Abstract
Cytokines regulate the innate and adaptive immune responses and are pleiotropic, redundant and multifunctional. Expression of most cytokines, including TNF-α and IL-1α/ß, is very low in normal brain. Metabolism of lipids is of particular interest due to their high concentration in the brain. Inflammatory response after stroke suggests that cytokines (TNF-α, IL-1 α/ß, IL-6), affect the phospholipid metabolism and subsequent production of eicosanoids, ceramide, and ROS that may potentiate brain injury. Phosphatidylcholine and sphingomyelin are source for lipid messengers. Sphingomyelin synthase serves as a bridge between metabolism of glycerolipids and sphingolipids. TNF-α and IL-1 α/ß can induce phospholipases (A2, C, and D) and sphingomyelinases, and concomitantly proteolyse phosphatidylcholine and sphingomyelin synthesizing enzymes. Together, these alterations contribute to loss of phosphatidylcholine and sphingomyelin after stroke that can be attenuated by inhibiting TNF-α or IL-1 α/ß signaling. Inflammatory responses are instrumental in the formation and destabilization of atherosclerotic plaques. Secretory PLA2 IIA is found in human atherosclerotic lesions and is implicated in initiation, progression and maturation of atherosclerosis, a risk factor for stroke. Lipoprotein-PLA2, part of apolipoprotein B-100 of LDL, plays a role in vascular inflammation and coronary endothelial dysfunction. Cytokine antagonism attenuated secretory PLA2 IIA actions, suggesting cytokine-lipid integration studies will lead to new concepts contributing to bench-to-bedside transition for stroke therapy.
Keywords: Atherosclerosis, Arachidonic acid, Ceramide, CNS injury, Cytokines, Inflammation, Interleukin-1ß, Lipid metabolism, Lipoprotein-PLA2, Phospholipases, Phosphatidylcholine, Sphingomyelin, Stroke, TNF-α, Review
2. STROKE
2.1. Stroke or “brain attack”: a problem of vast clinical importance
Cerebral ischemia or stroke is characterized by an obstruction of blood flow to the brain and is the leading cause of long-term disability, third leading cause of death. It is estimated that about 700,000 Americans suffer from stroke each year, and approximately 4.7 million Americans are stroke survivors. The economic impact of stroke amounts to more than $62.7 billion in direct health care costs and due to loss of productivity annually (1).
Stroke generally refers to a local interruption of blood flow to the brain due to blockage of a cerebral artery. Approximately 12% of strokes are hemorrhagic (rupture of a cerebral blood vessel; 9% intracranial, 3% subarachnoid), whereas the remaining 88% are ischemic and result from occlusion of a cerebral artery (either thrombolic or embolic). Thrombolic stroke results from the formation of a clot or thrombus in a cerebral artery that blocks blood flow at the site of formation. Embolic stroke occurs when a cerebral artery is blocked by a clot that formed elsewhere and was carried to the brain through the circulation. Cerebral ischemia can also be global. i.e., total loss of blood flow to the brain caused by events such as cardiac arrest.
Stroke results in disruption of glucose and oxygen supply that ultimately leads to apoptotic and necrotic cell death, and development of an infarction. Focal ischemia is characterized by an ischemic core surrounded by a “penumbra” region that has partial reduction in blood flow due to presence of collateral arteries. If left untreated, the infarct can propagate into the penumbra. The ischemic core is generally considered unsalvageable, whereas the penumbra may be rescued by timely intervention and poses a target for the development of therapeutic treatment. Inflammation poses as one the high risk factors for stroke in the initiation, progression and maturation of atherosclerosis. CNS inflammation plays a dual role in the patho-physiological processes of stroke: deleterious during the early acute phase while participating in repair processes during the late stage.
