Abstract
Objective
To describe the short‐term outcome of critically ill HIV‐infected children with access to highly active antiretroviral therapy (HAART) in a developing region.
Methods
Prospective observational study conducted in a paediatric teaching hospital in Cape Town, South Africa. All children admitted to the paediatric intensive care unit (PICU) with suspected HIV infection were screened. Data are n (%) with 95% confidence intervals.
Results
Sixty eight of 96 HIV antibody‐positive children, median age 3 months, were confirmed HIV‐infected. Predicted PICU mortality was 0.42. Fifty one children (75%; 95% CI 65 to 85%) survived to PICU discharge, but hospital survival was only 51% (95% CI 40 to 63%). Limitation of intervention (LOI) decisions were a factor in the majority of PICU and ward deaths. Twenty one PICU survivors (31%; 95% CI 20 to 42%) commenced HAART, and two children were already on treatment. Nineteen children (28%) were considered to be established on HAART after 1 month. Thirteen HIV‐infected children (19%; 95% CI 10 to 28%), representing 25% (95% CI 14 to 37%) of all PICU survivors, and 68% (95% CI 48 to 89%) of those PICU survivors who were established on HAART remain well on treatment after median 350 days.
Conclusion
The majority of HIV‐infected children survived to discharge from PICU, but only half survived to hospital discharge. LOI decisions, usually made in PICU, directly influenced short‐term survival and the opportunity to commence HAART. Although few critically ill HIV‐infected children survived to become established on HAART, the long‐term outcome of children on HAART is encouraging and warrants further investigation.
UNAIDS (the Joint United Nations Programme on HIV/AIDS) estimated that in 2003 almost 38 million people were living with HIV, including 2.1 million children, and approximately 630 000 children were newly infected in that year.1 In developed countries, prevention‐of‐mother‐to‐child‐transmission (PMTCT) programmes have led to a reduction in vertical transmission rates from 15–20% to less than 2%.2,3,4 However, the incidence of perinatal HIV infection remains high in developing countries, where large numbers of undiagnosed, antiretroviral‐naïve children continue to present to state health services.5,6,7
HIV‐infected infants frequently present to health services for the first time with a life‐threatening critical illness.8 This problem is magnified several fold in high‐prevalence developing regions, where rationing of resources for those who might derive maximal benefit is an inescapable necessity.9 One hundred and thirty six HIV antibody‐positive children were admitted to the paediatric intensive care unit (PICU) at this institution in 2002, prior to the availability of highly active antiretroviral therapy (HAART) in the public sector. Seventy three per cent of children survived to PICU discharge, 46% to hospital discharge, but only 14% were known to be alive one year later (personal communication, AA).
The high incidence of HIV infection and lack of access to HAART, coupled with resource constraints, led to debate over whether the public health service could afford a deontological approach to health care for critically ill HIV‐infected children. In a recent review of these ethical issues, it has been suggested that “pragmatic adherence to a policy of refusing to offer ventilation [to HIV‐infected children] … has to be followed”, in order to avoid overwhelming the regional paediatric critical care services.9
HAART has become available in developing and transitional countries, but the success of HAART programmes in regions with adequate healthcare resources, and relatively low incidence of paediatric HIV infection, cannot be taken for granted in developing regions with a much greater burden of HIV disease.10,11,12,13 Data are urgently required to guide policy, resource allocation, and ethical decision‐making in this setting. The decision to offer intensive care, as for any complex medical intervention, would depend partly on available resources and partly on the likelihood of successful outcome.9 In the case of critically ill HIV‐infected children, successful outcome would be defined as survival to become established on long‐term HAART, not merely survival to PICU discharge.
This prospective observational study was conducted to describe the short‐term outcome of HIV‐infected children, who were admitted to intensive care with the intention of starting HAART on resolution of their critical illness; to identify obstacles to successful implementation of long‐term HAART for such children; and to guide the allocation of critical care resources for HIV‐infected children in a developing or transitional region
Methods
Setting
The study was conducted over a 16‐month period, commencing in February 2003, in a 288‐bed paediatric teaching hospital in Cape Town, South Africa. Enrolment took place over the nine‐month period to October 2003 in the multidisciplinary PICU, which is routinely staffed for 18 beds and accommodates approximately 1200 admissions per annum. Follow‐up in the general hospital wards and Infectious Diseases Clinic (IDC) was completed in May 2004.
Study population
Children admitted to PICU in whom HIV infection was suspected, or who were known to be HIV infected, or HIV exposed (that is, maternal HIV infection or child HIV enzyme‐linked immunosorbent assay positive (ELISA)), were screened for enrolment. Definitive diagnosis of HIV status was made by HIV polymerase chain reaction (PCR) in children less than 18 months of age and by confirmatory ELISA in older children.
