Abstract
Recent studies show that children who die from fulminant meningococcaemia have very low cortisol:adrenocorticotrophic hormone (ACTH) ratios within the first 8 h of presentation to emergency facilities compared with survivors. This observation supports the possibility that adrenal insufficiency may contribute to rapid cardiovascular collapse in these children. In recent years, the use of hydrocortisone treatment has become increasingly popular in the care of adult and paediatric patients with septic shock. In this review, the classical adrenal insufficiency literature is presented and the existing rationale for using titrated hydrocortisone treatment (2–50 mg/kg/day) to reverse catecholamine‐resistant shock in children who have absolute adrenal insufficiency (defined by peak cortisol level <18 μg/dl after ACTH challenge) or pituitary, hypothalamic or adrenal axis insufficiency is provided. In addition, the concept of relative adrenal insufficiency (basal cortisol >18 μg/dl but a peak response to ACTH <9 μg/dl) is reviewed. Although there is a good rationale supporting the use of 7 days of low‐dose hydrocortisone treatment (about 5 mg/kg/day) in adults with this condition and catecholamine resistant septic shock, the paediatric literature suggests that it is prudent to conduct more studies before recommending this approach in children.
When the lesion is acute and rapid, I believe the anaemia, prostration, and peculiar condition of the skin will present a corresponding character and that whether acute or chronic, provided the lesion involve the entire structure of both organs (suprarenal glands), death will inevitably be the consequence. (Thomas Addison, MD 1855)
In 1855, Dr T Addison reported 12 adult patients who died with rather curious findings.1 These patients had hyperpigmentation and diseased suprarenal glands. All the deceased patients had similar clinical history of progressive malaise, fatigue and prostration before death. The diseases afflicting the suprarenal gland included tuberculosis, cancer and haemorrhage. As medical knowledge at the time was not aware of the role of cortisol, aldosterone or adrenocorticotrophic hormone (ACTH), and the term adrenal gland was not born, this syndrome was known as Addison's disease.2 Similar reports over the next decade documented the prevalence of Addison's disease outside Dr Addison's hospital.3 Dr Addison and others soon reported partial improvement in patients who were given suprarenal gland extracts.4 In 1894, Voelcker reported three patients who died with acute bacterial infection and suprarenal disease who did not have hyperpigmentation.5 He described this syndrome as “adrenal insufficiency related to acute infection” and hypothesised that hyperpigmentation was related to chronic adrenal insufficiency. Soon afterwards, Waterhouse and Friderischsen reported patients who died of bilateral adrenal haemorrhage, systemic fibrin thrombosis and acute bacterial infection.6,7
Insight into the mechanism by which suprarenal extract gave partial improvement in Addison's disease accelerated with the advent of analytical and synthetic organic chemistry.8,9,10 Administration of adrenaline caused short‐lived improvement; however, it was not until the late 1920s, 30s and 40s, and the discovery of steroid chemistry that therapeutic advances were made. Great excitement abounded as a multitude of case reports documented miraculous recovery in patients with Addison's disease who were treated with hydrocortisone.11,12 Animal and human investigations soon determined that the adrenal hormone cortisol is necessary to maintain glucose and cardiovascular function, and aldosterone to maintain cardiovascular and salt metabolism. The role of the hypothalamic and pituitary hormones—corticotrophin‐releasing hormone and ACTH—in stimulating the release of cortisol and aldosterone from the adrenal gland was also delineated.13 In paediatric medicine, this period also ushered the discovery of congenital adrenal hyperplasia and adrenal insufficiency as a reversible cause of death in newborns.14 Chronic administration of hydrocortisone to these infants reversed hypotension, hypoglycaemia and salt wasting in the same dramatic manner as it did in Addison's disease.
