Abstract
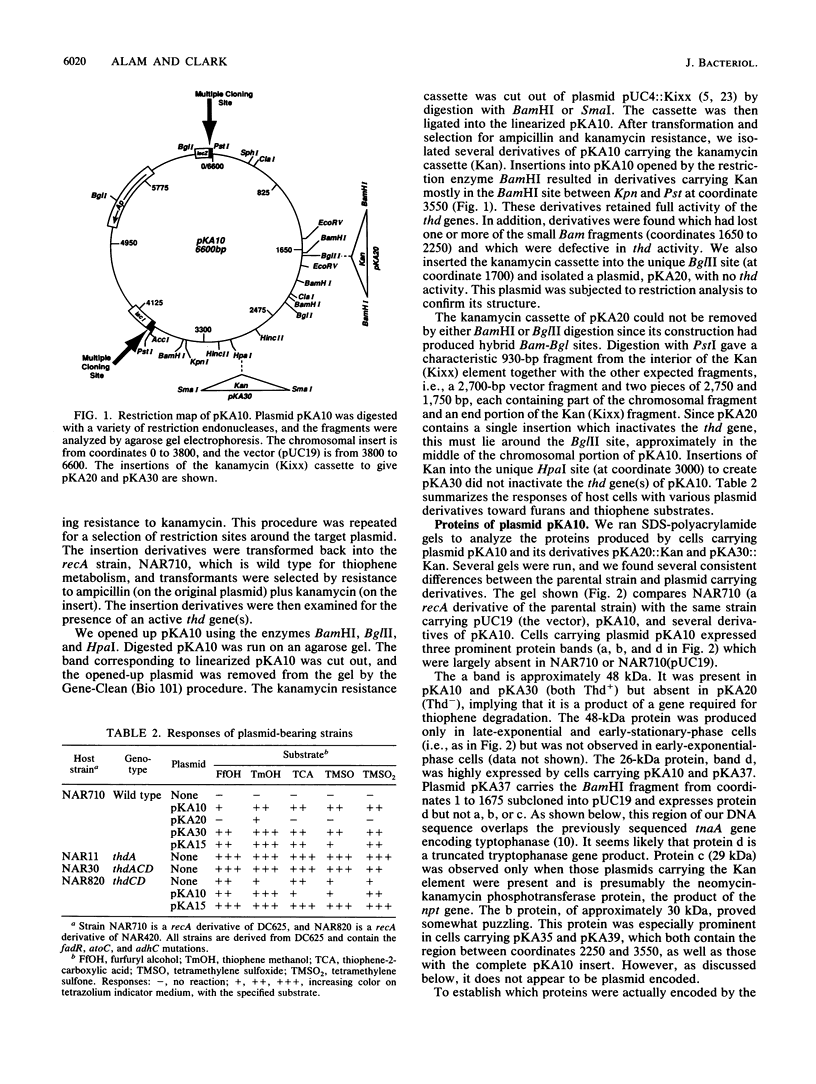
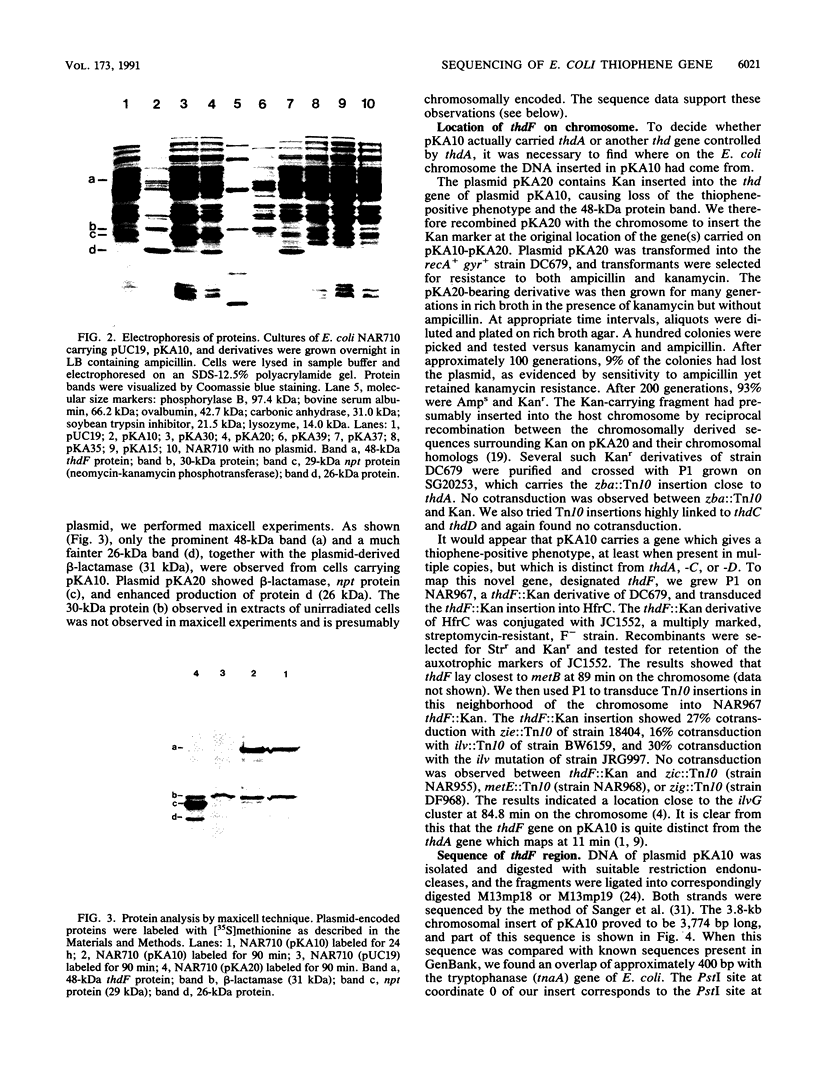
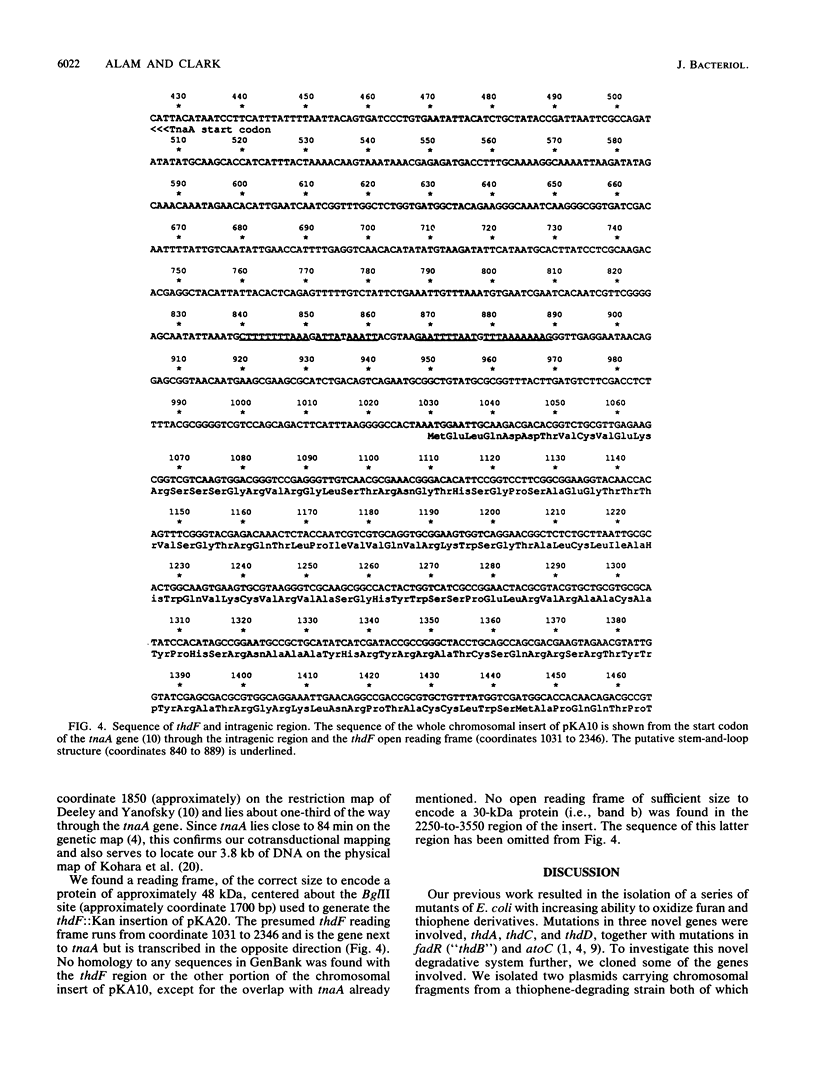
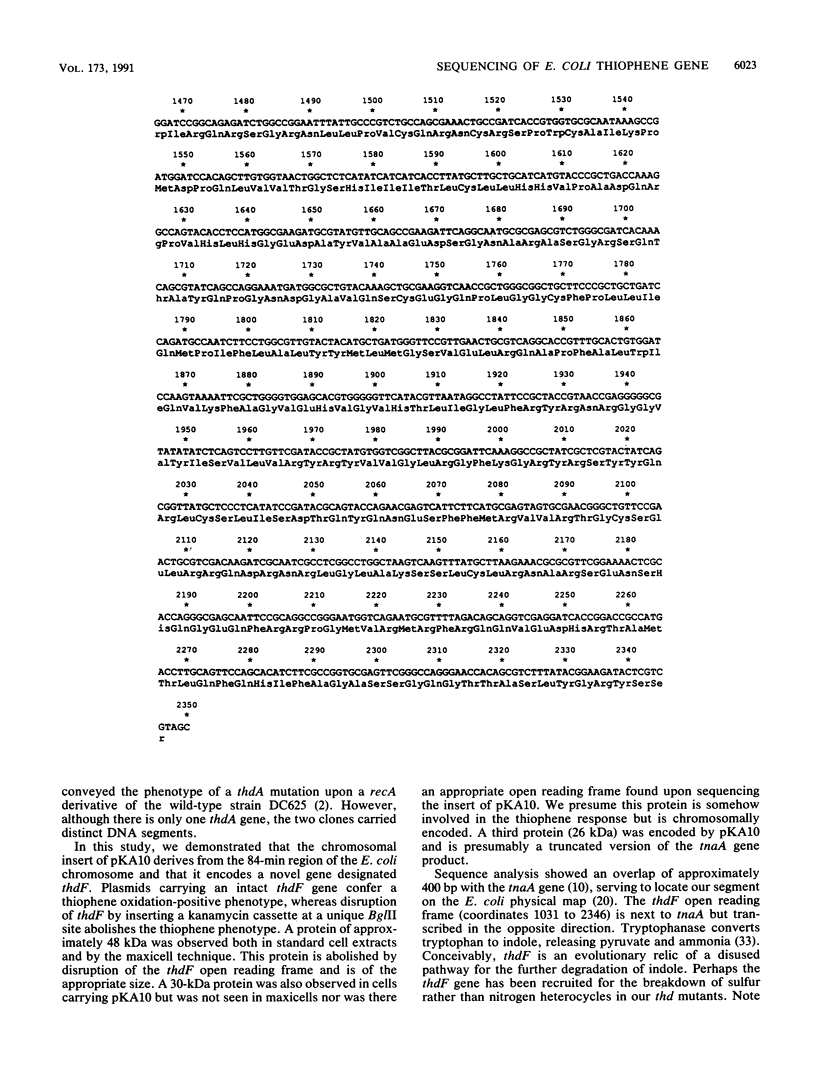
Our previous work resulted in the isolation of mutant strains of Escherichia coli K-12 which were able to oxidize furans and thiophenes as a result of mutations in several novel genes. Some of the genes involved in thiophene oxidation were cloned into the multicopy vector pUC19. The plasmid pKA10 carries a 3.8-kb chromosomal fragment which encodes a previously undiscovered gene involved in thiophene oxidation. Three proteins with approximate molecular sizes of 48, 30, and 26 kDa were overproduced by cells carrying pKA10. Maxicell experiments and DNA sequence analysis indicated that the 48- and 26-kDa proteins are encoded by pKA10, whereas the 30-kDa protein is apparently chromosomally derived. A cassette specifying kanamycin resistance was inserted into various sites on pKA10. An insertion which abolished the 48-kDa protein also abolished thiophene oxidation. Chromosomal integration of pKA10::Kan allowed us to locate the chromosomal insert of pKA10 at 84 min on the E. coli genetic map by transduction. Since no previously identified genes involved in thiophene metabolism are located in this region, we designated the gene for the 48-kDa protein as thdF. Sequencing of the 3.8-kb insert revealed an overlap of several hundred bases with the regulatory and structural regions of the tnaA gene, which is also located at 84 min. The 26-kDa protein is probably truncated tnaA protein. An open reading frame corresponding to the 48-kDa thdF protein was located next to the tnaA gene, which encodes tryptophanase, but was transcribed in the opposite sense.