Data collection
Sixty eight HIV‐infected children, median age 3 months (0.1–85 months), form the study population for which further data were collected. Data were collected from routine hospital and laboratory records, and included: duration of hospital and PICU admission; knowledge of HIV status at time of admission to PICU; clinical diagnosis during PICU admission; Centres for Disease Control and Prevention (CDC) clinical (A, B or C) and immunological categories (I, II or III); baseline CD4+ lymphocyte count; predicted mortality by Paediatric Index of Mortality I, and the PICU and hospital outcome.14,15
Intervention in PICU
Children requiring mechanical ventilation for severe pneumonia would routinely undergo diagnostic non‐directed bronchoalveolar lavage, and would be treated for Pneumocystis jirovecii pneumonia (PJP) with high‐dose intravenous cotrimoxazole and oral prednisone. There was a low threshold for the use of second‐line antibiotics and antifungals for severely immunosuppressed children with suspected nosocomial sepsis. A high positive end‐expiratory pressure, low tidal volume, “lung protective” ventilatory strategy would be used routinely, with a low threshold for use of high‐frequency oscillatory ventilation in severe lung disease.
Limitation of intervention
In view of resource limitations, HIV‐infected children with advanced disease and recurrent hospital admissions would not usually have been referred for PICU admission in the first instance. After admission to PICU, LOI decisions for HIV‐infected children with a poor prognosis might include a decision not to escalate the level of support (for example, inotropic agents, intubation, or re‐intubation); to withhold cardiopulmonary resuscitation (CPR); or to limit duration of ventilatory support (withdrawal of life‐prolonging therapy). In practice, HIV‐infected children would rarely be offered mechanical ventilation for longer than 2–3 weeks before an elective trial of extubation would be carried out, with the express understanding that readmission to PICU would not be offered in the event of subsequent deterioration. These difficult management decisions were made in an ethical framework similar to that recently described by Jeena et al.9
Highly active antiretroviral treatment
All HIV‐infected children (n = 68) were considered for highly active antiretroviral treatment (HAART), as part of the IDC's HAART programme, if they survived to discharge from the ICU. The inclusion criteria for this programme were based on modified PENTA (Paediatric European Network for the Treatment of AIDS) guidelines.16 Clinical and immunological criteria for starting HAART included children classified as CDC clinical category C or immunological category III; or children classified as clinical category B with a CD4+ count <20%. Social criteria required the identification of a responsible caregiver, committed to long‐term therapy and adherence monitoring, with a permanent residence in Cape Town. Informed consent was obtained from the caregiver before the child was commenced on HAART.
First‐line therapy included two nucleoside reverse transcriptase inhibitors (NRTIs) (one thymidine analogue and one non‐thymidine analogue), and a protease inhibitor or a non‐nucleoside reverse transcriptase inhibitor (NNRTI). The choice of the third agent was largely determined by cost considerations at the time when the antiretroviral programme was started: children >3 years of age or >10 kg received efavirenz (EFV) and those <3 years of age or <10 kg were given ritonavir. The preferred protease inhibitor for the protease inhibitor‐containing regimen would have been lopinavir/ritonavir (kaletra), but cost implications prevented its use at that time.17 For the purposes of the outcome analysis, establishment on HAART was defined as adherence to at least 1 month of therapy.
Data are expressed as median (range), n (%), and 95% confidence intervals (CI) for proportions. Outcome data are expressed as % of all HIV‐infected PICU admissions, and as % of all HIV‐infected PICU survivors, because the decision to admit to PICU was on the basis of an “intention to treat” all HIV‐infected PICU survivors with HAART. The study was approved by the institutional ethics committee.
Results
Ninety six children, out of a total of 1036 PICU admissions during the study period (9%), were confirmed to be HIV ELISA positive. Of these, we confirmed that 71% (n = 68) were HIV‐infected, 16% (n = 15) were non‐infected and the remaining 14% (n = 13) had unconfirmed status at time of death or discharge. Of those children whose status was never confirmed, PCR was not performed in the seven children less than 6 weeks of age; two children died; three children were discharged before confirmatory tests could be done; and parental consent for testing was not granted for one child.
HIV status prior to PICU admission
Of the 96 HIV ELISA positive children screened, 60 were children of mothers who were aware of their antenatal HIV positive status—that is, they were known to be HIV‐exposed in utero. However, at the time of admission to PICU only 24% of children (n = 16/68) were known to be HIV‐infected, including five children already on HAART. Seventy six per cent (n = 52) of HIV‐infected children were newly diagnosed in PICU, of whom 52% (n = 27) were not known to be HIV‐exposed at birth, or tested HIV ELISA positive, before presentation with this critical illness.
Diagnoses and CDC category
In the HIV‐infected study group (n = 68), the most common primary diagnosis requiring PICU admission was lower respiratory tract infection (LRTI) (72%; n = 49), including 41% (n = 28) who had proven PJP. Other diagnoses (n = 19) included hypovolaemic shock secondary to gastroenteritis (n = 7); septic shock (n = 5); upper airway obstruction (n = 3); postoperative care (n = 3); and cardiomyopathy (n = 1).
All HIV‐infected children were categorised clinically according to the CDC classification system. Fifty seven per cent (n = 39) were categorised as CDC clinical category C, 41% (n = 28) as CDC clinical category B, and 2% (n = 1) as CDC clinical category A. Thirty two children (47%) had documented CD4+ counts allowing for CDC immunological staging. Half of these (n = 16) demonstrated severe immunosuppression, placing them in CDC immune category III, while 28% (n = 9) and 22% (n = 7) were immune categories II and I, respectively. The median CD4% was 21% (2–48%) and median absolute count 0.831×109/l (0.023–2.953×109/l).
