Abstract
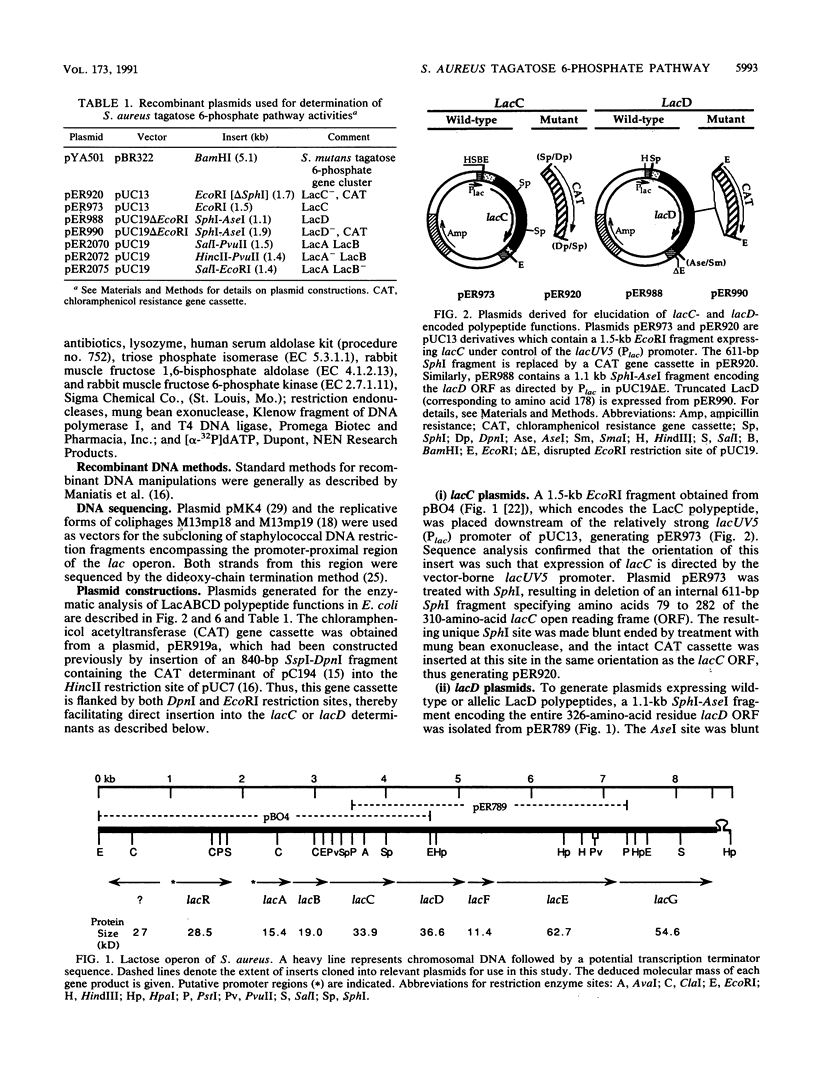
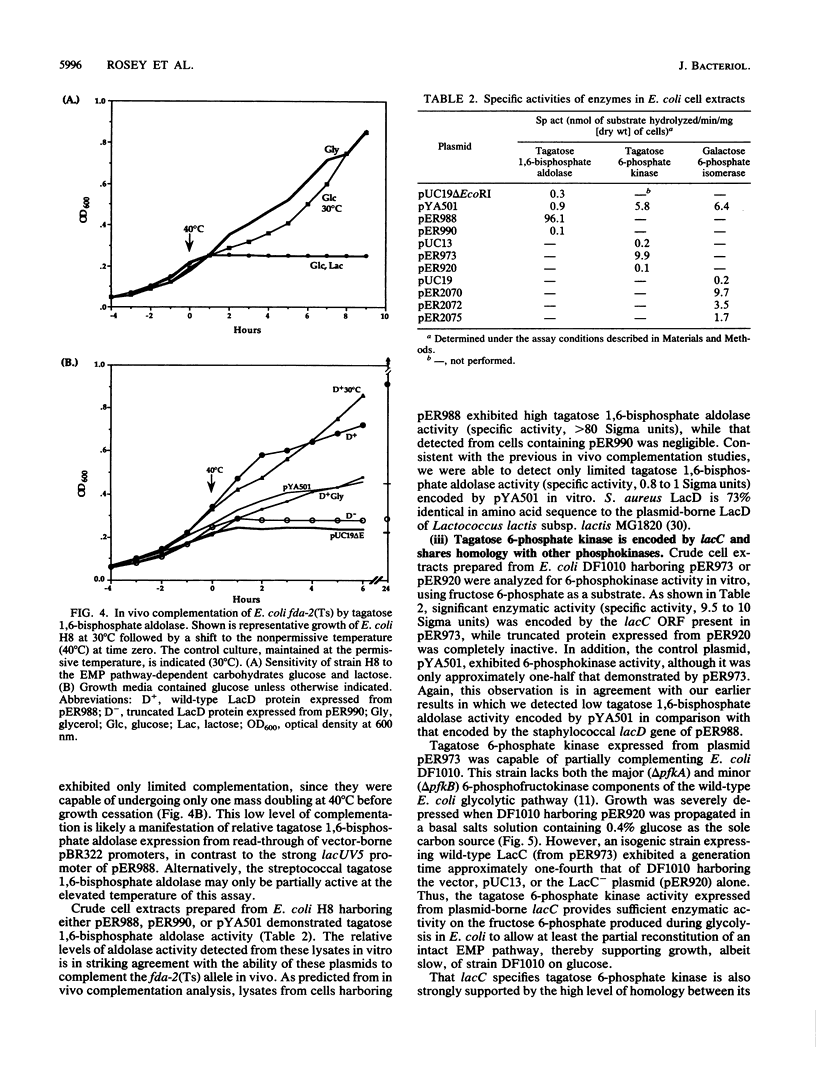
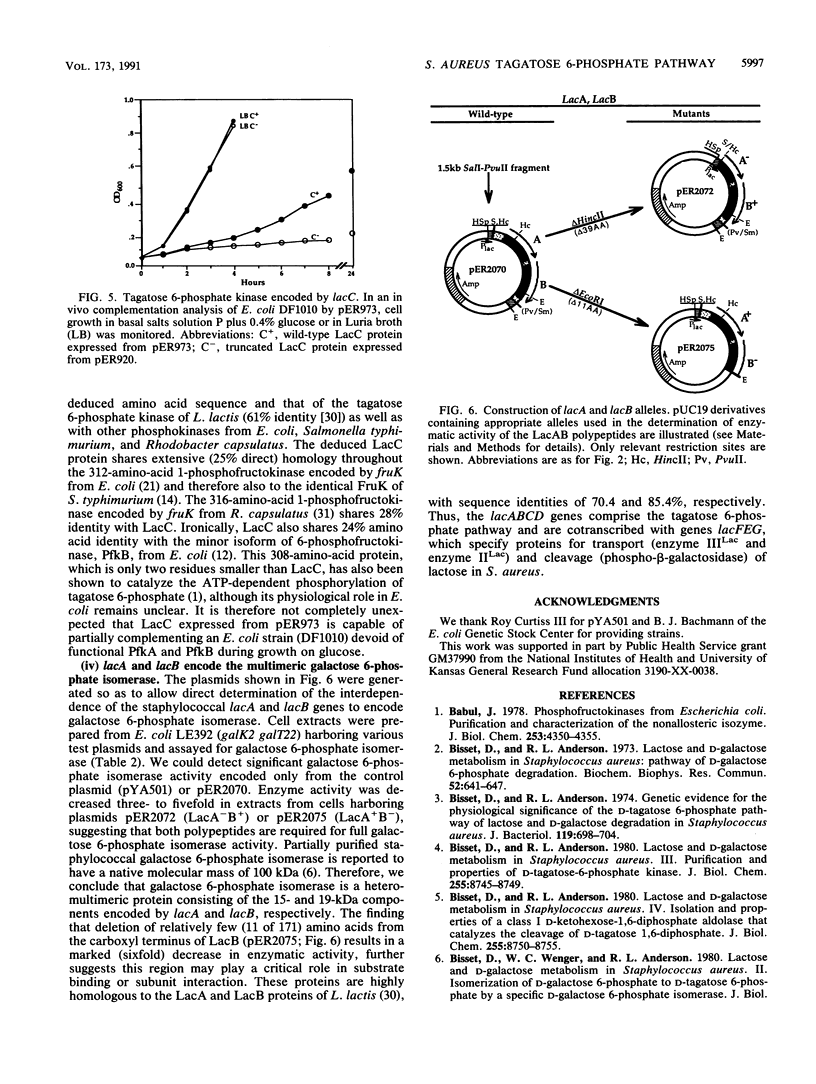
The nucleotide and deduced amino acid sequences of the lacA and lacB genes of the Staphylococcus aureus lactose operon (lacABCDFEG) are presented. The primary translation products are polypeptides of 142 (Mr = 15,425) and 171 (Mr = 18,953) amino acids, respectively. The lacABCD loci were shown to encode enzymes of the tagatose 6-phosphate pathway through both in vitro studies and complementation analysis in Escherichia coli. A serum aldolase assay, modified to allow detection of the tagatose 6-phosphate pathway enzymes utilizing galactose 6-phosphate or fructose phosphate analogs as substrate, is described. Expression of both lacA and lacB was required for galactose 6-phosphate isomerase activity. LacC (34 kDa) demonstrated tagatose 6-phosphate kinase activity and was found to share significant homology with LacC from Lactococcus lactis and with both the minor 6-phosphofructokinase (PfkB) and 1-phosphofructokinase (FruK) from E. coli. Detection of tagatose 1,6-bisphosphate aldolase activity was dependent on expression of the 36-kDa protein specified by lacD. The LacD protein is highly homologous with LacD of L. lactis. Thus, the lacABCD genes comprise the tagatose 6-phosphate pathway and are cotranscribed with genes lacFEG, which specify proteins for transport and cleavage of lactose in S. aureus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babul J. Phosphofructokinases from Escherichia coli. Purification and characterization of the nonallosteric isozyme. J Biol Chem. 1978 Jun 25;253(12):4350–4355. [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Genetic evidence for the physiological significance of the D-tagatose 6-phosphate pathway of lactose and D-galactose degradation in staphylococcus aureus. J Bacteriol. 