Periodontitis sites at baseline and 6-month visits (p<0.0001).
A total of 19 sites in 13 patients showed 2 mm or more of new attachment loss during the 6-month study period. The mean attachment loss, probing depth, PGE2 values (Fig. 2) of these 19 active sites measured at baseline and 6-month visits were higher than the mean attachment loss, probing depth, PGE2 values of inactive sites (Table 5). It was also noted that the mean baseline attachment loss, probing depth, PGE2 values (Fig. 2) of active sites increased significantly at 6-month visits (0.0001<p<0.001).
Fig. 2.
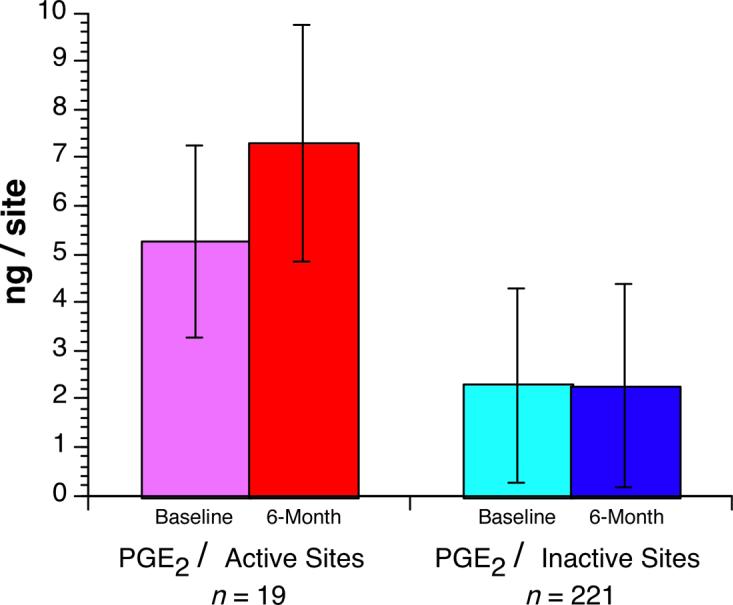
Mean values of gingival crevicular fluid prostaglandin E2 (PGE2) with 95% confidence intervals at active versus inactive sites at baseline and 6-month visits.
Table 5.
Mean and SD of Attachment Loss (AL), Probing Depth (PD), PGE2 values in active and inactive sites.
| Active sites n = 19 |
Inactive sites n = 221 |
|||
|---|---|---|---|---|
| Baseline | 6-month | Baseline | 6-month | |
| AL (mm) | 4.42±1.26 | 6.42±1.26 | 1.74±1.85 | 1.84±1.88 |
| PD (mm) | 5.84±0.76 | 7.63±0.83 | 3.23±1.84 | 3.35±1.89 |
| PGE2 (ng/site) | 5.26±1.97 | 7.29±2.46 | 2.29±2.02 | 2.27±2.09 |
There were significant differences in mean AL, PD, and PGE2 values between active and inactive sites at baseline and 6-month visits (p<0.0001). In active sites, the mean PGE2 values were significantly higher at 6-month compared to baseline visits.
Discussion
The results of this longitudinal study support the hypothesis that a higher GCF level of PGE2 is associated with periodontal disease progression in HIV+ patients. In the present study, 19 of 240 study sites (7.91%) had 2 mm or more attachment loss during the 6-month study period. The mean PGE2 values of these 19 active periodontitis sites at baseline and 6-month visits were significantly higher than the mean PGE2 values of inactive periodontitis sites (p<0.0001), suggesting a potential role for PGE2 in the progression of established periodontitis sites. There was a significant increase in the mean PGE2 level of active periodontitis sites at the 6-month visit compared to the mean of PGE2 level at baseline (p<0.0001). It has been reported that PGE2 is produced by macrophages following uptake of apoptotic cells, and may contribute to immunosuppressive responses. 6,7 PGE2 and platelet-activating factor (PAF) contribute to increasing viral replication in HIV-infected macrophages exposed to apoptotic cells. 9 It has also been reported that PGE2 production is up-modulated in HIV infection.9 In the present study, GCF PGE2 levels were correlated positively with viral load values at baseline (r:0.79 ; p<0.0001) and at 6-month visits (r:0.78 ; p<0.0001), supporting the notion that PGE2 may have contributed to the increased HIV-1 replication. PGE2 was correlated negatively with the CD4+ cell counts at baseline (r:−0.84 ; p<0.0001) and at the 6-month visits (r:−0.82 ; p<0.0001), suggesting that PGE2 may have played a significant role in the immunosuppressive responses which resulted in the progression of established periodontitis. Patients with AIDS show elevated levels of circulating PGE2.15 It has been suggested that PGE2 suppresses both Th1- and Th2-polarized antigen-specific human T-cell responses.16 Lima et al. reported that addition of PGE2 to HIV-1-infected macrophages induced the production of HIV-1. 