PICU and hospital survival
Predicted PICU mortality (PIM I score) was 0.42 and observed PICU mortality was 0.25 (n = 17), with a standardised mortality ratio (SMR) of 0.60. Seventy five per cent (CI 65–85%) of HIV‐infected children (n = 51) survived to PICU discharge. PICU survival of children with PJP was also 75% (n = 21). The median duration of PICU stay for the HIV‐infected PICU survivors and non‐survivors, was 5 days (1–55 days) and 9 days (1–19 days), respectively. The PICU course and clinical data of hospital non‐survivors (PICU and ward) are detailed in table 1.
Table 1 Hospital non‐survivors: PICU course and clinical data.
| Patient | Admission diagnoses | Co‐diagnoses | Predicted mortality | CDC clinical category | CDC immune category | Nosocomial sepsis (PICU) | Antimicrobial therapy | Ventilatory support (days) | HFOV (days) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Pneumonia (PJP, Candida albicans) | 0.26 | C | III | Enterobacter cloacae, Klebsiella pneumoniae (pulmonary) | Piptazobactam, amikacin, meropenem, CTX, fluconazole | 18 | 8 | |
| 2 | Pneumonia (PJP, CMV, suspected TB) | Pneumothorax | 0.13 | C | N/D | Suspected | Piptazobactam, amikacin, meropenem, CTX | 13 | 3 |
| 3 | Meningitis (Streptococcal pneumoniae) | Chronic lung disease, pulmonary hypertension | 0.33 | C | N/D | Nil | Cefotaxime | 1 | 0 |
| 4 | Pneumonia (PJP, suspected CMV) | 0.17 | C | N/D | Nil | Piptazobactam, amikacin, CTX | 11 | 4 | |
| 5 | Pneumonia (Candida albicans) | 0.66 | B | N/D | Burkholderia cepacia (pulmonary) | Piptazobacatm, amikacin, meropenem, CTX, amphotericin B | 10 | 10 | |
| 6 | Pneumonia (adenovirus, RSV, suspected CMV, suspected TB) | Septicaemia (Staphylococcus aureus, Alcaligenes dentrificans) | 0.34 | B | N/D | Nil | Ciprofloxacin, vancomycin, meropenem, CTX, fluconazole, anti‐TB agents | 9 | 0 |
| 7 | Pneumonia (suspected PJP) | Septicaemia (Candida fomata), peritonitits (Escherichia coli, Enterococcus faecium) | 0.99 | B | N/D | Nil | Meropenem, CTX, amphotericin B | 8 | 6 |
| 8 | Pneumonia (PJP) | 0.66 | C | N/D | Nil | Cefotaxime, CTX, fluconazole | 11 | 0 | |
| 9 | Pneumonia (PJP, CMV, Escherichia coli) | 0.50 | C | N/D | Nil | Cefotaxime, piptazobactam, amikacin, CTX | 9 | 3 | |
| 10 | Pneumonia (PJP, CMV, Klebsiella pneumoniae) | 0.51 | C | N/D | Nil | Ampicillin, gentamicin, CTX, fluconazole | 4 | 3 | |
| 11 | Dilated cardiomyopathy | 0.22 | B | N/D | Nil | Nil | 0 | 0 | |
| 12 | Pneumonia (RSV, Candida albicans, suspected CMV) | Severe failure to thrive | 0.25 | C | N/D | Suspected (pulmonary) | Piptazobactam, amikacin, fluconazole | 7 | 7 |
| 13 | Pneumonia (PJP) | Neck abscess (Staphylococcus aureus) | 0.42 | C | N/D | Klebsiella pneumoniae/Enterobacter cloacae (pulmonary) | Piptazobactam, amikacin, imipenem, CTX, cloxacillin | 11 | 6 |
| 14 | Pneumonia (suspected PJP) | Tracheostomy | 0.76 | B | N/D | Nil | Ampicillin, gentamicin, CTX | <1 | 0 |
| 15 | Atypical LTB, pneumonia | 0.08 | C | N/D | Nil | Nil | 0 | 0 | |
| 16 | Pneumonia (Suspected PJP, Klebsiella pneumoniae) | 0.90 | B | N/D | Nil | Ampicillin, gentamicin, piptazobactam, amikacin, CTX | 2 | 1 | |
| 17 | Septic shock, gastroenteritis | 0.75 | B | N/D | M/R | M/R | M/R | M/R | |
| 18 | Staphylococcal sepsis (MRSA) | Pneumonia, gastroenteritis | 0.47 | B | I | Nil | Piptazobactam, amikacin, vancomycin, ciprofloxacin, CTX | 6 | 0 |
| 19 | Staphylococcal sepsis (MRSA), suspected PJP | Gastroenteritis (Salmonella Group C), renal impairment | 0.49 | B | III | Nil | Piptazobactam, amikacin, CTX vancomycin, ciprofloxacin | 5 | 2 |
| 20 | Pneumonia | Reye's syndrome, hypoxic brain injury | 0.37 | B | N/D | Nil | Cefotaxime, CTX, acyclovir, fluconazole | 12 | 0 |
