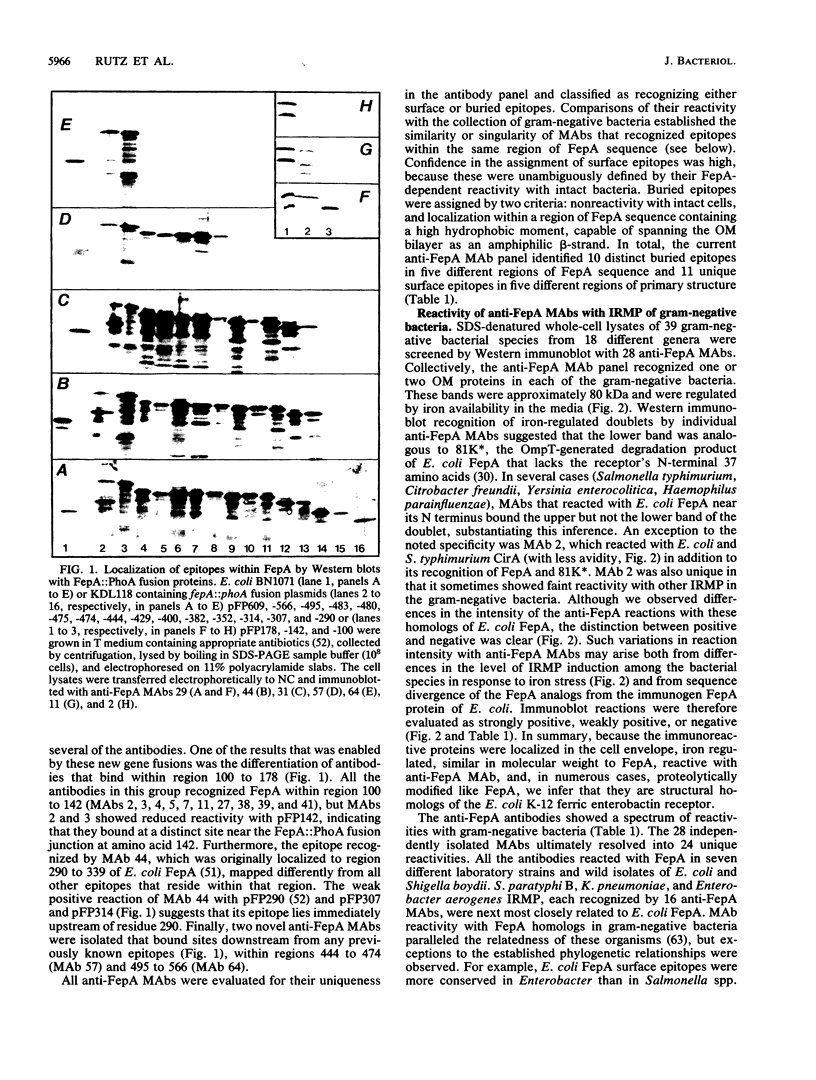
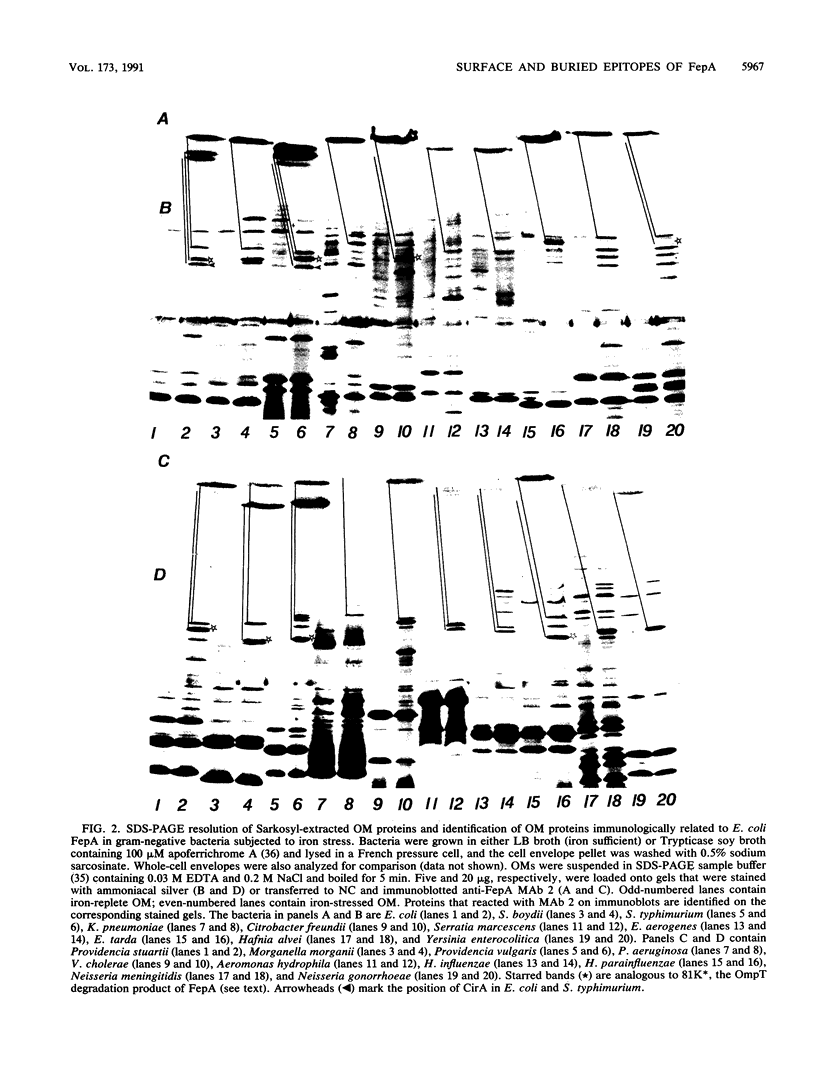
Abstract
Using sodium dodecyl sulfate-polyacrylamide gel electrophoresis of iron-deficient and replete cell envelopes, 59Fe-siderophore uptake studies, and Western immunoblots and cytofluorimetric analyses with monoclonal antibodies (MAbs), we surveyed a panel of gram-negative bacteria to identify outer membrane proteins that are structurally related to the Escherichia coli K-12 ferric enterobactin receptor, FepA. Antibodies within the panel identified FepA epitopes that are conserved among the majority of the bacteria tested, as well as epitopes present in only a few of the strains. In general, epitopes of FepA that are buried in the outer membrane bilayer were more conserved among gram-negative bacteria than epitopes that are exposed on the bacterial cell surface. The surface topology and tertiary structure of FepA are quite similar in E. coli and Shigella flexneri but differ in Salmonella typhimurium. Of the 18 different genera tested, 94% of the bacteria transported ferric enterobactin, including members of the previously unrecognized genera Citrobacter, Edwardsiella, Enterobacter, Haemophilus, Hafnia, Morganella, Neisseria, Proteus, Providencia, Serratia, and Yersinia. The ferric enterobactin receptor contains at least one buried epitope, recognized by MAb 2 (C. K. Murphy, V. I. Kalve, and P. E. Klebba, J. Bacteriol. 172:2736-2746, 1990), that is conserved within the structure of an iron-regulated cell envelope protein in all the bacteria that we have surveyed. With MAb 2, we identified and determined the Mr of cell envelope antigens that are immunologically related to E. coli FepA in all the gram-negative bacteria tested. Collectively, the library of anti-FepA MAbs showed unique patterns of reactivity with the different bacteria, allowing identification and discrimination of species within the following gram-negative genera: Aeromonas, Citrobacter, Edwardsiella, Enterobacter, Escherichia, Haemophilus, Hafnia, Klebsiella, Morganella, Neisseria, Proteus, Providencia, Pseudomonas, Salmonella, Serratia, Shigella, Vibrio, and Yersinia.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaro C., Aznar R., Alcaide E., Lemos M. L. Iron-binding compounds and related outer membrane proteins in Vibrio cholerae non-O1 strains from aquatic environments. Appl Environ Microbiol. 1990 Aug;56(8):2410–2416. doi: 10.1128/aem.56.8.2410-2416.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong S. K., Francis C. L., McIntosh M. A. Molecular analysis of the Escherichia coli ferric enterobactin receptor FepA. J Biol Chem. 1990 Aug 25;265(24):14536–14543. [PubMed] [Google Scholar]
- Banerjee-Bhatnagar N., Frasch C. E. Expression of Neisseria meningitidis iron-regulated outer membrane proteins, including a 70-kilodalton transferrin receptor, and their potential for use as vaccines. Infect Immun. 1990 Sep;58(9):2875–2881. doi: 10.1128/iai.58.9.2875-2881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley A. T., Klebba P. E. Effect of lipopolysaccharide structure on reactivity of antiporin monoclonal antibodies with the bacterial cell surface. J Bacteriol. 1988 Mar;170(3):1063–1068. doi: 10.1128/jb.170.3.1063-1068.