In the 1940s and 50s, Lillehei and others began evaluation of the role of hydrocortisone treatment in experimental models of endotoxic and septic shock.15,16,17,18,19 These investigators showed survival benefit with a dosage of 20–50 mg/kg/day. A multitude of case reports and series followed, showing miraculous reversal of previously fatal septic shock in children and young adults in the 1950s. So impressive and desired were these results that subsequent textbooks recommended the administration of 20–50 mg/kg of hydrocortisone followed by the same dose as a 24‐h continuous infusion for children with meningococcaemia and purpura.20,21 With the immediate need of saving the lives of patients with Addison's disease, congenital adrenal hyperplasia or acute infection‐related adrenal insufficiency accomplished, clinical investigations turned to better understanding of clinical diagnosis and dosing using information obtained by measuring cortisol levels in patients under conditions of stress, infection and shock.
In healthy volunteers, basal levels of cortisol were found to be 15 µg/dl in the morning. Under conditions of stress, including fever or seizures, these levels rose to >18 µg/dl. Similar to these times of stress, administration of 1 U ACTH increased cortisol levels above 18 µg/dl. Hence, adrenal insufficiency could be diagnosed by basal cortisol levels <4 µg/dl or ACTH stimulated levels <18 µg/dl. Indeed, patients with the clinical diagnosis of Addison's disease or congenital hyperplasia were found to meet these criteria with basal cortisol levels <4 µg/dl and ACTH stimulated levels <18 µg/dl. The dose of hydrocortisone treatment required has been based in part on these findings. Humans produce and excrete 0.5 mg/kg/day of cortisol; hence the physiological hydrocortisone replacement dose is 12.5 mg/m2/day or 0.5 mg/kg/day. The corroboration of this physiological replacement dose is found in its ability to adequately suppress increased ACTH levels and hyperpigmentation in patients with Addison's disease. Stress dose has been considered as four times this dose (50 mg/m2/day or 2 mg/kg/day). The adequacy of this dose has been shown by the clinical experience that patients with Addison's disease given this dose tolerate dental procedures and surgical stress. In general, physiological dosing maintains cortisol levels at 7–8 µg/dl, whereas stress doses maintain levels at 18–30 µg/dl. One important caveat exists when using ACTH stimulated cortisol levels to diagnose adrenal insufficiency. If the patient has a hypothalamic or pituitary‐based inability to stimulate adrenal cortical steroid production and excretion, then the ACTH test can be normal but the basal levels should be low. These patients can be diagnosed using the metapyrone test, which is described later.
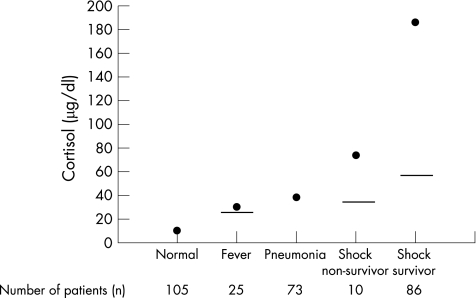
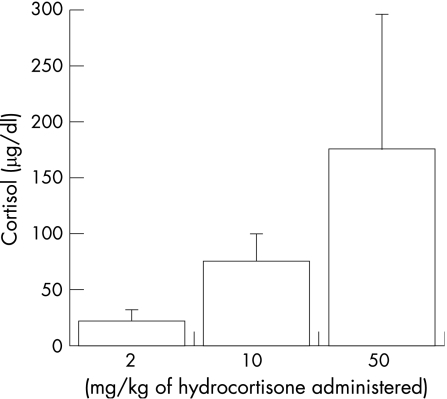
Basal cortisol levels were found to be higher in patients with fever, infection, severe infection and septic shock than in healthy volunteers. Children with fever have threefold higher cortisol levels (18–30 µg/dl),22 those with pneumonia have fivefold higher levels (30–50 µg/dl),23 and survivors of septic shock or meningitis have cortisol levels 3–90‐fold higher than basal levels (14–417 µg/dl; fig 1).24,25 Interestingly, the dosage recommended by Lillehei and others for use in patients with purpura‐related adrenal insufficiency, 25–50 mg/kg/day, attains cortisol levels between 150 and 300 µg/dl (fig 2). Administration of ACTH stimulation to these patients also showed differences in cortisol response compared with normal volunteers. Some patients with low basal levels did not reach a peak of 18 µg/dl, showing adrenal insufficiency similar to that found in patients with Addison's disease (absolute adrenal insufficiency). These patients would be expected to benefit from hydrocortisone treatment as they have both cortisol and aldosterone deficiency. However, among patients with basal cortisol levels >18 µg/dl, those with more severe disease had a blunted cortisol response with an incremental increase <9 µg/dl. This introduced the concept that patients with infection could have a diminished adrenal reserve even when they did not have Addisonian adrenal insufficiency (relative adrenal insufficiency). The conventional wisdom of the time from these observations was that patients with infection related adrenal insufficiency and septic shock should be treated with 25–50 mg/kg of hydrocortisone. A randomised controlled trial evaluated this approach in children with dengue shock and reported that randomisation to 35 mg/kg/day of hydrocortisone reduced mortality from 25% to 7%.26
Figure 1 Comparison of peak and median cortisol levels reported in paediatric illness, septic shock survivors and non‐survivors.21,22,24,25 Solid line represent median cartisol values for the study and are reported if data are available.