| 21 | Pneumonia (Candida albicans, suspected TB) | ARF UTI (Escherichia coli), cardiomyopathy, gastroenteritis | 0.42 | B | I | Nil | Cefotaxime, CTX, fluconazole, anti‐TB agents | 5 | 0 |
| 22 | Pneumonia (PJP, RSV) | Septicaemia (Acinetobacter baumannii) | 0.35 | B | N/D | Nil | Piptazobactam, amikacin, CTX, fluconazole | 18 | 9 |
| 23 | Pneumonia (PJP, Klebsiella pneumoniae, CMV) | 0.78 | C | N/D | Suspected | Ampicillin, gentamicin, piptazobactam, amikacin, CTX, ganciclovir, fluconazole | 5 | 0 | |
| 24 | Pneumonia (PJP) | 0.76 | C | N/D | Suspected | Piptazobactam, amikacin, CTX, meropenem, fluconazole | 12 | 9 | |
| 25 | Pneumonia (PJP, Candida albicans) | 0.29 | C | N/D | Pseudomonas aeruginosa (pulmonary) | Cefuroxime, gentamicin, piptazobactam, amikacin, CTX | 14 | 0 | |
| 26 | Pneumonia (PJP, CMV) | 0.25 | C | N/D | Nil | Ampicillin, gentamicin, CTX | 1 | 0 | |
| 27 | Pneumonia (PJP, Escherichia coli) | 0.24 | C | III | M/R | M/R | M/R | M/R | |
| 28 | Pneumonia (PJP, CMV) | Septicaemia (Klebsiella pneumoniae, Pseudomnas aeruginosa), suspected meningitis | 0.47 | C | III | Nil | Cefotaxime, CTX, fluconazole, anti‐TB agents | 3 | 0 |
| 29 | Pneumonia (PJP, CMV) | 0.12 | C | III | Nil | Piptazobactam, amikacin, CTX | 2 | 0 | |
| 30 | Pneumonia (PJP, CMV, Klebsiella pneumoniae) | 0.42 | C | N/D | Nil | Piptazobactam, amikacin, CTX, fluconazole | 5 | 0 | |
| 31 | Pneumonia (Suspected PJP, Klebsiella pneumoniae) | Gastroenteritis | 0.26 | B | N/D | Nil | Piptazobactam, amikacin, CTX | 4 | 3 |
| 32 | Pneumonia (PJP, CMV, Candida albicans) | 0.69 | C | N/D | Nil | Ampicillin, gentamicin, CTX, fluconazole | 2 | 0 | |
| 33 | Pneumonia (PJP, CMV, Enterobacter cloacae) | 0.57 | C | III | Nil | Ampicillin, gentamicin, piptazobactam, amikacin, CTX, ganciclovir | 5 | 4 |
CMV, cytomegalovirus; CTX, cotrimoxazole; LTB, laryngotracheobronchitis; M/R, missing record; N/D, not done; PJP, pneumocystis jirovecii pneumonia; RSV, respiratory syncytial virus; TB, tuberculosis.
In 94% (n = 16) of the PICU non‐survivors (n = 17), a limitation of intervention (LOI) decision was made: to withhold re‐intubation after trial of extubation (n = 4); to withhold CPR in the event of a cardiac arrest (n = 7); or to withdraw life‐prolonging ventilatory support (n = 5). Data on limitation of medical intervention and hospital mortality (PICU and ward) are detailed in table 2.
Table 2 Hospital non‐survivors: limitation of intervention (LOI) and mortality data.
| Patient | Type of LOI | Where LOI made | Reason for LOI | When LOI made (days post PICU admission) | Time from LOI to death (days) | Where died | Time from PICU discharge to death (days) | Opiate palliation | Cause of death |
|---|---|---|---|---|---|---|---|---|---|
| 1 | WLPT | PICU | FTW | 18 | <1 | PICU | N/A | Yes | Pneumonia |
| 2 | NFR | PICU | PPP | 13 | <1 | PICU | N/A | Yes | Pneumonia |
| 3 | WLPT | PICU | BD | 1 | <1 | PICU | N/A | No | Meningitis |
| 4 | WLPT | PICU | FTW | 11 | <1 | PICU | N/A | Yes | Pneumonia |
| 5 | WLPT | PICU | FTW | 10 | <1 | PICU | N/A | Yes | Pneumonia |
| 6 | NFR | PICU | FTW | 9 | <1 | PICU | N/A | Yes | Pneumonia, septic shock |
| 7 | NFC | PICU | FMT, FTW | 6 | <1 | PICU | N/A | Yes | MOF (sepsis) |
| 8 | NFC | PICU | FTW | 7 | 4 | PICU | N/A | Yes | Pneumonia |
| 9 | WLPT | PICU | FTW | 9 | <1 | PICU | N/A | Yes | Pneumonia |
| 10 | NFR | PICU | PPP | 4 | <1 | PICU | N/A | Yes | Pneumonia |
| 11 | NFC | PICU | PPP | 1 | <1 | PICU | N/A | No | Cardiomyopathy |
| 12 | NFC | PICU | PPP | 1 | 7 | PICU | N/A | Yes | Pneumonia |
| 13 | NFC | PICU | PPP | 9 | 2 | PICU | N/A | Yes | Pneumonia |