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin C. A., Jensen A. E. Passive immunization with antibodies against iron-regulated outer membrane proteins protects turkeys from Escherichia coli septicemia. Infect Immun. 1987 May;55(5):1239–1242. doi: 10.1128/iai.55.5.1239-1242.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauner A., Källenius G., Wrangsell G., Wretlind B., Svenson S. B. Antibody responses to Escherichia coli J5 lipopolysaccharide and to Salmonella porin in patients with bacteremia. Microb Pathog. 1986 Oct;1(5):475–481. doi: 10.1016/0882-4010(86)90009-4. [DOI] [PubMed] [Google Scholar]
- Carniel E., Antoine J. C., Guiyoule A., Guiso N., Mollaret H. H. Purification, location, and immunological characterization of the iron-regulated high-molecular-weight proteins of the highly pathogenic yersiniae. Infect Immun. 1989 Feb;57(2):540–545. doi: 10.1128/iai.57.2.540-545.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carniel E., Mazigh D., Mollaret H. H. Expression of iron-regulated proteins in Yersinia species and their relation to virulence. Infect Immun. 1987 Jan;55(1):277–280. doi: 10.1128/iai.55.1.277-280.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Charbit A., Gehring K., Nikaido H., Ferenci T., Hofnung M. Maltose transport and starch binding in phage-resistant point mutants of maltoporin. Functional and topological implications. J Mol Biol. 1988 Jun 5;201(3):487–496. doi: 10.1016/0022-2836(88)90630-4. [DOI] [PubMed] [Google Scholar]
- Chart H., Buck M., Stevenson P., Griffiths E. Iron regulated outer membrane proteins of Escherichia coli: variations in expression due to the chelator used to restrict the availability of iron. J Gen Microbiol. 1986 May;132(5):1373–1378. doi: 10.1099/00221287-132-5-1373. [DOI] [PubMed] [Google Scholar]
- Chart H., Griffiths E. Antigenic and molecular homology of the ferric enterobactin receptor protein of Escherichia coli. J Gen Microbiol. 1985 Jun;131(6):1503–1509. doi: 10.1099/00221287-131-6-1503. [DOI] [PubMed] [Google Scholar]
- Chart H., Stevenson P., Griffiths E. Iron-regulated outer-membrane proteins of Escherichia coli strains associated with enteric or extraintestinal diseases of man and animals. J Gen Microbiol. 1988 Jun;134(6):1549–1559. doi: 10.1099/00221287-134-6-1549. [DOI] [PubMed] [Google Scholar]
- Crosa J. H. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol Rev. 1989 Dec;53(4):517–530. doi: 10.1128/mr.53.4.517-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneer H. G., Potter A. A. Iron-repressible outer-membrane proteins of Pasteurella haemolytica. J Gen Microbiol. 1989 Feb;135(Pt 2):435–443. doi: 10.1099/00221287-135-2-435. [DOI] [PubMed] [Google Scholar]
- Ernst J. F., Bennett R. L., Rothfield L. I. Constitutive expression of the iron-enterochelin and ferrichrome uptake systems in a mutant strain of Salmonella typhimurium. J Bacteriol. 1978 Sep;135(3):928–934. doi: 10.1128/jb.135.3.928-934.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanylo L. P., Kadis S., Maudsley J. R. Siderophore production by Proteus mirabilis. Can J Microbiol. 1984 Aug;30(8):1046–1051. doi: 10.1139/m84-163. [DOI] [PubMed] [Google Scholar]