9 We have previously shown the positive association between viral load and Fusobacterium nucleatum and Prevotella intermedia in HIV+ patients, indicating that the subtle changes in the immune system may allow proliferation of more virulent clones of periodontal pathogens.1 Hessle et al. reported that gram-negative bacteria induced strong PGE2 production in human monocytes. 14 In the present study, the strong associations between PGE2 and probing depth, and PGE2 and attachment loss at baseline and 6-month visits also support our hypothesis. The increased levels of PGE2 in GCF of systemically healthy subjects with periodontitis were reported by other investigators. 10,19 In a systemically healthy patient population, it has been reported that the levels of PGE2 were two-fold higher in the LPS-stimulated whole blood cell cultures from periodontitis patients than from controls. 20 Data also indicate that immuno-compromised diabetic patients with periodontitis may possess a hyperinflammatory monocytic response to bacterial challenge, resulting in elevated GCF levels of inflammatory mediators such as PGE2, interleukin-1 beta, and tumor necrosis factor alpha which mediates increased inflammation and tissue destruction.11-13 Araya et al. reported that the means of LPS-induced PGE2 levels in whole blood samples from Type-1 diabetic patients were significantly higher than the levels observed for systemically healthy controls. 21 In a systemically healthy study population, Nakashima et al. reported that the discriminant function including total amounts of PGE2, collagenase, alkaline phosphatase, α-2 macroglobulin, osteocalcin and antigenic elastase was found to possess higher predictability as compared to any single parameter. 22
The strong association between cigarette smoking and periodontal status was well established in the earlier studies. 23-27 Cigarette smoking has been shown to alter host response mechanisms by adversely affecting the PMN function, thereby depressing phagocyte-mediated protective responses to periodontopathic bacteria. 28 In the present study, smoking pack-years was positively correlated with PGE2 , probing depth, attachment loss, viral load, and negatively correlated with CD4+ cell counts at baseline and 6-month visits. Tanaka et al. suggested that nicotine and lipopolysaccharide (LPS) stimulate the formation of osteoclast-like cells via an increase in macrophage colony-stimulating factor and PGE2 production by osteoblasts. 29 Wewers et al. reported a decrease in CD4+/CD8+ cell ratios and significant depressions in both the percentage and absolute numbers of CD4+ and CD8+ cells in bronchoalveolar lavage fluid of smokers. 30 It has been reported that ex-smokers with chronic obstructive pulmonary disease (COPD) had higher CD4+ cell counts than current smokers with COPD. 31 Our data with respect to the positive association between viral load values and smoking support the observation made by Clarke et al. 32 Low CD4 levels may not only allow excessive viral replication during opportunistic infections, but also predispose to an inadequate host response to infections.
Age was significantly associated with pack-years, viral load, probing depth, attachment loss, and PGE2 indicating the potential role of age as a risk factor for periodontal disease (0.0001<p<0.01). Higher prevalence and severity of periodontal disease with increasing age has been reported previously. 1,26,27,33,34
In summary, these data indicate that sites with high GCF levels of PGE2 are at risk for progression of established periodontitis in HIV-infected individuals. PGE2 can be considered as risk factor for periodontitis in HIV+ patients.