| 14 | NFC | PICU | PPP | 1 | <1 | PICU | N/A | Yes | Pneumonia, septic shock |
| 15 | NFR | PICU | PPP | 1 | 2 | PICU | N/A | Yes | Pneumonia, septic shock |
| 16 | NFC | PICU | FMT | 1 | <1 | PICU | N/A | Yes | Pneumonia, septic shock |
| 17 | M/R | M/R | M/R | M/R | M/R | PICU | N/A | M/R | Septic shock |
| 18 | NFC | PICU | PPP | 8 | 2 | Ward | 2 | Yes | Pneumonia |
| 19 | NFC, NFR | PICU | PPP | 8 | 8 | Ward | 8 | No | MOF (sepsis) |
| 20 | NFC, NFR | PICU | PPP | 14 | 26 | Ward | 26 | No | Pneumonia |
| 21 | NFC, NFR | PICU | PPP | 5 | 1 | Ward | 1 | Yes | Pneumonia, cardiomyopathy |
| 22 | NFR | PICU | PPP | 18 | 47 | Ward | 47 | No | Chronic subglottic airway obstruction |
| 23 | NFC, NFR | PICU | PPP | 4 | 12 | Ward | 9 | Yes | Pneumonia |
| 24 | NFR | PICU | PPP | 12 | 6 | Ward | 5 | No | Pneumonia |
| 25 | NFC, NFR | PICU | PPP | 14 | 9 | Ward | 8 | No | Pneumonia |
| 26 | NFPR | Ward | PPP | 13 | 3 | Ward | 15 | No | Pneumonia |
| 27 | M/R | M/R | M/R | M/R | M/R | Ward | 4 | M/R | Pneumonia |
| 28 | NFPR | Ward | PPP | 7 | 2 | Ward | 5 | No | Pneumonia |
| 29 | NFR | PICU | PPP | 2 | 8 | Ward | 8 | No | Pneumonia |
| 30 | NFR | PICU | PPP | 5 | 8 | Ward | 8 | Yes | Pneumonia |
| 31 | NFC, NFR | PICU | PPP | 1 | 10 | Ward | 6 | No | Pneumonia |
| 32 | NFR | Ward | PPP | 17 | 4 | Ward | 17 | Yes | Pneumonia |
| 33 | NFR | PICU | PPP | 5 | 13 | Ward | 13 | No | Pneumonia |
BD, brain death; CMV, cytomegalovirus; CTX, cotrimoxazole; FTW, failure to wean (in PICU); LTB, laryngotracheobronchitis; MOF, multiiple organ failure; M/R, missing record; N/A, not applicable; N/D, not done; NFC, not for CPR (in PICU); NFPR, not for PICU readmission; NFR, not for re‐intubation (in PICU); PJP, pneumocystis jirovecii pneumonia; PPP, perceived poor prognosis (before established on HAART); RSV, respiratory syncytial virus; TB, tuberculosis; WLPT, withdrawal of life‐prolonging treatment (in PICU).
Almost a third of the PICU survivors (31%; n = 16), representing 24% of all HIV‐infected children, subsequently died in the hospital wards without being offered readmission to PICU. The median time to death of these ward non‐survivors was nine days (1–47 days) after PICU discharge, with two children dying within three days. Cause of death was judged to be due to progression of the initial disease process (that is, present at the time of admission) in 12 children, deterioration secondary to a nosocomial disease process in three children, and an uncertain cause in one child (see table 2).
Sixty nine per cent of the PICU survivors (n = 35) survived to hospital discharge, with an overall hospital survival of 51% (95% CI 35 to 68%). Hospital survival of children with PJP was 57% (n = 16). The median duration of hospital stay for the HIV‐infected PICU survivors, from time of admission to PICU to discharge from hospital, was 17 days (3–376 days).
Highly active antiretroviral therapy
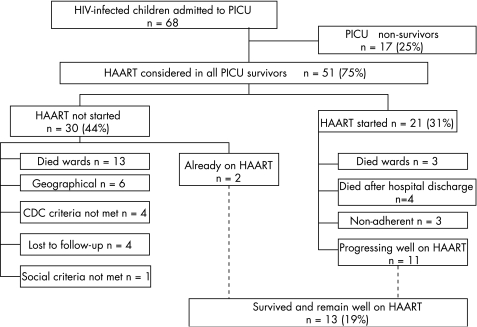
All PICU survivors (n = 51) were reviewed in terms of the IDC eligibility criteria for starting HAART (fig 1). Fifty nine per cent (n = 30) were not commenced on HAART for the following reasons: n = 13 died in the wards subsequent to PICU discharge; n = 6 were excluded on geographical grounds (they were either resident outside of the Western Cape or were referred back to an institution with access to HAART); n = 4 did not meet the CDC criteria (either clinical or immunological) for enrolment onto the IDC programme; n = 2 had already commenced HAART prior to PICU admission; n = 4 were lost to follow‐up; and one child was excluded due to unfavourable social circumstances.
Figure 1 Critically ill HIV‐infected children: survival and HAART.