- Fenwick B. W., Cullor J. S., Osburn B. I., Olander H. J. Mechanisms involved in protection provided by immunization against core lipopolysaccharides of Escherichia coli J5 from lethal Haemophilus pleuropneumoniae infections in swine. Infect Immun. 1986 Aug;53(2):298–304. doi: 10.1128/iai.53.2.298-304.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Beros M. E., Gonzalez C., McIntosh M. A., Cabello F. C. Immune response to the iron-deprivation-induced proteins of Salmonella typhi in typhoid fever. Infect Immun. 1989 Apr;57(4):1271–1275. doi: 10.1128/iai.57.4.1271-1275.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay J., Benson S., Schwartz M. Genetic mapping of antigenic determinants on a membrane protein. J Biol Chem. 1983 Feb 25;258(4):2410–2414. [PubMed] [Google Scholar]
- Getzoff E. D., Geysen H. M., Rodda S. J., Alexander H., Tainer J. A., Lerner R. A. Mechanisms of antibody binding to a protein. Science. 1987 Mar 6;235(4793):1191–1196. doi: 10.1126/science.3823879. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Tainer J. A., Rodda S. J., Mason T. J., Alexander H., Getzoff E. D., Lerner R. A. Chemistry of antibody binding to a protein. Science. 1987 Mar 6;235(4793):1184–1190. doi: 10.1126/science.3823878. [DOI] [PubMed] [Google Scholar]
- Goldberg M. B., DiRita V. J., Calderwood S. B. Identification of an iron-regulated virulence determinant in Vibrio cholerae, using TnphoA mutagenesis. Infect Immun. 1990 Jan;58(1):55–60. doi: 10.1128/iai.58.1.55-60.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterman S. K., Dann L. Excretion of enterochelin by exbA and exbB mutants of Escherichia coli. J Bacteriol. 1973 Jun;114(3):1225–1230. doi: 10.1128/jb.114.3.1225-1230.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterman S. K., Dann L. Excretion of enterochelin by exbA and exbB mutants of Escherichia coli. J Bacteriol. 1973 Jun;114(3):1225–1230. doi: 10.1128/jb.114.3.1225-1230.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom R. C., Pavlovskis O. R., Galloway D. R. Antibody response of infected mice to outer membrane proteins of Pseudomonas aeruginosa. Infect Immun. 1984 Jan;43(1):49–53. doi: 10.1128/iai.43.1.49-53.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen A. Z., Maeland J. A. The porin protein of the outer membrane of Escherichia coli: reactivity in immunoblotting, antibody-binding by the native protein, and cross-reactivity with other enteric bacteria. Acta Pathol Microbiol Immunol Scand B. 1987 Oct;95(5):315–321. doi: 10.1111/j.1699-0463.1987.tb03131.x. [DOI] [PubMed] [Google Scholar]
- Hofstra H., Van Tol J. D., Dankert J. Cross-reactivity of major outer membrane proteins of Enterobacteriaceae, studied by crossed immunoelectrophoresis. J Bacteriol. 1980 Jul;143(1):328–337. doi: 10.1128/jb.143.1.328-337.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollifield W. C., Jr, Fiss E. H., Neilands J. B. Modification of a ferric enterobactin receptor protein from the outer membrane of Escherichia coli. Biochem Biophys Res Commun. 1978 Jul 28;83(2):739–746. doi: 10.1016/0006-291x(78)91051-3. [DOI] [PubMed] [Google Scholar]
- Ikeda J. S., Hirsh D. C. Antigenically related iron-regulated outer membrane proteins produced by different somatic serotypes of Pasteurella multocida. Infect Immun. 1988 Sep;56(9):2499–2502. doi: 10.1128/iai.56.9.2499-2502.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns M., Skehill A., McCabe W. R. Immunization with rough mutants of Salmonella minnesota. IV. Protection by antisera to O and rough antigens against endotoxin. J Infect Dis. 1983 Jan;147(1):57–67. doi: 10.1093/infdis/147.1.57. [DOI] [PubMed] [Google Scholar]
