Abstract
About 1 in 715 young adults is a survivor of childhood malignancy, but these individuals are at increased risk of considerable treatment‐related morbidity or even mortality. A recent study suggests that at least 60% have one or more chronic health problems, whilst about 20% have three or more. The principle goal of long‐term follow‐up (LTFU) of survivors is to decrease the severity of late treatment complications by performing appropriate surveillance to detect incipient toxicity, and by facilitating timely diagnosis and management of emerging or established late adverse effects. The content of LTFU is dictated by the type and amount of treatment for the malignancy, and has been defined in recent clinical guidelines. Moreover, LTFU allows provision of survivor education, psychosocial support and health promotion advice. However, considerable variation exists in how LTFU is performed, with several alternative models involving a range of professionals in a variety of locations, depending on numerous clinical and organisational factors. There is increasing utilisation of multidisciplinary teams, and recognition of the importance of effective transition strategies whereby care is transferred to more age‐appropriate providers, usually after a period of joint care in adolescence. It is of paramount importance to ascertain and meet the needs of survivors themselves.
As the treatment of childhood malignancy has continued to improve, with the overall 5‐year survival rate increasing from 25% for children diagnosed in the 1960s to about 75% for those diagnosed in the 1990s,1 the number of long‐term survivors has risen. More than 26 000 people are alive in Britain after childhood malignancy, and 1 in 715 of the current young adult population is a survivor.1,2
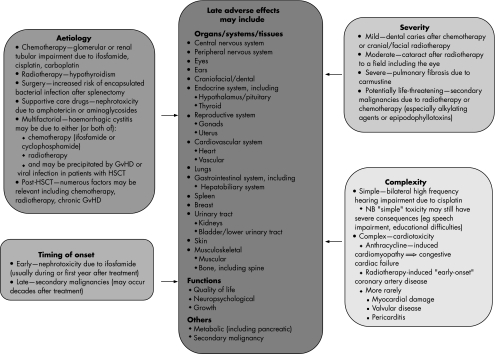
However, survivors are at increased risk of considerable morbidity and even mortality as a result of late adverse effects of their previous treatment. A cohort study of >2000 five‐year survivors from a large UK centre found that at least 60% had one or more chronic health problems and about 20% had three or more.3 More intensively treated patients may develop late toxicity even more frequently, as exemplified by survivors of haemopoietic stem cell transplantation (HSCT), of whom >90% had at least one late effect and >70% had at least three.4 The range of late effects encountered is very wide, affecting any organ, system, tissue or function, with variable aetiology, timing of onset, severity and complexity5,6 (fig 1), and may encompass psychological problems including difficulties with interpersonal relationships and post‐traumatic stress disorder.7,8 Increased late mortality has been documented in the North American Childhood Cancer Survivor Study, where >20 000 long‐term survivors had a standardised mortality ratio of 10.8, about 20% of which was due to late side effects, especially secondary malignancy, and pulmonary and cardiac toxicity.9
Figure 1 Late adverse effects in long‐term survivors of childhood malignancy, with illustrative examples of the wide variety of manifestations and broad range of aetiological factors, timing of onset, severity and complexity. GvHD, graft versus host disease; HSCT, haemopoietic stem cell transplantation.
With increasing recognition of the frequency and importance of late adverse effects, long‐term follow‐up (LTFU) has become an integral part of the care of children and adolescents previously treated for malignancy or who have undergone HSCT. It is not difficult to identify examples of late effects that may be diagnosed and managed in a LTFU clinic with the expectation of definite benefit, but it is less easy to be certain exactly what impact LTFU programmes have on the overall physical and psychosocial health of the total population of long‐term survivors.
Therefore, it is instructive to consider the specific goals of LTFU. The principle aim is to decrease the severity of late complications of treatment, thereby hopefully reducing excess morbidity or even mortality. This may be achieved by undertaking appropriate surveillance to detect incipient late effects, by diagnosing emerging or established late effects promptly, or by facilitating timely management of chronic toxicity. Surveillance may include clinical assessment by medical history and physical examination, the performance of appropriate investigations, or both. However, there are several other important roles of LTFU, notably the delivery of ongoing health education about the original malignancy, its treatment and their consequences, including potential late adverse effects (physical, psychological and psychosocial), and the provision of support and advice to help survivors deal with these consequences. In addition, LTFU permits the provision of relevant health promotion advice, and may offer important opportunities for research into late adverse effects.