Figure 2 Serum cortisol levels observed after administration of various doses of hydrocortisone in children with septic shock.
The late 1970s and 1980s saw the widespread implementation of intensive care medicine and a remarkable twist in the steroid story. In 1974, Weitzman and Berger systematically reviewed 32 original clinical investigations, published from 1950 to 1971, that dealt with the use of corticosteroids in bacterial infections.27 The authors concluded that properly designed studies must be carried out before the controversy can be resolved. Investigators showed that baboons pretreated with supra‐pharmacological doses of methylprednisolone or dexamethasone, a synthetic steroid without any mineralocorticoid (aldosterone) effect but with a pronounced glucocorticoid effect, prevented mortality. The proposed mechanism of this pharmacology was not reversal of adrenal insufficiency, but rather the inhibition of tumour necrosis factor (TNF) gene expression through pharmacological stimulation of the glucocorticoid hormone responsive element. The glucocorticoid dose given (30 mg/kg Solu‐Medrol q 8 h × 3) is equivalent to 450 mg/kg/day of hydrocortisone, or >9 times the recommended dose in the paediatric literature of the time (table 1).
Table 1 Comparison of hydrocortisone dosages recommended for stress and shock with synthetic steroid dosages used to treat asthma, croup and spinal cord injury.
| Disease | Steroid used | Dose used | Equivalent dose of hydrocortisone |
|---|---|---|---|
| Asthma | Methylprednisolone | 4 mg/kg/day | 20 mg/kg/day |
| Airway oedema | Dexamethasone | 2 mg/kg/day | 53 mg/kg/day |
| Spinal cord injury | Methylprednisolone | 154 mg/kg (24 h) | 770 mg/kg (24 h) |
| Stress dose steroids | Hydrocortisone | 2 mg/kg/day | 2 mg/kg/day |
| Shock dose steroids | Hydrocortisone | 50 mg/kg/day | 50 mg/kg/day |
Three randomised controlled trials were carried out in adults with septic shock. The first study randomly assigned 59 patients with septic shock to receive either methylprednisolone or dexamethasone as compared with a control group. Although the mortality was comparable in all groups, patients who received steroids showed early reversal of shock. Two other large prospective, randomised, double‐blind, placebo‐controlled studies failed to show mortality benefit in patients with septic shock treated with methylprednisolone sodium succinate. One study showed higher mortality at 14 days in a subgroup of patients with raised serum creatinine.28,29,30 In response to these three studies, synthetic glucocorticoid use was stopped for the purpose of reducing inflammation. Unfortunately, these studies also seem to have extinguished memory of the previous Addisonian literature, and clinicians stopped using the natural steroid hydrocortisone for infection‐related adrenal insufficiency.