1974 Sep;119(3):698–704. doi: 10.1128/jb.119.3.698-704.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Lactose and D-galactose metabolism in Staphylococcus aureus. III. Purification and properties of D-tagatose-6-phosphate kinase. J Biol Chem. 1980 Sep 25;255(18):8745–8749. [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Lactose and D-galactose metabolism in Staphylococcus aureus. IV. Isolation and properties of a class I D-ketohexose-1,6-diphosphate aldolase that catalyzes the cleavage of D-tagatose 1,6-diphosphate. J Biol Chem. 1980 Sep 25;255(18):8750–8755. [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Lactose and D0galactose metabolism in Staphylococcus aureus: pathway of D-galactose 6-phosphate degradation. Biochem Biophys Res Commun. 1973 May 15;52(2):641–647. doi: 10.1016/0006-291x(73)90761-4. [DOI] [PubMed] [Google Scholar]
- Breidt F., Jr, Stewart G. C. Cloning and expression of the phospho-beta-galactosidase gene of Staphylococcus aureus in Escherichia coli. J Bacteriol. 1986 Jun;166(3):1061–1066. doi: 10.1128/jb.166.3.1061-1066.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidt F., Jr, Stewart G. C. Nucleotide and deduced amino acid sequences of the Staphylococcus aureus phospho-beta-galactosidase gene. Appl Environ Microbiol. 1987 May;53(5):969–973. doi: 10.1128/aem.53.5.969-973.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böck A., Neidhardt F. C. Isolation of a Mutant of Escherichia coli with a Temperature-sensitive Fructose-1,6-Diphosphate Aldolase Activity. J Bacteriol. 1966 Aug;92(2):464–469. doi: 10.1128/jb.92.2.464-469.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daldal F. Molecular cloning of the gene for phosphofructokinase-2 of Escherichia coli and the nature of a mutation, pfkB1, causing a high level of the enzyme. J Mol Biol. 1983 Aug 5;168(2):285–305. doi: 10.1016/s0022-2836(83)80019-9. [DOI] [PubMed] [Google Scholar]