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdulrashid N., Clark D. P. Isolation and genetic analysis of mutations allowing the degradation of furans and thiophenes by Escherichia coli. J Bacteriol. 1987 Mar;169(3):1267–1271. doi: 10.1128/jb.169.3.1267-1271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam K. Y., Worland M. J., Clark D. P. Analysis and molecular cloning of genes involved in thiophene and furan oxidation by E. coli. Appl Biochem Biotechnol. 1990 Spring-Summer;24-25:843–855. doi: 10.1007/BF02920299. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E., Ludwig G., Auerswald E. A., Reiss B., Schaller H. Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. Gene. 1982 Oct;19(3):327–336. doi: 10.1016/0378-1119(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Savageau M. A. Generalized indicator plate for genetic, metabolic, and taxonomic studies with microorganisms. Appl Environ Microbiol. 1977 Feb;33(2):434–444. doi: 10.1128/aem.33.2.434-444.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlage R. S., Sayler G. S., Larimer F. Monitoring of naphthalene catabolism by bioluminescence with nah-lux transcriptional fusions. J Bacteriol. 1990 Sep;172(9):4749–4757. doi: 10.1128/jb.172.9.4749-4757.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. P., Alam K. Y., Abdulrashid N., Klubek B. Successive mutation of E. coli for improved thiophene degradation. Scientific note. Appl Biochem Biotechnol. 1988 Aug;18:393–401. doi: 10.1007/BF02930842. [DOI] [PubMed] [Google Scholar]
