Abstract
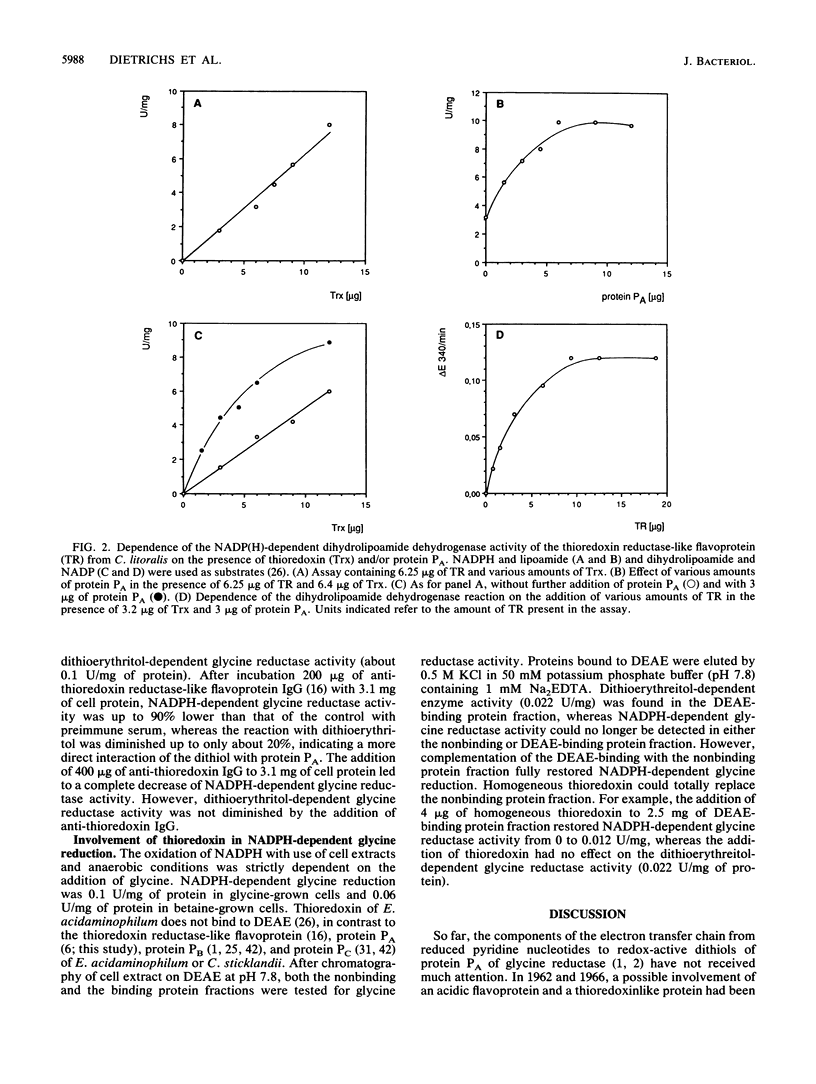
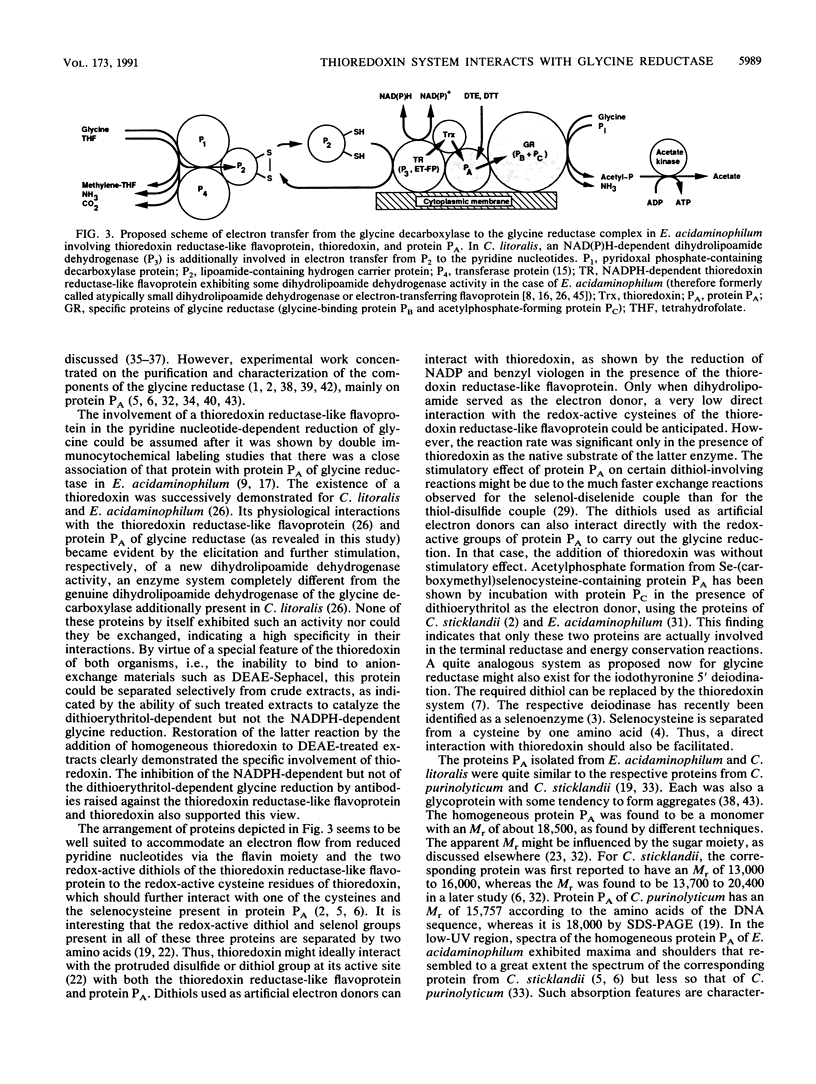
Purification of protein PA of the glycine reductase complex from Eubacterium acidaminophilum and Clostridium litorale [corrected] was monitored by a new spectrophotometric assay. The procedure depended on a specific two- to threefold stimulation of a dihydrolipoamide dehydrogenase activity that is elicited by the interaction of a thioredoxin reductase-like flavoprotein and thioredoxin from both organisms. Protein PA isolated from E. acidaminophilum by 75Se labeling and monitoring of the dithioerythritol-dependent glycine reductase activity was identical in its biochemical, structural, and immunological properties to the protein isolated by using the stimulation assay. Proteins PA from both organisms were glycoproteins of Mr about 18,500 and exhibited very similar N-terminal amino acid sequences. Depletion of thioredoxin from crude extracts of E. acidaminophilum totally diminished the NADPH-dependent but not the dithioerythritol-dependent glycine reduction. The former activity could be fully restored by adding thioredoxin. Antibodies raised against the thioredoxin reductase-like flavoprotein or thioredoxin inhibited to a high extent NADPH-dependent but not dithioerythritol-dependent glycine reductase activity. These results indicate the involvement of the thioredoxin system in the electron flow from reduced pyridine nucleotides to glycine reductase.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behne D., Kyriakopoulos A., Meinhold H., Köhrle J. Identification of type I iodothyronine 5'-deiodinase as a selenoenzyme. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1143–1149. doi: 10.1016/s0006-291x(05)80905-2. [DOI] [PubMed] [Google Scholar]
- Berry M. J., Banu L., Larsen P. R. Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature. 1991 Jan 31;349(6308):438–440. doi: 10.1038/349438a0. [DOI] [PubMed] [Google Scholar]
- Cone J. E., Del Río R. M., Davis J. N., Stadtman T. C. Chemical characterization of the selenoprotein component of clostridial glycine reductase: identification of selenocysteine as the organoselenium moiety. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2659–2663. doi: 10.1073/pnas.73.8.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone J. E., del Río R. M., Stadtman T. C. Clostridial glycine reductase complex. Purification and characterization of the selenoprotein component. J Biol Chem. 1977 Aug 10;252(15):5337–5344. [PubMed] [Google Scholar]
- Das A. K., Hummel B. C., Gleason F. K., Holmgren A., Walfish P. G. Bacterial and mammalian thioredoxin systems activate iodothyronine 5'-deiodination. Biochem Cell Biol. 1988 May;66(5):460–464. doi: 10.1139/o88-057. [DOI] [PubMed] [Google Scholar]