Clinicians involved in LTFU agree that the content of follow‐up is dictated by the nature (drugs, radiotherapy, etc) and amount (dose and intensity) of treatment previously received. Historically, these have been the most important determinants of the frequency and type of late effects, although there is increasing interest in the potential importance of genetic predisposition.10 In the last 2 years, three groups from the UK and the USA have produced guidelines for the content of LTFU.6,11,12 However, these documents do not attempt to deal with issues regarding the most appropriate organisational model for performing LTFU, as this will vary from centre to centre, and over time, depending on several factors including local resources, expertise and interests, centre size and regional geography.
Therefore, LTFU incorporates different strategies in different centres, even within a single country. Indeed, given the treatment‐based approach to determining its content, LTFU often varies considerably between different patient groups in the same centre. However, there are common strands to LTFU that are applicable to and potentially beneficial for all survivors, particularly the provision of health education, psychosocial support and health promotion advice. Strategies to meet the variable additional needs of particular groups of survivors may be best illustrated by a range of selected examples, although it should be borne in mind that other approaches may be applicable to each scenario described below.
Some patients (eg, those with low stage Wilms' tumour) may be cured of their malignancy by relatively simple treatment with very little risk of long‐term toxicity. Historically many such patients have been discharged from follow‐up, and contemporary practice usually embraces a low intensity of LTFU. This may be delivered in several different ways, but one suggested strategy is regular (eg, every 2 years) postal or telephone contact.
Most children treated for acute lymphoblastic leukaemia have received 2 or 3 years of combination chemotherapy, with a moderate risk of causing late adverse effects, including a low but definite risk of severe toxicity (eg, anthracycline‐induced cardiomyopathy). Most survivors of acute lymphoblastic leukaemia probably require LTFU by an appropriately trained or experienced individual, but this need not necessarily be a paediatric oncologist or related specialist. Indeed “protocol‐driven” LTFU may be delivered by a nurse or a primary care doctor in a variety of locations, ranging from the referring paediatric oncology unit (or allied age‐appropriate hospital clinic) to primary care. Provision of appropriate information for non‐specialist carers (such as general practitioners) is important to ensure that they are aware of the specific late effects that may occur in particular patients, and may be facilitated by the use of individualised patient‐held records.
Some groups of survivors have greater LTFU needs as a result of their initial diagnosis and its treatment (eg, survivors of brain tumours), or because they have received more intensive treatment (eg, HSCT). Most such patients require medically supervised LTFU, often involving considerable additional specialist input (eg, from endocrinologists), usually hospital based, on a regular (at least annual) basis. Finally, occasional patients who have already developed a particular late effect (eg, pulmonary or renal damage) or who are at high risk of an adverse outcome (eg, speech delay or educational difficulties due to severe cisplatin‐induced hearing loss) may require specialist follow‐up in a variety of settings depending on local circumstances.
These examples illustrate the range of intensities of LTFU that may be appropriate for different groups of survivors. Although the different models of LTFU mentioned above have been proposed previously,13 considerable heterogeneity in provision remains, with a wide range of alternative yet appropriate strategies. In several cases, there is probably no right or wrong approach. What is important is that the strategy implemented is capable of fulfilling the aims of LTFU and that it allows the appropriate content of LTFU surveillance to be provided. Recent evidence implies that it is not difficult to identify the intensity of LTFU care needed by individual patients.14 If the appropriate level of care is given, then the issues of “who provides LTFU, where and how often” become less critical, recognising that more than one different model may be appropriate. Nevertheless, much more research is required, with the aim of facilitating the development of LTFU strategies that are widely applicable and demonstrably effective.15
Pending such research, the approach proposed by the National Institute for Clinical Excellence in “Improving outcomes in children and young people with cancer”16 involves a LTFU multidisciplinary team, including a lead clinician with expertise in LTFU (usually an oncologist, but not necessarily paediatric), a specialist nurse, an endocrinologist, an appropriate allied health professional (eg social worker) and a psychologist. Further, the guidance suggests that an appropriate “key worker” should be identified to provide information and support for, and coordinate the care of, each patient. Although not specified in the guidance, specialist nurses may fulfil this role effectively in many cases.