Twenty one children, representing 31% (95% CI 20 to 42%) of all HIV‐infected children admitted to PICU, and 41% (95% CI 28 to 55%) of PICU survivors, initially commenced HAART. Of those children in whom HAART was started, 90% (n = 19) had required intensive care for respiratory illness, and 71% (n = 15) had proven PJP. Almost half (48%; n = 10) had severe immunosuppression with severe clinical disease and were categorised as CDC category C III. Median time to starting HAART was 16.5 days (4–244 days) from the date of PICU admission. One child was initially assessed as CDC category B II, but progressed to B III, and was started on HAART within the study period.
Short‐term outcome on HAART
Of the 21 PICU survivors who initially commenced HAART, three children died in the medical wards within four days of starting therapy, leaving 18 children who survived to hospital discharge on HAART. Another child did not return for follow‐up at 4 weeks and was lost to the treatment programme. Thus 17 children, representing 25% of HIV‐infected admissions, 33% of PICU survivors, and 81% of those initially commenced on treatment, were considered to be established on HAART after completing at least one month of treatment.
Thereafter, one child died at home of unknown causes after 32 days of treatment. Two children did not return for follow‐up at 8 weeks and were subsequently lost to the treatment programme. Thus, 14 children, representing 21% of HIV‐infected PICU admissions, 27% of PICU survivors, and 67% of those initially commenced on treatment, remained on HAART after two months.
However, two children who were already on HAART also survived to PICU discharge; and four children were considered not to have been established on HAART, due to early death or loss to follow‐up within the first month of treatment. Therefore, 16 children, representing 24% of HIV‐infected PICU admissions, 31% of PICU survivors, and 84% of the 19 children who had received at least 30 days of treatment, remained established on HAART beyond two months.
Medium‐term outcome on HAART
Three children subsequently died after 150, 201 and 327 days on treatment respectively (one at home; one after failed resuscitation in the emergency department; and one in the medical wards). The precise cause of death was not determined in all cases, although disseminated Bacillus Calmette‐Guerin (BCG) was suspected in one case, and presumed sepsis in another.
Thus, 13 children, representing 19% (95% CI 10 to 28%) of HIV‐infected PICU admissions, 25% (95% CI 14 to 37%) of PICU survivors, and 68% (95% CI 48 to 89%) of the 19 children established on HAART, remain on therapy and are progressing well, after a median duration of treatment of 350 days (278–476 days).
Discussion
Paediatric HIV infection is potentially preventable if pregnant women are able to access interventions to prevent vertical transmission.11 However, failure to achieve adequate screening coverage results in a population of HIV‐infected children who have not benefited from such measures, whose definitive diagnosis of HIV infection is delayed, and who may not access appropriate healthcare, such as PJP prophylaxis, until first presentation with a critical illness.
Universal PMTCT coverage for this region was thought to have been achieved in 2003. However, this study highlights the fact that more than one third of HIV‐antibody positive children admitted to intensive care were born to mothers with unknown HIV status, and only a quarter of the HIV‐infected children had been definitively diagnosed prior to PICU admission, a percentage similar to that in 1998.18 HAART became available at this centre in 2002, funded initially by non‐governmental organisations, pharmaceutical companies, and research initiatives.10 Since HAART has become more accessible, investigation of perinatally‐exposed infants by PCR has become routine at 14 weeks of age. We speculate that both acceptance of antenatal screening and definitive diagnosis rates will increase once HAART is routinely available to HIV‐infected individuals.19
The 75% PICU survival for critically ill HIV‐infected children, including those with PJP, is higher than that reported previously in Southern Africa, and compares favourably with reports from developed regions.8,18,20 This finding is encouraging, as it demonstrates that PICU outcome for HIV‐infected children is not as poor as has been supposed, even without receiving HAART during the acute illness.9 It follows that the assumptions underpinning ethical decision‐making and resource allocation need to be viewed in light of these new data.9
However, we acknowledge that although the observed PICU mortality (0.25) is lower than the PICU predicted mortality (0.42), the predicted mortality is similar to the eventual hospital mortality (0.49). Since clinical practice limited the duration of ventilatory support in HIV‐infected children with a poor prognosis, premature ward transfer might have resulted in higher ward mortality and artificially low PICU mortality. This factor may not have played a major role, as only two children died within three days of PICU discharge. However, subsequent limitation on PICU readmission may have had a greater influence on ward survival, since, despite relatively good PICU survival for PJP, only 57% of these children survived to leave hospital.
We were disappointed to find that less than a third of children who were admitted to PICU, with the intention of starting HAART, actually commenced treatment. It is worth noting that many PICU survivors who were eligible for HAART did not start treatment. Many of these children were not started on HAART for operational reasons, and we acknowledge that children transferred to other geographical regions may subsequently have accessed HAART in local institutions. However, almost half of the PICU survivors who did not commence HAART did not show clinical improvement after discharge from PICU, and died in the wards without being offered antiretroviral treatment. The immediate cause of death was frequently continued deterioration or secondary exacerbation of pneumonia, and limitation of PICU readmission criteria would have contributed directly to the ward mortality in this group. Therefore, it must be acknowledged that early mortality, both in PICU and after PICU discharge, has considerable impact in reducing the chances of an HIV‐infected child commencing HAART.