- Kadurugamuwa J. L., Anwar H., Brown M. R., Shand G. H., Ward K. H. Media for study of growth kinetics and envelope properties of iron-deprived bacteria. J Clin Microbiol. 1987 May;25(5):849–855. doi: 10.1128/jcm.25.5.849-855.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Klebba P. E., Benson S. A., Bala S., Abdullah T., Reid J., Singh S. P., Nikaido H. Determinants of OmpF porin antigenicity and structure. J Biol Chem. 1990 Apr 25;265(12):6800–6810. [PubMed] [Google Scholar]
- Klebba P. E., McIntosh M. A., Neilands J. B. Kinetics of biosynthesis of iron-regulated membrane proteins in Escherichia coli. J Bacteriol. 1982 Mar;149(3):880–888. doi: 10.1128/jb.149.3.880-888.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T., Kawata T. Composition of major outer membrane proteins of Vibrio vulnificus isolates: effect of different growth media and iron deficiency. Microbiol Immunol. 1986;30(3):193–201. doi: 10.1111/j.1348-0421.1986.tb00935.x. [DOI] [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Saxen H., Valtonen M., Mäkelä P. H. Immunization with major outer membrane proteins in experimental salmonellosis of mice. Infect Immun. 1979 Sep;25(3):857–862. doi: 10.1128/iai.25.3.857-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Saxén H., Mäkelä P. H. Immunization with major outer membrane protein (porin) preparations in experimental murine salmonellosis: effect of lipopolysaccharide. Infect Immun. 1981 Nov;34(2):328–332. doi: 10.1128/iai.34.2.328-332.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos M. L., Salinas P., Toranzo A. E., Barja J. L., Crosa J. H. Chromosome-mediated iron uptake system in pathogenic strains of Vibrio anguillarum. J Bacteriol. 1988 Apr;170(4):1920–1925. doi: 10.1128/jb.170.4.1920-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J., Neilands J. B. Mechanisms of siderophore iron transport in enteric bacteria. J Bacteriol. 1976 May;126(2):823–830. doi: 10.1128/jb.126.2.823-830.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge J. M., Williams P., Brown M. R. Influence of growth rate and iron limitation on the expression of outer membrane proteins and enterobactin by Klebsiella pneumoniae grown in continuous culture. J Bacteriol. 1986 Feb;165(2):353–356. doi: 10.1128/jb.165.2.353-356.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lundrigan M. D., Kadner R. J. Nucleotide sequence of the gene for the ferrienterochelin receptor FepA in Escherichia coli. Homology among outer membrane receptors that interact with TonB. J Biol Chem. 1986 Aug 15;261(23):10797–10801. [PubMed] [Google Scholar]
- Mackie E. B., Longenecker B. M., Rabin H. R., Di Ninno V. L., Bryan L. E. Immune response of the mouse to gram-negative bacterial outer membrane extracts as assessed with monoclonal antibodies. J Immunol. 1982 Aug;129(2):829–832. [PubMed] [Google Scholar]