- Daldal F. Nucleotide sequence of gene pfkB encoding the minor phosphofructokinase of Escherichia coli K-12. Gene. 1984 Jun;28(3):337–342. doi: 10.1016/0378-1119(84)90151-3. [DOI] [PubMed] [Google Scholar]
- FRAENKEL D. G., NEIDHARDT F. C. Use of chloramphenicol to study control of RNA synthesis in bacteria. Biochim Biophys Acta. 1961 Oct 14;53:96–110. doi: 10.1016/0006-3002(61)90797-1. [DOI] [PubMed] [Google Scholar]
- Geerse R. H., Izzo F., Postma P. W. The PEP: fructose phosphotransferase system in Salmonella typhimurium: FPr combines enzyme IIIFru and pseudo-HPr activities. Mol Gen Genet. 1989 Apr;216(2-3):517–525. doi: 10.1007/BF00334399. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse M. L., Hill K. L., Egan J. B., Hengstenberg W. Metabolism of lactose by Staphylococcus aureus and its genetic basis. J Bacteriol. 1968 Jun;95(6):2270–2274. doi: 10.1128/jb.95.6.2270-2274.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Orchard L. M., Kornberg H. L. Sequence similarities between the gene specifying 1-phosphofructokinase (fruK), genes specifying other kinases in Escherichia coli K12, and lacC of Staphylococcus aureus. Proc Biol Sci. 1990 Nov 22;242(1304):87–90. doi: 10.1098/rspb.1990.0108. [DOI] [PubMed] [Google Scholar]
- Oskouian B., Stewart G. C. Cloning and characterization of the repressor gene of the Staphylococcus aureus lactose operon. J Bacteriol. 1987 Dec;169(12):5459–5465. doi: 10.1128/jb.169.12.5459-5465.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskouian B., Stewart G. C. Repression and catabolite repression of the lactose operon of Staphylococcus aureus. J Bacteriol. 