This raises the question of whether some children might have benefited from early HAART—that is, started before resolution of their acute illness. Although all these children were critically ill, the majority had single organ system respiratory disease, and in the absence of feed intolerance, haemodynamic instability, or metabolic acidaemia, it is difficult to pinpoint specific contraindications to HAART. Although there are no data to show that the introduction of HAART to antiretroviral‐naïve children improves the short‐term outcome of an acute illness, adult data suggest that ICU survival is improved in patients already receiving HAART.22 We did not expect children to derive virological, immunological, or clinical benefit until several weeks after starting HAART.17 Three children died within days of commencing HAART, but it is difficult to separate the effects of their acute illness from potential adverse effects of therapy.23 It should also be noted that these children were severely immunosuppressed, and potentially at increased risk of intercurrent infections and adverse outcome, although we acknowledge that an acute decline in CD4 cells might occur during an opportunistic infection.
What is already known on this topic
Large numbers of critically ill HIV‐infected children present to public sector health services in developing countries.
Before the availability of HAART in this region, the outcome of critically ill HIV‐infected children was poor.
What this study adds
The PICU outcome of critically ill HIV‐infected children in this developing region has improved.
LOI decisions are common and directly influence both PICU and hospital mortality.
Although short‐term mortality is a major obstacle to establishing children on HAART, the outcome of those children who become established on HAART is encouraging and warrants further investigation.
Despite the fact that less than a quarter of the original cohort of HIV‐infected children remain well on long‐term HAART, it is important to examine the reasons for this finding. We have pointed out that short‐term mortality considerably reduced the number of children to whom HAART was offered in the first instance, and that the majority of early deaths on HAART occurred in children who were not considered to be established on treatment.
In high prevalence developing regions, it is unlikely that healthcare resources would be channeled into critical care, in order to cope with all HIV‐infected children who might require such advanced support.9 If this is the case, then the restrictive criteria for PICU admission, readmission, and limitation of intervention are likely to remain unchanged, and short‐term mortality may not improve. Therefore, despite encouraging early PICU outcomes, few of the critically ill HIV‐infected children presenting to PICU are likely to achieve a successful long‐term outcome—that is, survive to become established on long‐term HAART. This factor should also be incorporated within the ethical framework, which guides clinical decision‐making for such children.9
We wish to stress that the disappointing short‐term mortality data do not reflect the efficacy or effectiveness of HAART in these children, because many of them were never established on antiretroviral treatment. In developed countries, HAART has resulted in marked reductions in both morbidity and mortality, and one might expect a child commencing HAART to have a good long‐term outcome.12,13,24,25,26 In developing countries, commencement of HAART in the setting of advanced disease, poor access to healthcare services, lack of infrastructure, inadequate nutrition and poor living standards may result in poorer outcomes compared to developed regions. Therefore, given that most of our patients were children with severe immunological compromise, who were recovering from a critical illness, we were encouraged that more than two thirds of those who were established on HAART remain in the treatment programme. In developing and transitional countries, the long‐term outcome of antiretroviral‐naïve HIV‐infected children, who are commenced on HAART after a critical illness, remains an issue for further investigation.
We suggest that efforts should be focused on decreasing the need for intensive care in the first instance, by improving antenatal diagnosis, ensuring prevention of vertical transmission, earlier definitive diagnosis, cotrimoxazole prophylaxis, and early antiretroviral treatment of HIV‐infected infants.11,19 Other public health approaches, including prevention of primary HIV infections in adults, should also be a priority. Community‐based measures might be a more appropriate use of scarce healthcare resources than provision of high‐cost intensive care for HIV‐infected children, the majority of whom do not survive to become established on long‐term HAART.27
Conclusion
The majority of HIV‐infected children in this study survived to discharge from PICU, although only half survived to hospital discharge. Limitation of intervention decisions, which were usually made in PICU, directly influenced PICU survival, hospital survival and the opportunity to commence HAART. Unless institutional and critical care resources are expanded, few critically ill HIV‐infected children are likely to survive to become established on HAART. Healthcare priorities in developing countries might be better concentrated on reducing the need for intensive care admission in the first instance. However, the long‐term outcome of those children who did survive to become established on HAART is encouraging, and warrants further investigation.
Abbreviations
CDC - Centers for Disease Control and Prevention
CPR - cardiopulmonary resuscitation
EFV - efavirenz
HAART - highly active antiretroviral therapy
IDC - infectious diseases clinic
LOI - limitation of intervention
LRTI - lower respiratory tract infection
NNRTI - non‐nucleoside reverse transcriptase inhibitor
NRTI - nucleoside reverse transcriptase inhibitor
PICU - paediatric intensive care unit
PMTCT - prevention of mother‐to‐child transmission
SMT - standardised mortality ratio
Footnotes
Funded by a self‐initiated research grant from the Medical Research Council of South Africa (MH).
Competing interests: None.