- Dietrichs D., Andreesen J. R. Purification and comparative studies of dihydrolipoamide dehydrogenases from the anaerobic, glycine-utilizing bacteria Peptostreptococcus glycinophilus, Clostridium cylindrosporum, and Clostridium sporogenes. J Bacteriol. 1990 Jan;172(1):243–251. doi: 10.1128/jb.172.1.243-251.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrichs D., Meyer M., Schmidt B., Andreesen J. R. Purification of NADPH-dependent electron-transferring flavoproteins and N-terminal protein sequence data of dihydrolipoamide dehydrogenases from anaerobic, glycine-utilizing bacteria. J Bacteriol. 1990 Apr;172(4):2088–2095. doi: 10.1128/jb.172.4.2088-2095.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürre P., Andreesen J. R. Purine and glycine metabolism by purinolytic clostridia. J Bacteriol. 1983 Apr;154(1):192–199. doi: 10.1128/jb.154.1.192-199.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürre P., Andreesen J. R. Selenium-dependent growth and glycine fermentation by Clostridium purinolyticum. J Gen Microbiol. 1982 Jul;128(7):1457–1466. doi: 10.1099/00221287-128-7-1457. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Freudenberg W., Andreesen J. R. Purification and partial characterization of the glycine decarboxylase multienzyme complex from Eubacterium acidaminophilum. J Bacteriol. 1989 Apr;171(4):2209–2215. doi: 10.1128/jb.171.4.2209-2215.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg W., Dietrichs D., Lebertz H., Andreesen J. R. Isolation of an atypically small lipoamide dehydrogenase involved in the glycine decarboxylase complex from Eubacterium acidaminophilum. J Bacteriol. 1989 Mar;171(3):1346–1354. doi: 10.1128/jb.171.3.1346-1354.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gander J. E. Gel protein stains: glycoproteins. Methods Enzymol. 1984;104:447–451. doi: 10.1016/s0076-6879(84)04112-4. [DOI] [PubMed] [Google Scholar]
- Garcia G. E., Stadtman T. C. Selenoprotein A component of the glycine reductase complex from Clostridium purinolyticum: nucleotide sequence of the gene shows that selenocysteine is encoded by UGA. J Bacteriol. 1991 Mar;173(6):2093–2098. doi: 10.1128/jb.173.6.2093-2098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V., Weber K. Calcium-dependent conformational changes in the 36-kDa subunit of intestinal protein I related to the cellular 36-kDa target of Rous sarcoma virus tyrosine kinase. J Biol Chem. 1985 Feb 10;260(3):1688–1695. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989 Aug 25;264(24):13963–13966. [PubMed] [Google Scholar]
- Meyer M., Dietrichs D., Schmidt B., Andreesen J. R. Thioredoxin elicits a new dihydrolipoamide dehydrogenase activity by interaction with the electron-transferring flavoprotein in Clostridium litoralis and Eubacterium acidaminophilum. J Bacteriol. 1991 Feb;173(4):1509–1513. doi: 10.1128/jb.173.4.1509-1513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann E., Hippe H., Gottschalk G. Betaine: New Oxidant in the Stickland Reaction and Methanogenesis from Betaine and l-Alanine by a Clostridium sporogenes-Methanosarcina barkeri Coculture. Appl Environ Microbiol. 1983 Feb;45(2):474–483. doi: 10.1128/aem.45.2.474-483.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racusen D. Glycoprotein detection in polyacrylamide gel with thymol and sulfuric acid. Anal Biochem. 1979 Nov 1;99(2):474–476. doi: 10.1016/s0003-2697(79)80035-4. [DOI] [PubMed] [Google Scholar]