1990 Jul;172(7):3804–3812. doi: 10.1128/jb.172.7.3804-3812.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosey E. L., Stewart G. C. The nucleotide sequence of the lacC and lacD genes of Staphylococcus aureus. Nucleic Acids Res. 1989 May 25;17(10):3980–3980. doi: 10.1093/nar/17.10.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Nakazawa T., Hays J. B., Roseman S. Sugar transport. IV. Isolation and characterization of the lactose phosphotransferase system in Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):932–940. [PubMed] [Google Scholar]
- Smorawinska M., Hsu J. C., Hansen J. B., Jagusztyn-Krynicka E. K., Abiko Y., Curtiss R., 3rd Clustered genes for galactose metabolism from Streptococcus mutans cloned in Escherichia coli. J Bacteriol. 1983 Feb;153(2):1095–1097. doi: 10.1128/jb.153.2.1095-1097.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M. A., Yasbin R. E., Young F. E. New shuttle vectors for Bacillus subtilis and Escherichia coli which allow rapid detection of inserted fragments. Gene. 1984 Jul-Aug;29(1-2):21–26. doi: 10.1016/0378-1119(84)90161-6. [DOI] [PubMed] [Google Scholar]
- Wu L. F., Reizer A., Reizer J., Cai B., Tomich J. M., Saier M. H., Jr Nucleotide sequence of the Rhodobacter capsulatus fruK gene, which encodes fructose-1-phosphate kinase: evidence for a kinase superfamily including both phosphofructokinases of Escherichia coli. J Bacteriol. 1991 May;173(10):3117–3127. doi: 10.1128/jb.173.10.3117-3127.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooijen R. J., van Schalkwijk S., de Vos W. M. Molecular cloning, characterization, and nucleotide sequence of the tagatose 6-phosphate pathway gene cluster of the lactose operon of Lactococcus lactis. J Biol Chem. 1991 Apr 15;266(11):7176–7181. [PubMed] [Google Scholar]