Acknowledgments
The authors wish to thank Senait Gebremedhin in the Department of Microbiology, Arthur A. Dugoni School of Dentistry, for her excellent technical assistance. This study was supported by NIDCR grant DE12417 and funds (DRES06-046) form Arthur A. Dugoni School of Dentistry, University of the Pacific.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alpagot T, Duzgunes N, Wolff LF, Lee A. Risk factors for periodontitis in HIV+ patients. J Periodontal Res. 2004;39:149–157. doi: 10.1111/j.1600-0765.2004.00718.x. [DOI] [PubMed] [Google Scholar]
- 2.Alpagot T, Suzara V, Bhattacharyya M. The associations between gingival crevice fluid matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1 and periodontitis in human immunodeficiency virus-positive patients. J Periodontal Res. 2006;41:491–497. doi: 10.1111/j.1600-0765.2006.00887.x. [DOI] [PubMed] [Google Scholar]
- 3.Wahl SM, Greenwell-Wild T, Peng GMG, Orenstein JM, Vasquez N. Viral and host co-factors facilitate HIV-1 replication in macrophages. J Leukoc Biol. 2003;74:726–735. doi: 10.1189/jlb.0503220. [DOI] [PubMed] [Google Scholar]
- 4.Crowe S, Zhu T, Muller WA. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J Leukoc Biol. 2003;74:635–641. doi: 10.1189/jlb.0503204. [DOI] [PubMed] [Google Scholar]
- 5.Lima RG, Van Weyenbergh J, Saraiva EMB, Barral-Netto M, Galvao-Castro B, Bou-Habib DC. The replication of human immunodeficiency virus type 1 in macrophages is enhanced after phagocytosis of apoptotic cells. J Infect Dis. 2002;185:1561–1566. doi: 10.1086/340412. [DOI] [PubMed] [Google Scholar]
- 6.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2 and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freire-de-Lima CG, Nascimento DO, Soares MB, Bozza PT, Castro-Faria-Neto HC, de Mello FG, DosReis GA, Lopes MF. Uptake of Apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature. 2000;403:199–203. doi: 10.1038/35003208. [DOI] [PubMed] [Google Scholar]
- 8.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 9.Lima RG, Moreira L, Paes-Leme J, Barreto-De-Souza V, Castro-Faria-Neto HC, Bozza P, Bou-Habib DC. Interaction of Macrophages with apoptotic cells enhances HIV type 1 replication through PGE2, PAF, and vitronectin receptor. AIDS Res Hum Retroviruses. 2006;22:763–769. doi: 10.1089/aid.2006.22.763. [DOI] [PubMed] [Google Scholar]
- 10.Offenbacher S, Odle BM, Van Dyke TE. The use of crevicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. J Periodontal Res. 1986;21:101–112. doi: 10.1111/j.1600-0765.1986.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 11.Salvi GE, Yalda B, Collins JG, Jones BH, Smith FW, Arnold RR, Offenbacher S. Inflammatory mediator response as a potential risk marker for periodontal diseases in insulin-dependent diabetes mellitus patients. J Periodontol. 1997;68:127–135. doi: 10.1902/jop.1997.68.2.127. [DOI] [PubMed] [Google Scholar]
- 12.Salvi GE, Beck JD, Offenbacher S. PGE2, IL-1β and TNF-α responses in diabetics as modifiers of periodontal disease expression. Ann Periodontol. 1998;3:40–50. doi: 10.1902/annals.1998.3.1.40. [DOI] [PubMed] [Google Scholar]
- 13.Salvi GE, Brown EC, Fujihashi K, Kiyono H, Smith FW, Beck JD, Offenbacher S. Inflammatory mediators of the terminal dentition in adult and early onset periodontitis. J Periodontal Res. 1998;33:212–225. doi: 10.1111/j.1600-0765.1998.tb02193.x. [DOI] [PubMed] [Google Scholar]
- 14.Hessle CC, Andersson B, Wold AE. Gram-negative, but not Gram-positive, bacteria elicit strong PGE2 production in human monocytes. Inflammation. 2003;27:329–332. doi: 10.1023/b:ifla.0000006700.41614.21. [DOI] [PubMed] [Google Scholar]
- 15.Foley P, Kazazi F, Biti R, Sorrell TC, Cunningham AL. HIV infection of monocytes inhibits the T-lymphocyte proliferative response to recall antigens, via production of eicosanoids. Immunology. 1992;75:391–397. [PMC free article] [PubMed] [Google Scholar]
- 16.Okano M, Sugata Y, Fujiwara T, Matsumoto R, Nishibori M, Shimizu K, Maeda M, Kimura Y, Kariya S, Hattori H, Yokoyama M, Kino K, Nishizaki K. E prostanoid 2 (EP2)/EP4-mediated suppression of antigen-specific human T-cell responses by prostaglandin E2. Immunology. 2006;118:343–352. doi: 10.1111/j.1365-2567.2006.02376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silness J, Loe H. Periodontal disease in pregnancy (II). Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 18.Loe H, Silness J. Periodontal disease in pregnancy (1). Prevalence and severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. 1963. [DOI] [PubMed] [Google Scholar]