It is important to recognise that LTFU will usually extend well beyond childhood, and that transitional strategies should be developed whereby adolescent survivors are transferred to the care of a more age‐appropriate provider (and environment) at a defined age, usually after a period of joint care.17 Many of the challenges associated with transition are relevant to the care of adolescents with other chronic illnesses (eg, cystic fibrosis) managed by paediatricians and therefore well documented.18 However, it is particularly important to avoid losing long‐term survivors of cancer to follow‐up at this stage, as many of the potentially more serious late effects may not manifest until a decade after completion of treatment or even later. For example, the cumulative incidence of secondary malignancies continues to rise for at least 25 years,19 while there is increasing awareness of the development of late cardiotoxicity up to 20 years after anthracycline treatment in previously asymptomatic patients.20
There are several barriers to optimal provision of LTFU, including factors related to survivors themselves (eg, social or cultural background) and their healthcare providers (eg, attitudes and beliefs).21 One of the most important obstacles is lack of knowledge, among both survivors and non‐specialist clinicians.22 It is salutary to acknowledge that provision of information about their illness, its treatment and future health risks was one of the most important needs highlighted by a focus group of survivors.23
The same focus group also identified the need for strategies to deal with everyday but difficult issues such as seeking employment or obtaining insurance.23 In addition, a modified Delphi panel of survivors highlighted the need for guidance in developing self‐advocacy skills and accessing sources of support.24 However, there is an increasing amount of information designed to meet the specific information needs of individual survivors, such as the United Kingdom Children's Cancer Study Group's After cure package, which is available as a booklet and also accessible on the internet.25 It covers general issues about cancer and its treatment, providing advice about lifestyle, health promotion, education and employment, disability, life insurance and mortgages, travel and vaccinations, fertility and sexual function, and is appropriate for all survivors. Specific inserts are available describing particular aspects of late toxicity relevant to individual survivors on the basis of their previous treatment. Finally, it provides information about childhood cancer survivor groups and several relevant websites. A further suggestion deriving from one of the consensus views of a workshop of survivors is that care should be patient led with support from a key worker.26 Hence, there is increasing awareness of the importance of providing information for survivors, as well as seeking and acting on their views when developing future models of LTFU.22
Research will continue to have a central role in the evolution of LTFU. For many years, paediatric oncologists have expended much effort in intensifying treatment for poor prognosis malignancies in an attempt to cure more children, while remaining mindful of the need for reduced treatment strategies that can maintain high cure rates with less late toxicity in good prognosis malignancies.27 Indeed, there are several ongoing randomised controlled trials with reduced treatment arms (lower doses or dose intensity, or reduced duration). Nevertheless, inevitably, the continued use of intensive treatment will be associated with a pronounced frequency of potentially severe late effects, although it is often many years before the full effect is evident. It is vital to continue careful prospective evaluation looking for previously unrecognised late effects, especially after novel treatments, including newer biological agents (eg, targeted protein kinase inhibitors). Similarly, it is important to seek better understanding of risk factors, including genetic predisposition, although the multifactorial aetiology of many late effects complicates preventive strategies. However, research should play an equally important part in developing effective LTFU strategies to meet the needs of the ever‐increasing population of survivors of childhood cancer.
Abbreviations
LTFU - long‐term follow‐up
HSCT - haemopoietic stem cell transplantation
Footnotes
Competing interests: None.
References
- 1.Kroll M E, Passmore S J, Stiller C A.et al Childhood cancer—UK. In: Toms JR, ed. CancerStats monograph 2004. London: Cancer Research UK, 200463–72.
- 2.Campbell J, Wallace W H B, Bhatti L A.et alChildhood cancer in Scotland: Trends in incidence, mortality and survival 1975–1999. Edinburgh: NHS Scotland Information and Statistics Division, 2004
- 3.Curry H L, Parkes S E, Powell J E.et al Caring for survivors of childhood cancers: the size of the problem. Eur J Cancer 200642501–508. [DOI] [PubMed] [Google Scholar]
- 4.Skinner R, Leiper A D. Bone marrow transplantation. In: Wallace WHB, Green DM, eds. Late effects of childhood cancer. London: Arnold, 2004304–320.