2.2. Atherosclerosis is a risk factor for stroke
Atherosclerosis has traditionally been viewed as simply the deposition and accumulation of lipids and cellular debris within the wall of medium to large arteries, resulting in plaque formation and disturbance of blood flow. The role of cholesterol in atherosclerosis is well established and has been elegantly reviewed elsewhere (2). In atherosclerosis, it is now believed that a complex endothelial dysfunction induced by a variety of factors results in an inflammatory response that is instrumental in the formation and destabilization of plaques, one of the greatest risk factors for ischemic stroke (3,4). Gene expression profile and understanding dynamic nature of atherosclerotic vascular wall will pave path for genomics, proteomics, and metabolomics ultimately leading to therapeutics and makes bench-to-bedside transition (201). Inflammatory cytokines have a central role in atherogenesis and plaque rupture (202,205). Increased levels of tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) up-regulate expression of adhesion molecules and promote monocyte recruitment into developing atherosclerotic lesions. Expression of macrophage matrix metalloproteinase 9 (MMP-9) degrades extracellular matrix components including the fibrous cap of atheromatous plaques. Destabilization of this aggregate can result in release of an embolus and induction of ischemic stroke (3-8). Statins inhibit cytokine expression independent of cholesterol lowering in both in vivo and in vitro models, suggesting that they might attenuate the pathogenesis of chronic progressive diseases (coronary diseases, AD, diabetes, MS, osteoporosis, chronic pancreatitis, pulmonary fibrosis and much more) that generally accompany atherosclerosis (202). Data from clinics provide the basis to hypothesize that statins, given for cardiovascular disease, might have the secondary benefit of inhibiting these chronic progressive diseases. A systemic inflammation also aggravates brain injury after stroke (9).
2.2.1 Initiation of atherosclerosis: apolipoprotein B-100 (apoB-100) of low-density lipoprotein (LDL)
Atherosclerosis is defined by the accumulation in the arterial intima of mainly LDL-derived lipids along with apoB-100. LDL is the major carrier of cholesterol in the circulation and is composed of one apoB-100 together with phosphatidylcholine (PC), sphingomyelin (SM) and unesterified cholesterol (500:200:400 molecules respectively) constituting a surface film surrounding a core of cholesteryl esters and triacylglycerols.
2.2.2. Lipoprotein-PLA2 (Lp-PLA2) is also known as platelet activating factor (PAF) acetylhydrolase
Lp-PLA2, 45 kDa protein, is a member of PLA2 family classified as group VIIA PLA2 and is also known as plasma PAF acetylhydrolases (10). This enzyme is found in blood circulation in most animals, and in humans is associated with apoB-100 of LDL and is also found in atherosclerotic plaques (10, 11). Higher levels of Lp-PLA2 are also associated with coronary heart disease, stroke and dementia (11, 12). Lp-PLA2 is produced and secreted by cells of monocyte-macrophage series, T-lymphocytes and mast cells. The enzyme is best known for its PAF acetylhydrolase activity but also hydrolyzes oxidized phospholipids such as oxidized PC of LDL to generate oxidized fatty acids and lyso-phosphatidylcholine (lyso-PC) (13). Local coronary lyso-PC formation is also associated with endothelial dysfunction and supports the role of this enzyme in vascular inflammation and atherosclerosis in humans (11). Lp-PLA2 apparently plays a dual role; the anti-inflammatory function arises by hydrolyzing PAF, which is known to activate platelets, monocytes and macrophages.
2.2.3. Sphingomyelinase (SMase) activity of LDL: A link between atherosclerosis and ceramide
LDL possesses SMase activity, which hydrolyzes SM to release ceramide. Sequence analogy with bacterial SMase suggests that this activity may be intrinsic to apoB-100. Ceramide is elevated in atherosclerotic plaques as well as in LDL isolated from these lesions. Aggregation of LDL within the arterial wall is considered to be a critical step in the initiation of atherosclerosis and ceramide is believed to play an important role in this process (14).
2.2.4. Atherosclerosis and group IIA secretory PLA2 (inflammatory PLA2)
Group IIA phospholipase A2 (secretory PLA2 also known as inflammatory PLA2) has been found in human atherosclerotic lesions (15, 16). sPLA2 IIA is implicated in chronic inflammatory conditions such as arthritis and may also contribute to atherosclerosis (17), one of the risk factors for stroke. sPLA2 IIA is a pro-atherogenic factor and it has been suggested that this enzyme regulates collagen deposition in the plaque and fibrotic cap development (18). sPLA2 is one of the enzymes responsible for the release of lyso-PC via its catalytic action and these two play a crucial role in the development of atherosclerosis (19). Non-catalytic (non-enzymatic) atherogenic effects of sPLA2 II are thought to involve binding to a muscular-type (M-type) sPLA2 receptor (see section 6.1.3. on sPLA2 receptors).
2.3. Energy failure is the initial metabolic event in stroke
The energy needs of the brain are supplied by metabolism of glucose and oxygen for the phosphorylation of ADP to ATP. Most of the ATP generated is utilized in the brain in maintaining intracellular homeostasis and transmembrane ion gradients of sodium, potassium, and calcium. Energy failure results in rapid loss of ATP and uncontrolled leakage of ions across the cell membrane that results in membrane depolarization and release of the neurotransmitters such as glutamate and dopamine (20, 21). Excess glutamate release and stimulation of its receptors results in activation of phospholipases/sphingomyelinases (22-26), phospholipid hydrolysis and release of ceramide and free fatty acids (FFA) including arachidonic acid (ArAc) (26, 27). Ultimately these processes lead to apoptotic or necrotic cell death (28) (Figure 1, next page).