The late 1990s and 2000s produced a plethora of renewed investigation, which recalled, rediscovered and refined this memory.31,32,33,34,35,36,37,38,39,40,41 Investigators have shown that the modern child with infection, sepsis and septic shock similar to the child of the past can have one of three adrenal function states: (1) adrenal insufficiency similar to that observed in Addison's disease (about 20%); (2) a high cortisol level, but blunted adrenal response to ACTH stimulation, now called relative adrenal insufficiency (about 25%); or (3) a normal adrenal response to ACTH stimulation (cortisol increases by >9 µg/dl).42,43 In the remainder of this discussion, we will update the reader on what is known about children in each of these categories of adrenal function and provide our recommendations on hydrocortisone treatment for each (table 2).
Table 2 Recommendation summary.
| Cortisol levels | Recommendation | |
|---|---|---|
| Absolute adrenal insufficiency | Peak cortisol level <18 µg/dl after ACTH stimulation | Hydrocortisone 2–50 mg/kg as bolus, followed by infusion or intermittent doses (2–50 mg/kg/24 h titrated to haemodynamic homoeostasis) |
| Relative adrenal insufficiency | Basal cortisol level >18 µg/dl and ACTH response <9 µg/dl | Needs to be studied further |
| Pituitary failure | Basal cortisol level <5 µg/dl and peak >18 µg/dl | Hydrocortisone 2–50 mg/kg as bolus, followed by infusion of 2–50 mg/kg/24 h titrated to haemodynamic homoeostasis |
| Diagnosed with metapyrone test |
ACTH, adrenocorticotrophic hormone.
Recommendations
Children with septic shock and Addison's disease, with or without hyperpigmentation (peak cortisol after ACTH response <18 µg/dl) should be treated, according to tradition, with hydrocortisone directed to pharmacodynamic response and the goal of reversal of shock. Hydrocortisone will reverse warm shock and cold shock equally in these children. De Kleijn et al recently showed that non‐survivors of meningococcal septic shock have very low cortisol:ACTH ratios during the first 8 h of admission before their death (fig 3).44 Similarly, two other studies also showed considerably higher cortisol levels in meningococcal sepsis survivors as compared with non‐survivors.25,45
Figure 3 Reduced first 8‐hour cortisol:ACTH ratio in shock non‐survivors compared with shock survivors in meningococcal sepsis.44
The haemodynamic response to hydrocortisone in patients with septic shock is neither subtle nor delayed. We can see the desired haemodynamic response for a given level of hydrocortisone infusion as early as 15 min after initiation. Depending on the degree of extremis, the dose of hydrocortisone should be titrated to effect. The initial bolus of hydrocortisone can be between 2 and 50 mg/kg, followed by an infusion of 2–50 mg/kg/24 h. As hydrocortisone has a biological half‐life of 8–12 h, it can also be given intermittently. Although there are no randomised trials supporting this recommendation, the logic of the treatment appears compelling to us. If the adrenal gland is non‐functional and the child is dying from shock, then hydrocortisone should be given at whatever dose is necessary to restore cardiovascular homoeostasis (table 2).
For children with septic shock and high basal cortisol levels with a blunted response to ACTH, there is a need to understand whether long‐term treatment with intermittent stress dose hydrocortisone treatment improves outcome. Annane and others noted that adults with poor outcome from septic shock commonly had high cortisol levels with an ACTH stimulated cortisol response <9 µg/dl.46 In a large randomised controlled trial, adults with septic shock who required norepinephrine were randomised to usual treatment or a 7‐day course of intermittent hydrocortisone (100 mg thrice daily) and fludrocortisone (0.1 mg/day).47 These patients attained a cortisol level of approximately 100 µg/dl with this treatment (D Annane, personal communication). Overall, there was no difference in outcome between treatment groups. However, in an a priori planned retrospective analysis, patients with high basal cortisol levels and treated with steroids had their mortality reduced from 50% to 40%. The authors concluded that this regimen of “stress plus” dose glucocorticoids or mineralocorticoids should be given for 7 days to improve outcome in patients with relative adrenal insufficiency.47 Interestingly, none of these adult patients who had septic shock had Addison's disease. All reached peak cortisol levels well above 18 µg/dl with ACTH stimulation. Subsequent investigations have now shown that the leading risk factor for relative adrenal insufficiency in adult patients with septic shock is the use of etomidate for intubation.48 This hypnotic is a CYP450 and esterase inhibitor which reduces cortisol synthesis.49,50 Studies have shown that even one dose of etomidate in the emergency room reduces the cortisol increment response to ACTH and increases the incidence of relative adrenal insufficiency 12‐fold among patients in the intensive care unit.51 With this new information, Dr Annane reviewed the hydrocortisone and fludrocortisone trial47 and found that most of the patients in his trial who had relative adrenal insufficiency had received etomidate.48 Dr Annane's assessment of these observations is clear from the title of his editorial “ICU physicians should abandon the use of etomidate”.52 This call has been reinforced by an editorial on anaesthesia in which the authors recommend the use of ketamine rather than etomidate for emergent induction.53 Ketamine reduces systemic inflammation while maintaining cortisol production. We agree with the above editorial and recommend that ketamine and not etomidate should be used as the induction agent for intubation in children with septic shock.