- Malouin F., Campbell G. D., Halpenny M., Becker G. W., Parr T. R., Jr Outer membrane and porin characteristics of Serratia marcescens grown in vitro and in rat intraperitoneal diffusion chambers. Infect Immun. 1990 May;58(5):1247–1253. doi: 10.1128/iai.58.5.1247-1253.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe W. R., Bruins S. C., Craven D. E., Johns M. Cross-reactive antigens: their potential for immunization-induced immunity to Gram-negative bacteria. J Infect Dis. 1977 Aug;136 (Suppl):S161–S166. doi: 10.1093/infdis/136.supplement.s161. [DOI] [PubMed] [Google Scholar]
- McIntosh M. A., Earhart C. F. Coordinate regulation by iron of the synthesis of phenolate compounds and three outer membrane proteins in Escherichia coli. J Bacteriol. 1977 Jul;131(1):331–339. doi: 10.1128/jb.131.1.331-339.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton D. J., Williams P. Characterization of the outer-membrane proteins of Haemophilus parainfluenzae expressed under iron-sufficient and iron-restricted conditions. J Gen Microbiol. 1989 Feb;135(Pt 2):445–451. doi: 10.1099/00221287-135-2-445. [DOI] [PubMed] [Google Scholar]
- Morton D. J., Williams P. Siderophore-independent acquisition of transferrin-bound iron by Haemophilus influenzae type b. J Gen Microbiol. 1990 May;136(5):927–933. doi: 10.1099/00221287-136-5-927. [DOI] [PubMed] [Google Scholar]
- Murphy C. K., Kalve V. I., Klebba P. E. Surface topology of the Escherichia coli K-12 ferric enterobactin receptor. J Bacteriol. 1990 May;172(5):2736–2746. doi: 10.1128/jb.172.5.2736-2746.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C. K., Klebba P. E. Export of FepA::PhoA fusion proteins to the outer membrane of Escherichia coli K-12. J Bacteriol. 1989 Nov;171(11):5894–5900. doi: 10.1128/jb.171.11.5894-5900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunnariwo J. A., Schryvers A. B. Iron acquisition in Pasteurella haemolytica: expression and identification of a bovine-specific transferrin receptor. Infect Immun. 1990 Jul;58(7):2091–2097. doi: 10.1128/iai.58.7.2091-2097.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozenberger B. A., Nahlik M. S., McIntosh M. A. Genetic organization of multiple fep genes encoding ferric enterobactin transport functions in Escherichia coli. J Bacteriol. 1987 Aug;169(8):3638–3646. doi: 10.1128/jb.169.8.3638-3646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M. Synthesis and utilization of siderophores by Shigella flexneri. J Bacteriol. 1980 Sep;143(3):1420–1424. doi: 10.1128/jb.143.3.1420-1424.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson A., Kuipers B., Pelzer M., Verhagen E., Tiesjema R. H., Tommassen J., Poolman J. T. Monoclonal antibodies against the 70-kilodalton iron-regulated protein of Neisseria meningitidis are bactericidal and strain specific. Infect Immun. 1990 Sep;58(9):3036–3041. doi: 10.1128/iai.58.9.3036-3041.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. R., Earhart C. F. Escherichia coli K-12 envelope proteins specifically required for ferrienterobactin uptake. J Bacteriol. 1986 Jun;166(3):930–936. doi: 10.1128/jb.166.3.930-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K., Young L., Neshat S. Enterobactin-mediated iron transport in Pseudomonas aeruginosa. J Bacteriol. 1990 Dec;172(12):6991–6996. doi: 10.1128/jb.172.12.6991-6996.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins-Browne R. M., Prpic J. K., Stuart S. J. Yersiniae and iron. A study in host-parasite relationships. Contrib Microbiol Immunol. 1987;9:254–258. [PubMed] [Google Scholar]