- Deeley M. C., Yanofsky C. Nucleotide sequence of the structural gene for tryptophanase of Escherichia coli K-12. J Bacteriol. 1981 Sep;147(3):787–796. doi: 10.1128/jb.147.3.787-796.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlove P. E., Cunningham P. R., Parker J., Clark D. P. Cloning and sequence analysis of the fermentative alcohol-dehydrogenase-encoding gene of Escherichia coli. Gene. 1989 Dec 21;85(1):209–214. doi: 10.1016/0378-1119(89)90483-6. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hoffmann M. R., Faust B. C., Panda F. A., Koo H. H., Tsuchiya H. M. Kinetics of the removal of iron pyrite from coal by microbial catalysis. Appl Environ Microbiol. 1981 Aug;42(2):259–271. doi: 10.1128/aem.42.2.259-271.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhl M. J., Clark D. P. Thiophene-degrading Escherichia coli mutants possess sulfone oxidase activity and show altered resistance to sulfur-containing antibiotics. Appl Environ Microbiol. 1990 Oct;56(10):3179–3185. doi: 10.1128/aem.56.10.3179-3185.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel J. A., Vossen J. P., Venema G. A general method for the construction of Escherichia coli mutants by homologous recombination and plasmid segregation. Mol Gen Genet. 1987 May;207(2-3):294–301. doi: 10.1007/BF00331592. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mazodier P., Cossart P., Giraud E., Gasser F. Completion of the nucleotide sequence of the central region of Tn5 confirms the presence of three resistance genes. Nucleic Acids Res. 1985 Jan 11;13(1):195–205. doi: 10.1093/nar/13.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Monticello D. J., Finnerty W. R. Microbial desulfurization of fossil fuels. Annu Rev Microbiol. 1985;39:371–389. doi: 10.1146/annurev.mi.39.100185.002103. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Nunn W. D. A molecular view of fatty acid catabolism in Escherichia coli. Microbiol Rev. 1986 Jun;50(2):179–192. doi: 10.1128/mr.50.2.179-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli G., Overath P. ato Operon: a highly inducible system for acetoacetate and butyrate degradation in Escherichia coli. Eur J Biochem. 1972 Sep 25;29(3):553–562. doi: 10.1111/j.1432-1033.1972.tb02021.x. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell E. E. Tryptophanase: structure, catalytic activities, and mechanism of action. Adv Enzymol Relat Areas Mol Biol. 1975;42:287–333. doi: 10.1002/9780470122877.ch6. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yen K. M., Serdar C. M. Genetics of naphthalene catabolism in pseudomonads. Crit Rev Microbiol. 1988;15(3):247–268. doi: 10.3109/10408418809104459. [DOI] [PubMed] [Google Scholar]
- Zhou D., White R. H. Biosynthesis of caldariellaquinone in Sulfolobus spp. J Bacteriol. 1989 Dec;171(12):6610–6616. doi: 10.1128/jb.171.12.6610-6616.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]