Figure 1.
Lipid metabolism in ischemic neuronal death. Activation of phospholipases (PLA2, PC-PLC, PI-PLC, PLD) sphingomyelinases (N-SMase and A-SMase) following cerebral ischemia results in release of lipid second messengers DAG, phosphatidic acid (PA), docosahexaenoic acid (DHA), ArAc and ceramide. PI-PLC releases inositol-trisphosphate (IP3) in addition to DAG; IP3 stimulates intracellular Ca++ release. PA and DAG can be readily interconverted by phosphatases and DAG-kinases. ArAc undergoes further metabolism by LOX to generate important vasoactive eicosanoids and ROS. Recent studies suggest that COX does not directly produce ROS during ArAc oxidative metabolism, but does form free radicals (i.e., carbon-centered radicals on ArAc) (148, 200). DHA (204) is metabolized to 10,17S-docosatriene (Neuroprotectin D1), an endogenous neuroprotectant. DAG and ArAc stimulate A-SMase and N-SMase, respectively, to generate pro-apoptotic ceramide (148).
2.4. Animal models for stroke
The majority of ischemic strokes in patients result from blockage of the middle cerebral artery (MCA). One commonly used animal model for stroke uses transient MCA occlusion (tMCAO) in rats or mice (29). In this model, a nylon monofilament is advanced through the internal carotid artery to occlude the MCA. The drop in blood flow in the brain is determined by Doppler flowmetry with a probe placed over the MCA territory. Restoration of blood flow (reperfusion) is achieved by withdrawing the occluding suture. Permanent MCAO (no reperfusion) is achieved usually by electro-coagulation or photochemically induced thrombosis of the MCA. Other animal models induce a global or forebrain ischemia by four-vessel occlusion in rat or bilateral common carotid artery occlusion in gerbil, which lacks the circle of Willis and thus has no collateral blood flow. Global models mimic the complete loss of blood flow to the brain that occurs during cardiac arrest.
3. THE BIOLOGICAL MEMBRANE: STRUCTURE AND FUNCTION
Cellular membranes are composed of glycerophospholipids (PC, phosphatidylethanolamine (PE), phosphatidylserine (PS) and phosphatidylinositol (PI)), sphingolipids (SM, ceramide and gangliosides), cholesterol and cholesterol esters, acylglycerols, and fatty acids. The phospholipid bilayer and associated lipids provide not only a permeability barrier but also a structured environment that is essential for the proper functioning of membrane-bound proteins (2). Cholesterol is one of the most important regulators of lipid organization as its structure allows it to fill interstitial spaces between hydrophobic fatty acid chains of phospholipids. The neutral lipids such as PC and SM predominantly reside on the outer or exofacial leaflet, whereas anionic phospholipids PS (exclusively inner leaflet), PE, and PI reside on the inner or cytofacial leaflet of the biological membrane. The transbilayer distribution of cholesterol between the leaflets determines membrane fluidity and can alter the membrane function. The asymmetric distribution of phospholipids in the plasma membrane is shown in Figure 2. Cardiolipin is a phospholipid that is exclusively limited to the mitochondrial membrane and is essential for proper assembly and functioning of the mitochondrial respiratory chain and oxidative phosphorylation (30).
Figure. 2.
Asymmetric distribution of phospholipids (PL) in a plasma membrane
The classic fluid mosaic model of the cellular membrane was modified recently to accommodate the concept of distinct microdomains that serve signaling functions (31). The tight interaction of sphingolipids with each other and with cholesterol segregates these lipids into discreet membrane structures characterized by a liquid-ordered or gel-like phase, in contrast to the glycerophospholipids in the bulk cell membrane that reside in a more fluid liquid-disordered phase. These distinct sphingolipid- and cholesterol-enriched microdomains have been termed as “lipid rafts”.