Hatherill et al54 evaluated adrenal function in children and found a high degree of relative adrenal insufficiency. Steroid use was associated with weaning from catecholamine support, but no improvement in survival. In a larger survey, the PHIS database was analysed and use of steroids was associated with increased mortality.55 Adrenal function was not assessed in this study. On the basis of these findings of lack of benefit, we suggest that it is both ethical and prudent to further study the role of hydrocortisone treatment in paediatric patients with relative adrenal insufficiency.
Children with normal adrenal function are defined by an incremental increase in cortisol level >9 µg/dl. They may benefit from hydrocortisone treatment if they do not have a functioning pituitary axis. Lack of endogenous production of ACTH will prevent production of cortisol and aldosterone, but not a normal incremental cortisol response to exogenously administered ACTH. This population of children is growing as the use of acute and chronic steroid treatment is becoming common practice for patients with cancer, transplantation, asthma, croup, allergies and autoimmune disease. There is probably no busy paediatric primary care or emergency department physician who has a week go by without prescribing a steroid. In our own practice, 50% of children who present with shock have had prior steroid exposure (unpublished data). Studies now show that pituitary–adrenal axis suppression can remain for up to 6 weeks to 6 months after short‐term steroid use.56 Central nervous system disease can also provide a primary cause of ACTH failure. The metapyrone test can be used to assess the pituitary ACTH reserve.57 In the final step of adrenal steroidogenesis, the adrenocortical enzyme 11β hydroxylase converts 11‐deoxycortisol to cortisol. Inhibition of 11β hydroxylase by metapyrone leads to a decrease of circulating cortisol and accumulation of 11‐deoxycortisol. The decline in plasma cortisol stimulates ACTH production, thereby increasing adrenal steroidogenesis proximal to the enzyme blockade and causing 11‐deoxycortisol to accumulate further.58
We recommend hydrocortisone treatment in patients with pituitary or adrenal axis failure in the same manner as we do for patients with Addison's disease. Depending on the degree of extremis, pharmacological doses of hydrocortisone should be given as a bolus and then continuous infusion at a dosage of 2–50 mg/kg/day. Although there are no randomised controlled trials supporting this recommendation, the logic appears compelling to us. If the pituitary axis is unable to induce the adrenal gland to produce cortisol and aldosterone, and the patient is dying from shock, then hydrocortisone treatment should be given at whatever dose is necessary to reverse shock.
There are little data on the use of steroids for neonatal adrenal insufficiency. In a recent double‐blind, randomised controlled study, stress dose hydrocortisone (3 mg/kg/day) reduced the cumulative dose of dopamine and dobutamine, but did not improve survival in infants of very low birth weight.59 Dexamethasone use in neonates was associated with an increased incidence of neurodevelopmental delay and cerebral palsy, and led to the American Academy of Pediatrics recommending against its routine use to prevent or treat chronic lung disease.60 However, a single study that followed preterms treated with hydrocortisone did not show similar adverse effects. This study evaluated 25 preterm infants who received hydrocortisone 5 mg/kg/day for 7 days followed by a tapering course with a median duration of treatment of 26 days. A follow‐up of these children at 8 years of age with magnetic resonance imaging and standardised neurocognitive assessment showed no long‐term effects on either neurostructural brain development or neurocognitive outcomes.61
Abbreviations
ACTH - adrenocorticotrophic hormone
Footnotes
Competing interests: None.