- STADTMAN T. C., ELLIOTT P., TIEMANN L. Studies on the enzymic reduction of amino acids. III. Phosphate esterification coupled with glycine reduction. J Biol Chem. 1958 Apr;231(2):961–973. [PubMed] [Google Scholar]
- Sliwkowski M. X., Stadtman T. C. Purification and immunological studies of selenoprotein A of the clostridial glycine reductase complex. J Biol Chem. 1987 Apr 5;262(10):4899–4904. [PubMed] [Google Scholar]
- Sliwkowski M. X., Stadtman T. C. Selenium-dependent glycine reductase: differences in physicochemical properties and biological activities of selenoprotein A components isolated from Clostridium sticklandii and Clostridium purinolyticum. Biofactors. 1988 Dec;1(4):293–296. [PubMed] [Google Scholar]
- Sliwkowski M. X., Stadtman T. C. Selenoprotein A of the clostridial glycine reductase complex: purification and amino acid sequence of the selenocysteine-containing peptide. Proc Natl Acad Sci U S A. 1988 Jan;85(2):368–371. doi: 10.1073/pnas.85.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman T. C. Clostridial glycine reductase: protein C, the acetyl group acceptor, catalyzes the arsenate-dependent decomposition of acetyl phosphate. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7853–7856. doi: 10.1073/pnas.86.20.7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman T. C. Glycine reduction to acetate and ammonia: identification of ferredoxin and another low molecular weight acidic protein as components of the reductase system. Arch Biochem Biophys. 1966 Jan;113(1):9–19. doi: 10.1016/0003-9861(66)90151-2. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. Selenium biochemistry. Annu Rev Biochem. 1990;59:111–127. doi: 10.1146/annurev.bi.59.070190.000551. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. Selenium-dependent clostridial glycine reductase. Methods Enzymol. 1978;53:373–382. doi: 10.1016/s0076-6879(78)53043-7. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Stadtman T. C. Selenium-dependent clostridial glycine reductase. Purification and characterization of the two membrane-associated protein components. J Biol Chem. 1979 Jan 25;254(2):447–452. [PubMed] [Google Scholar]
- Turner D. C., Stadtman T. C. Purification of protein components of the clostridial glycine reductase system and characterization of protein A as a selenoprotein. Arch Biochem Biophys. 1973 Jan;154(1):366–381. doi: 10.1016/0003-9861(73)90069-6. [DOI] [PubMed] [Google Scholar]
- Wardi A. H., Michos G. A. Alcian blue staining of glycoproteins in acrylamide disc electrophoresis. Anal Biochem. 1972 Oct;49(2):607–609. doi: 10.1016/0003-2697(72)90472-1. [DOI] [PubMed] [Google Scholar]
- Zinoni F., Birkmann A., Leinfelder W., Böck A. Cotranslational insertion of selenocysteine into formate dehydrogenase from Escherichia coli directed by a UGA codon. Proc Natl Acad Sci U S A. 1987 May;84(10):3156–3160. doi: 10.1073/pnas.84.10.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]