References
- 1.UNAIDS Report on the global HIV/AIDS epidemic. Available at http://www.unaids.org (accessed October 2004)
- 2.Dunn D T, Newell M L, Ades A E.et al Risk of human immunodeficiency virus type 1 transmission through breastfeeding. Lancet 1992340585–588. [DOI] [PubMed] [Google Scholar]
- 3.Mofenson L M. Advances in the prevention of vertical transmission of human immunodeficiency virus. Sem Pediatr Infect Dis 200314295–308. [DOI] [PubMed] [Google Scholar]
- 4.Thome C, Newell M L. Prevention of mother‐to‐child transmission of HIV infection. Curr Opin Infect Dis 200417247–252. [DOI] [PubMed] [Google Scholar]
- 5.Guay L A, Musoke P, Fleming T.et al Intrapartum and neonatal single dose nevirapine compared with zidovudine for prevention of mother‐to‐child transmission of HIV‐1 in Kampala, Uganda:HIVNET 012 randomised trial. Lancet 1999354795–802. [DOI] [PubMed] [Google Scholar]
- 6.Zwi K, Pettifor J, Soderlund N.et al HIV infection and in‐hospital mortality at an academic hospital in South Africa. Arch Dis Child 200083227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goulder P J R, Jeena P, Tudor‐Williams G.et al Paediatric HIV infection: correlates of protective immunity and global perspectives in prevention and management. British Med Bulletin 20015889–108. [DOI] [PubMed] [Google Scholar]
- 8.Cooper S, Lyall H, Walters S.et al Children with human immunodeficiency virus admitted to a paediatric intensive care unit in the United Kingdom over a 10‐year period. Intensive Care Med 200430113–118. [DOI] [PubMed] [Google Scholar]
- 9.Jeena P M, McNally L M, Stobie M.et al Challenges in the provision of ICU services to HIV infected children in resource poor settings: a South African case study. J Med Ethics 200531226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eley B, Nuttall J, Davies M A.et al Initial experience of a public sector antiretroviral treatment programme for HIV infected children and their infected parents. S Afr Med J 200494643–646. [PubMed] [Google Scholar]
- 11.Lyall E G H. Paediatric HIV in 2002—a treatable and preventable infection. J Clin Virol 200225107–119. [DOI] [PubMed] [Google Scholar]
- 12.Sharland M, Gibb D M, Tudor‐Williams G. Advances in the prevention and treatment of paediatric HIV infection in the United Kingdom. Arch Dis Child 200287178–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibb D M, Duong T, Tookey P A.et al Decline in mortality, AIDS, and hospital admissions in perinatally HIV‐1 infected children in the United Kingdom and Ireland. BMJ 20033271019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR Morb Mortal Wkly Rep 1994431–12. [Google Scholar]
- 15.Shann F, Pearson G, Slater A.et al Paediatric index of mortality (PIM): a mortality prediction model for children in intensive care. Intensive Care Med 199723201–207. [DOI] [PubMed] [Google Scholar]
- 16.Sharland M, Castelli G, Ramos J T, on behalf of the PENTA steering committee et al Penta Guidelines for the use of antiretroviral therapy in paediatric HIV infection 2001. Available at http://www.ctu.mrc.ac.uk/penta (accessed December 2006)
- 17.Van Rossum A M, Fraaij P L, de Groot R. Efficacy of highly active antiretroviral therapy in HIV‐1 infected children. Lancet Infect Dis 2002293–102. [DOI] [PubMed] [Google Scholar]
- 18.Zar H J, Apolles P, Argent A.et al The etiology and outcome of pneumonia in human immunodeficiency virus‐infected children admitted to intensive care in a developing country. Pediatr Crit Care Med 20012108–112. [DOI] [PubMed] [Google Scholar]
- 19.Wilfret C. Prevention of mother‐to‐child transmission of HIV: reflections on implementation of PMTCT in the developing world. Acta Paediatr 200291863–865. [DOI] [PubMed] [Google Scholar]
- 20.Jeena P M, Coovadia H M, Bhagwanjee S. Prospective controlled study of the outcome of human immunodeficiency virus‐1 antibody‐positive children admitted to an intensive care unit. Crit Care Med 199624963–967. [DOI] [PubMed] [Google Scholar]
- 21.Jeena P M, Wesley A G, Coovadia H M. Admission patterns and outcomes in a paediatric intensive care unit in South Africa over a 25‐year period (1971–1995). Intensive Care Med 19992588–94. [DOI] [PubMed] [Google Scholar]
- 22.Morris A, Wachter R M, Luce J.et al Improved survival with highly active antiretroviral therapy in HIV‐infected patients with severe Pneumocystis carinii pneumonia. AIDS 20031773–80. [DOI] [PubMed] [Google Scholar]
- 23.Moyle G. Mitochondrial toxicity: myths and facts. HIV Ther 2004945–47. [PubMed] [Google Scholar]
- 24.Granados J M S, Amador J T R, De Miguel S F.et al Impact of highly active antiretroviral therapy on the morbidity and mortality in Spanish human immunodeficiency virus‐infected children. Pediatr Infect Dis J 200322863–867. [DOI] [PubMed] [Google Scholar]
- 25.de Martino M, Balducci M, Galli L.et al Reduction in mortality with availability of antiretroviral therapy for children with perinatal HIV‐1 infection. Italian Register for HIV infection in Children and the Italian National AIDS Registry. JAMA 2000284190–197. [DOI] [PubMed] [Google Scholar]
- 26.Gortmaker S L, Hughes M, Cervia J.et al Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV‐1. N Engl J Med 20013451522–1528. [DOI] [PubMed] [Google Scholar]
- 27.Fauci A S. The AIDS epidemic. Considerations for the 21st century. N Engl J Med 19993411046–1050. [DOI] [PubMed] [Google Scholar]