- 5.Wallace H, Green D. eds. Late effects of childhood cancer. London: Arnold, 2004
- 6.Skinner R, Wallace W H B, Levitt G A.Therapy based long term follow up: Practice Statement. Leicester: United Kingdom Childrens Cancer Study Group (Late Effects Group), 2005
- 7.Hobbie W L, Stuber M, Meeske K.et al Symptoms of post‐traumatic stress in young adult survivors of childhood cancer. J Clin Oncol 2000184060–4066. [DOI] [PubMed] [Google Scholar]
- 8.Mackie E, Hill J, Kondryn H.et al Adult psychosocial outcomes in long‐term survivors of acute lymphoblastic leukaemia and Wilm's tumour: a controlled study. Lancet 20003551310–1314. [DOI] [PubMed] [Google Scholar]
- 9.Mertens A C, Yasui Y, Neglia J P.et al Late mortality experience in five‐year survivors of childhood and adolescent cancer: The Childhood Cancer Survivor Study. J Clin Oncol 2001193163–3172. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia S, Meadows A T. Long‐term follow‐up of childhood cancer survivors: future directions for clinical care and research. Pediatr Blood Cancer 200646143–148. [DOI] [PubMed] [Google Scholar]
- 11.Scottish Collegiate Guidelines Network Long term follow up of survivors of childhood cancer. A national clinical guideline. 2004. http://www.sign.ac.uk/pdf/sign76.pdf (accessed 9 Nov 2006)
- 12.Childrens Oncology Group Long‐term follow‐up guidelines for survivors of childhood, adolescent, and young adult cancers. 2004. http://www.survivorshipguidelines.org (accessed 9 Nov 2006)
- 13.Wallace W H B, Blacklay A, Eiser C.et al Developing strategies for long term follow up of survivors of childhood cancer. BMJ 2001323271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eiser C, Absolom K, Greenfield D.et al Follow‐up after childhood cancer: evaluation of a three‐level model. Eur J Cancer. In press [DOI] [PubMed]
- 15.Friedman D L, Freyer D R, Levitt G A. Models of care for survivors of childhood cancer. Pediatr Blood Cancer 200646159–168. [DOI] [PubMed] [Google Scholar]
- 16.National Institute for Health and Clinical Excellence Improving outcomes in children and young people with cancer. Guidance on Cancer Services. 2005. http://www.nice.org.uk/page.aspx?o = 268841 (accessed 9 Nov 2006)
- 17.Ginsberg J P, Hobbie W L, Carlson C A.et al Delivering long‐term follow‐up care to pediatric cancer survivors: transitional care issues. Pediatr Blood Cancer 200646169–173. [DOI] [PubMed] [Google Scholar]
- 18.Michaud P A, Suris J C, Viner R. The adolescent with a chronic condition. Part II: healthcare provision. Arch Dis Child 200489943–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neglia J P, Friedman D L, Yasui Y.et al Second malignant neoplasms in five‐year survivors of childhood cancer: Childhood Cancer Survivor Study. J Natl Cancer Inst 200193618–629. [DOI] [PubMed] [Google Scholar]
- 20.Steinherz L J, Steinherz P G, Tan C. Cardiac failure and dysrhythmias 6–19 years after anthracycline therapy: A series of 15 patients. Med Pediatr Oncol 199524352–361. [DOI] [PubMed] [Google Scholar]
- 21.Oeffinger K C, Wallace W H B. Barriers to follow‐up care of survivors in the United States and the United Kingdom. Pediatr Blood Cancer 200646135–142. [DOI] [PubMed] [Google Scholar]
- 22.Skinner R, Wallace W H B, Levitt G A. Long‐term follow‐up of people who have survived cancer during childhood. Lancet Oncol 20067489–498. [DOI] [PubMed] [Google Scholar]
- 23.Earle E A, Davies H, Greenfield D.et al Follow‐up care for childhood cancer survivors: a focus group analysis. Eur J Cancer 2005412882–2886. [DOI] [PubMed] [Google Scholar]
- 24.Zebrack B J, Eshelman D A, Hudson M M.et al Health care for childhood cancer survivors. Insights and perspectives from a Delphi panel of young adult survivors of childhood cancer. Cancer 2004100843–850. [DOI] [PubMed] [Google Scholar]
- 25.Griffiths A. ed. After cure. Leicester: United Kingdom Childrens Cancer Study Group (Late Effects Group), 2005, http://www.aftercure.org (accessed 9 Nov 2006)
- 26.Levitt G, Aslett H, Richardson A.et al Models of long term follow up for cancer survivors: reaching a consensus. [Abstract G224]. Arch Dis Child 200691(Suppl 1)A80 [Google Scholar]
- 27.Craft A W, Pearson A D J. Three decades of chemotherapy for childhood cancer: From cure ‘at any cost' to cure ‘at least cost'. Cancer Surv 19898605–629. [PubMed] [Google Scholar]