References
- 1.Addison T. On the constitutional and local effects of disease of the supra‐renal capsules. Lond Med Gaz 184943517–518. [Google Scholar]
- 2.Wilson L. Internal secretions in disease: the historical relations of clinical medicine and scientific physiology. J Hist Med Allied Sci 198439263–302. [DOI] [PubMed] [Google Scholar]
- 3.Greenhow E.Addison's disease. London: JW Roche, 1866
- 4.Oliver G, Schafer E. The physiological effects of extracts of the suprarenal capsules. J Physiol 189518230–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voelcker A. Abstract of post mortem report. Middlesex Hospital Rep Med Surg Path Rregistrars 1894278
- 6.Waterhouse R. A case of suprarenal apoplexy. Lancet 19111577–578. [Google Scholar]
- 7.Friderichsen C. Waterhouse‐Friderichsen syndrome. Acta Endocrinol Suppl (Copenh) 195518482–492. [DOI] [PubMed] [Google Scholar]
- 8.Von Fiirth O. The catechol‐like substance of the suprarenal. J Chem Soc Abstr 190078292 [Google Scholar]
- 9.Takamine J. Adrenalin, the active principle of the suprarenal glands and its mode of preparation. Am J Pharm 190173523–531. [Google Scholar]
- 10.Aldrich T. A preliminary report on the active principle of the suprarenal gland. Am J Physiol 19015457–461. [Google Scholar]
- 11.Muirhead A. An autograph history of a case of Addison's disease. J Am Med Assoc 192279556–557. [Google Scholar]
- 12.Rowntree L. Subsequent course of a case of Addison's disease. J Am Med Assoc 192279556–557. [Google Scholar]
- 13.Smith P. J Am Med Assoc. 1927;66:158. [Google Scholar]
- 14.De Crecchio L. Sopra un caso di apparenzi virili in una donna. Morgagni 18657154–188. [Google Scholar]
- 15.Lillehei R, MacLean L. Physiological approach to successful treatment of endotoxin shock in the experimental animal. Arch Surg 195978464. [DOI] [PubMed] [Google Scholar]
- 16.Kass E H, Finland M. Adrenocortical hormones in infection and immunity. Annu Rev Microbiol 19537361–388. [DOI] [PubMed] [Google Scholar]
- 17.Kass E H, Finland M. Adrenocortical hormones and the management of infection. Annu Rev Med 195781–18. [DOI] [PubMed] [Google Scholar]
- 18.Kass E H, Finland M. Corticosteroids and infections. Adv Intern Med 1958945–80. [PubMed] [Google Scholar]
- 19.Robinson H J. Adrenal steroids and resistance to infection. Antibiot Chemother 19607199–240. [DOI] [PubMed] [Google Scholar]
- 20.Migeon C. Adrenal steroid therapy. In: Rudolph A, Barnett H, Einhorn A, eds. Pediatrics, 16th edn. New York: Appleton‐Century‐Crofts, 19771651–1662.
- 21.McEvoy G. Hormones and synthetic substitutes. The American Hospital Formulary. pp. 04–08.