- Rocque W. J., Coughlin R. T., McGroarty E. J. Lipopolysaccharide tightly bound to porin monomers and trimers from Escherichia coli K-12. J Bacteriol. 1987 Sep;169(9):4003–4010. doi: 10.1128/jb.169.9.4003-4010.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E. Genetic relatedness in the family Enterobacteriaceae. Annu Rev Microbiol. 1976;30:327–349. doi: 10.1146/annurev.mi.30.100176.001551. [DOI] [PubMed] [Google Scholar]
- Schmitt M. P., Payne S. M. Genetic analysis of the enterobactin gene cluster in Shigella flexneri. J Bacteriol. 1991 Jan;173(2):816–825. doi: 10.1128/jb.173.2.816-825.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Sutcliffe J. G., Green N., Lerner R. A. Synthetic peptide immunogens as vaccines. Annu Rev Microbiol. 1983;37:425–446. doi: 10.1146/annurev.mi.37.100183.002233. [DOI] [PubMed] [Google Scholar]
- Snipes K. P., Hansen L. M., Hirsh D. C. Plasma- and iron-regulated expression of high molecular weight outer membrane proteins by Pasteurella multocida. Am J Vet Res. 1988 Aug;49(8):1336–1338. [PubMed] [Google Scholar]
- Taplits M., Michael J. G. Immune response to Escherichia coli B surface antigens. Infect Immun. 1979 Sep;25(3):943–945. doi: 10.1128/iai.25.3.943-945.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H., Jähnig F. Models for the structure of outer-membrane proteins of Escherichia coli derived from raman spectroscopy and prediction methods. J Mol Biol. 1986 Jul 20;190(2):191–199. doi: 10.1016/0022-2836(86)90292-5. [DOI] [PubMed] [Google Scholar]
- Walter M. A., Potter S. A., Crosa J. H. Iron uptake system medicated by Vibrio anguillarum plasmid pJM1. J Bacteriol. 1983 Nov;156(2):880–887. doi: 10.1128/jb.156.2.880-887.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. C., Newton A. An additional step in the transport of iron defined by the tonB locus of Escherichia coli. J Biol Chem. 1971 Apr 10;246(7):2147–2151. [PubMed] [Google Scholar]
- Ward K. H., Anwar H., Brown R. W., Wale J., Gowar J. Antibody response to outer-membrane antigens of Pseudomonas aeruginosa in human burn wound infection. J Med Microbiol. 1988 Nov;27(3):179–190. doi: 10.1099/00222615-27-3-179. [DOI] [PubMed] [Google Scholar]
- Wayne R., Frick K., Neilands J. B. Siderophore protection against colicins M, B, V, and Ia in Escherichia coli. J Bacteriol. 1976 Apr;126(1):7–12. doi: 10.1128/jb.126.1.7-12.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. E., Sparling P. F. Aerobactin utilization by Neisseria gonorrhoeae and cloning of a genomic DNA fragment that complements Escherichia coli fhuB mutations. J Bacteriol. 1987 Aug;169(8):3414–3421. doi: 10.1128/jb.169.8.3414-3421.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect Immun. 1979 Dec;26(3):925–932. doi: 10.1128/iai.26.3.925-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsham P. L., Konisky J. Effect of growth temperature on the acquisition of iron by Salmonella typhimurium and Escherichia coli. J Bacteriol. 1984 Apr;158(1):163–168. doi: 10.1128/jb.158.1.163-168.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Fierer J., Glauser M. P., Sadoff J. C., Douglas H., Braude A. I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982 Nov 11;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]