- 19.Leibur E, Tuhkanen A, Pintson U, Soder PO. Prostaglandin E2 levels in blood plasma and crevicular fluid of advanced periodontitis before and after surgical therapy. Oral Diseases. 1999;5:223–228. doi: 10.1111/j.1601-0825.1999.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 20.Fokkema SJ, Loos BG, Slegte C, van der Velden U. A type 2 response in lipopolysaccharide (LPS)-stimulated whole blood cell cultures from periodontitis patients. Clinical and Experimental Immunology. 2002;127:374–378. doi: 10.1046/j.1365-2249.2002.01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Araya AV, Pavez V, Perez C, Gonzalez F, Columbo A, Aguirre A, Schiattino I, Aguillon JC. Ex vivo lipopolysaccharide (LPS)-induced TNF-alpha, IL-1beta, IL-6 and PGE2 secretion in whole blood from Type-1 diabetes mellitus patients with or without aggressive periodontitis. European Cytokine Network. 2003;14:128–133. [PubMed] [Google Scholar]
- 22.Nakashima K, Giannopoulou C, Andersen E, Roehrich N, Brochut P, Dubrez B, Cimasoni G. A longitudinal study of various crevicular fluid components as markers of periodontal disease activity. J Clin Periodontol. 1996;23:832–838. doi: 10.1111/j.1600-051x.1996.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 23.Stoltenberg JL, Osborn JB, Pihlstrom B, Herzberg MC, Aeppli DM, Wolff LF, Fischer GE. Association between cigarette smoking, bacterial pathogens, and periodontal status. J Periodontol. 1993;64:1225–1230. doi: 10.1902/jop.1993.64.12.1225. [DOI] [PubMed] [Google Scholar]
- 24.Alpagot T, Wolff LF, Smith QT, Tran SD. Risk indicators for periodontal disease in a racially diverse population. J Clin Periodontol. 1996;23:982–988. doi: 10.1111/j.1600-051x.1996.tb00524.x. 1996. [DOI] [PubMed] [Google Scholar]
- 25.Bergstrom J, Preber H. Tobacco use as a risk factor. J Periodontol. 1994;65:545–550. doi: 10.1902/jop.1994.65.5s.545. [DOI] [PubMed] [Google Scholar]
- 26.Grossi SG, Zambon JJ, Ho AW, Koch G, Dunford RG, Machtei EE, Norderyd OM, Genco RJ. Assessment of risk for periodontal disease. 1. Risk indicators for attachment loss. J Periodontol. 1994;65:260–267. doi: 10.1902/jop.1994.65.3.260. [DOI] [PubMed] [Google Scholar]
- 27.Alpagot T, Font K, Lee A. Longitudinal evaluation of GCF IFN-γ levels and periodontal status in HIV+ patients. J Clin Periodontol. 2003;30:944–948. doi: 10.1034/j.1600-051x.2003.00403.x. 2003. [DOI] [PubMed] [Google Scholar]
- 28.MacFarlane GD, Herzberg MC, Wolff LF, Hardie NA. Refractory periodontitis associated with abnormal polymorphonuclear leukocyte phagocytosis and cigarette smoking. J Periodontol. 1992;63:908–913. doi: 10.1902/jop.1992.63.11.908. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka H, Tanabe N, Shoji M, Suzuki N, Katono T, Sato S, Motohashi Km Maeno M. Nicotine and lipopolysaccharide stimulate the formation of osteoclast-like cells by increasing macrophage colony-stimulating factor and prostaglandin E2 production by osteoblasts. Life Sciences. 2006;78:1733–1740. doi: 10.1016/j.lfs.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Wewers MD, Diaz PT, Wewers ME, Lowe MP, Nagaraja HN, Clanton TL. Cigarette smoking in HIV infection induces a suppressive inflammatory environment in the lung. Am J Respir Crit Care Med. 1998;158:1543–1549. doi: 10.1164/ajrccm.158.5.9802035. [DOI] [PubMed] [Google Scholar]
- 31.Lapperre TS, Postma DS, Gosman MME, Snoeck-Stroband JB, ten Hacken NHT, Hiemstra PS, Timens W, Sterk PJ, Mauad T. Relation between duration of smoking cessation and bronchial inflammation in COPD. Thorax. 2006;61:115–121. doi: 10.1136/thx.2005.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke JR, Taylor IK, Fleming J, Nukuna A, Williamson JD, Mitchell DM. The epidemiology of HIV-1 infection of the lung in AIDS patients. AIDS. 1993;7:555–560. doi: 10.1097/00002030-199304000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Beck JD, Koch GG, Offenbacher S. Incidence of attachment loss over 3 years in older adults-new and progressing lesions. Com Dent Oral Epidemiol. 1995;23:291–296. doi: 10.1111/j.1600-0528.1995.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 34.Alpagot T, Bell C, Lundergan W, Chambers DW, Rudin R. Longitudinal evaluation of GCF MMP-3 and TIMP-1 levels as prognostic factors for progression of periodontitis. J Clin Periodontol. 2001;28:353–359. doi: 10.1034/j.1600-051x.2001.028004353.x. 2001. [DOI] [PubMed] [Google Scholar]