- 22.Singh U K, Jana U K. Serum prolactin and cortisol in children with some paroxysmal disorders. Indian J Pediatr 19946157–61. [DOI] [PubMed] [Google Scholar]
- 23.Nickels D A, Moore D C. Serum cortisol responses in febrile children. Pediatr Infect Dis J 1989816–20. [DOI] [PubMed] [Google Scholar]
- 24.Schein R M, Sprung C L, Marcial E.et al Plasma cortisol levels in patients with septic shock. Crit Care Med 199018259–263. [DOI] [PubMed] [Google Scholar]
- 25.Riordan F A, Thomson A P, Ratcliffe J M.et al Admission cortisol and adrenocorticotrophic hormone levels in children with meningococcal disease: evidence of adrenal insufficiency? Crit Care Med 1999272257–2261. [DOI] [PubMed] [Google Scholar]
- 26.Min M, UT, Aye M.et al Hydrocortisone in the management of dengue shock syndrome. Southeast Asian J Trop Med Public Health 19756573–579. [PubMed] [Google Scholar]
- 27.Weitzman S, Berger S. Clinical trial design in studies of corticosteroids for bacterial infections. Ann Intern Med 19748136–42. [DOI] [PubMed] [Google Scholar]
- 28.Sprung C L, Caralis P V, Marcial E H.et al The effects of high‐dose corticosteroids in patients with septic shock. A prospective, controlled study. N Engl J Med 19843111137–1143. [DOI] [PubMed] [Google Scholar]
- 29.The Veterans Administration Systemic Sepsis Cooperative Study Group Effect of high‐dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. N Engl J Med 1987317659–665. [DOI] [PubMed] [Google Scholar]
- 30.Bone R C, Fisher C J, Clemmer T P.et al A controlled clinical trial of high‐dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med 1987317653–658. [DOI] [PubMed] [Google Scholar]
- 31.Hinshaw L B, Beller B K, Chang A C.et al Corticosteroid/antibiotic treatment of adrenalectomized dogs challenged with lethal E. coli. Circ Shock 198516265–277. [PubMed] [Google Scholar]
- 32.Claussen M S, Landercasper J, Cogbill T H. Acute adrenal insufficiency presenting as shock after trauma and surgery: three cases and review of the literature. J Trauma 19923294–100. [DOI] [PubMed] [Google Scholar]
- 33.Lee L M, Gumowski J. Adrenocortical insufficiency: a medical emergency. AACN Clin Issues Crit Care Nurs 19923319–330. [DOI] [PubMed] [Google Scholar]
- 34.Briegel J, Kellermann W, Forst H.et al Low‐dose hydrocortisone infusion attenuates the systemic inflammatory response syndrome. The Phospholipase A2 Study Group. Clin Invest 199472782–787. [DOI] [PubMed] [Google Scholar]
- 35.Bouachour G, Tirot P, Gouello J P.et al Adrenocortical function during septic shock. Intensive Care Med 19952157–62. [DOI] [PubMed] [Google Scholar]
- 36.Soni A, Pepper G M, Wyrwinski P M.et al Adrenal insufficiency occurring during septic shock: incidence, outcome, and relationship to peripheral cytokine levels. Am J Med 199598266–271. [DOI] [PubMed] [Google Scholar]
- 37.Briegel J, Schelling G, Haller M.et al A comparison of the adrenocortical response during septic shock and after complete recovery. Intensive Care Med 199622894–899. [DOI] [PubMed] [Google Scholar]
- 38.Aygen B, Inan M, Doganay M.et al Adrenal functions in patients with sepsis. Exp Clin Endocrinol Diabetes 1997105182–186. [DOI] [PubMed] [Google Scholar]
- 39.Duggan M, Browne I, Flynn C. Adrenal failure in the critically ill. Br J Anaesth 199881468–470. [DOI] [PubMed] [Google Scholar]
- 40.Cronin L, Cook D J, Carlet J.et al Corticosteroid treatment for sepsis: a critical appraisal and meta‐analysis of the literature. Crit Care Med 1995231430–1439. [DOI] [PubMed] [Google Scholar]
- 41.Lefering R, Neugebauer E A. Steroid controversy in sepsis and septic shock: a meta‐analysis. Crit Care Med 1995231294–1303. [DOI] [PubMed] [Google Scholar]
- 42.Pizarro C F, Troster E J, Damiani D.et al Absolute and relative adrenal insufficiency in children with septic shock. Crit Care Med 200533855–859. [DOI] [PubMed] [Google Scholar]
- 43.Casartelli C H, Garcia P C, Piva J P.et al Adrenal insufficiency in children with septic shock. J Pediatr (Rio J) 200379(Suppl 2)S169–S176. [DOI] [PubMed] [Google Scholar]
- 44.De Kleijn E D, Joosten K F, Van Rijn B.et al Low serum cortisol in combination with high adrenocorticotrophic hormone concentrations are associated with poor outcome in children with severe meningococcal disease. Pediatr Infect Dis J 200221330–336. [DOI] [PubMed] [Google Scholar]
- 45.Joosten K F, de Kleijn E D, Westerterp M.et al Endocrine and metabolic responses in children with meningoccocal sepsis: striking differences between survivors and nonsurvivors. J Clin Endocrinol Metab 2000853746–3753. [DOI] [PubMed] [Google Scholar]
- 46.Annane D, Sebille V, Troche G.et al A 3‐level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA 20002831038–1045. [DOI] [PubMed] [Google Scholar]
- 47.Annane D, Sebille V, Charpentier C.et al Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002288862–871. [DOI] [PubMed] [Google Scholar]
- 48.Annane D, Sebille V, Bellissant E. Corticosteroids for patients with septic shock. JAMA 200328943–44. [DOI] [PubMed] [Google Scholar]
- 49.Wagner R L, White P F. Etomidate inhibits adrenocortical function in surgical patients. Anesthesiology 198461647–651. [DOI] [PubMed] [Google Scholar]
- 50.Wagner R L, White P F, Kan P B.et al Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N Engl J Med 19843101415–1421. [DOI] [PubMed] [Google Scholar]
- 51.den Brinker M, Joosten K F, Liem O.et al Adrenal insufficiency in meningococcal sepsis: bioavailable cortisol levels and impact of interleukin‐6 levels and intubation with etomidate on adrenal function and mortality. J Clin Endocrinol Metab 2005905110–5117. [DOI] [PubMed] [Google Scholar]
- 52.Annane D. ICU physicians should abandon the use of etomidate. Intensive Care Med 200531325–326. [DOI] [PubMed] [Google Scholar]
- 53.Morris C, McAllister C. Etomidate for emergency anaesthesia; mad, bad and dangerous to know? Anaesthesia 200560737–740. [DOI] [PubMed] [Google Scholar]
- 54.Hatherill M, Tibby S M, Hilliard T.et al Adrenal insufficiency in septic shock. Arch Dis Child 19998051–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Markovitz B P, Goodman D M, Watson R S.et al A retrospective cohort study of prognostic factors associated with outcome in pediatric severe sepsis: what is the role of steroids? Pediatr Crit Care Med 20056270–274. [DOI] [PubMed] [Google Scholar]
- 56.Rix M, Birkebaek N H, Rosthoj S.et al Clinical impact of corticosteroid‐induced adrenal suppression during treatment for acute lymphoblastic leukemia in children: a prospective observational study using the low‐dose adrenocorticotropic test. J Pediatr 2005147645–650. [DOI] [PubMed] [Google Scholar]
- 57.Liddle G W, Estep H L, Kendall J W., Jret al Clinical application of a new test of pituitary reserve. J Clin Endocrinol Metab 195919875–894. [DOI] [PubMed] [Google Scholar]
- 58.Berneis K, Staub J J, Gessler A.et al Combined stimulation of adrenocorticotropic and compound‐S by single dose metyrapone test as an outpatient procedure to assess hypothalamic‐pituitary‐adrenal function. J Clin Endocrinol Metab 2002875470–5475. [DOI] [PubMed] [Google Scholar]
- 59.Ng P C, Lee C H, Bnur F L.et al A double‐blind, randomized, controlled study of a “stress dose” of hydrocortisone for rescue treatment of refractory hypotension in preterm infants. Pediatrics 2006117367–375. [DOI] [PubMed] [Google Scholar]
- 60.Committee on Fetus and N Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics 2002109330–338. [DOI] [PubMed] [Google Scholar]
- 61.Lodygensky G A, Rademaker K, Zimine S.et al Structural and functional brain development after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics 20051161–7. [DOI] [PubMed] [Google Scholar]