4. PHOSPHOLIPIDS: SYNTHESIS AND HYDROLYSIS
4.1. PC homeostasis
PC is the most abundant phospholipid in mammalian cells and constitutes ∼50% of the total phospholipid content. PC homeostasis is regulated by a balance between hydrolysis and synthesis, which under normal conditions is tightly regulated to assure a constant amount of the phospholipid in the cell membrane (32, 33). Controlled hydrolysis of PC is required for signal transduction and efficient regulation of cellular functions (33). A limited amount of membrane damage can be handled by the cell provided the energy status (e.g. ATP production and ion homeostasis) is not severely impaired. A major loss of membrane integrity is incompatible with cellular viability. Patho-physiological breakdown of membrane PC causes growth arrest (34) and even a 10% loss is sufficient to threaten cell viability (32). Excessive breakdown of PC by phospholipases not only changes membrane permeability, uncontrollably allowing small ions and metabolites to cross the cell membrane, but also releases second messengers such as phosphatidates, 1,2-diacylglycerol (DAG), and ArAc (20:4) and docosahexaenoic (22:6) acids, among others (26, 32). ArAc metabolites prostaglandins and leukotrienes are implicated in the development of inflammatory conditions.
4.2. Cytidine triphosphate:phosphocholine cytidylyltransferase (CCT) and PC synthesis
PC can be synthesized by methylation of PE by PE-N-methyltransferase (PEMT) or through the CDP-choline/Kennedy pathway. The PEMT pathway is significant only in the liver (35, 36) and PC synthesis in the brain is predominantly through the CDP-choline pathway (Figure 3), which is regulated by the rate-limiting enzyme CCT (32, 37). The majority of choline is derived from dietary sources (32). In the Kennedy pathway, choline is first phosphorylated, and CCT (37) synthesizes CDP-choline from phosphocholine and cytidine triphosphate. PC is synthesized from CDP-choline and DAG by the action of CDP-choline:DAG phosphocholine transferase (CPT). Under certain conditions, the availability of DAG may become rate-limiting in PC synthesis (38). Several studies have shown that blocking PC synthesis causes cell death. A number of compounds (camptothecin, ceramide, chelerythrine, etoposide, farsensol, geranylgeraniol and hexadecylphosphocholine) that induce apoptosis also cause a build-up of CDP-choline (measured by 31P nuclear magnetic resonance) (39). The accumulation of CDP-choline implies that a block of CPT is a common event in apoptosis. Inactivation of CCT also induces cell death (32).
Figure. 3.
PC synthesis through CDP-choline/Kennedy pathway (148). CCT is down-regulated after stroke, impeding PC synthesis (41). CCT:cytidine triphosphate:phosphocholine
CCT activity decreased in transient forebrain (40) and focal (41) ischemia, which may have resulted from induction of TNF-α, since TNF-α inhibits CCT (42) (see section 6.1.7.3.). We have also shown up-regulation of phospholipases and down-regulation of CCT that collectively contributed to loss of PC following tMCAO (41). Our recent studies also indicate that loss of PC is a cause rather than a result of cell death (43). Thus preserving membrane PC may offer benefit after stroke (41).
4.3. PC hydrolysis by phospholipases
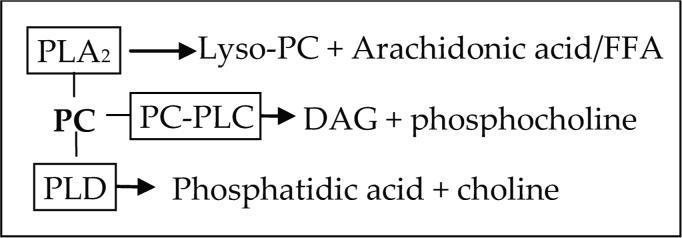
Phospholipases have received wide attention for their role in progression of stroke injury (25). PC is hydrolyzed by phospholipase A2 (PLA2), PC-phospholipase C (PC-PLC), and phospholipase D (PLD) to release lyso-PC, DAG, phosphatidic acid (PA), and FFA, especially ArAc. The immediate products formed from PC by each class of phospholipase are given in Figure 4.
Figure 4.
The immediate products formed from PC by each class of phospholipase.
4.3.1. PLA2
PLA2 isozymes occur in multiple forms (25, 44) in the mammalian cell and are classified as calcium independent (iPLA2, 84 kDa), and the calcium-dependent cytosolic (cPLA2, 85−110 kDa) and secretory (sPLA2, 14−18 kDa) forms. cPLA2 preferentially cleaves ArAc at the sn-2 position of PC and forms lyso-PC, whereas sPLA2s show no fatty acid preference and act on a broader range of phospholipids (45, 46). iPLA2s also are not selective for ArAc and are considered to be involved primarily in membrane remodeling (47, 48). Full activation of cPLA2 requires binding of Ca2+ for translocation from cytosol to the phospholipid membrane and phosphorylation of specific serine residues (49). Activation of PLA2 is harmful to neurons in several ways: 1) membrane phospholipid degradation, 2) increased Ca2+ influx, 3) formation of lyso-PC that inhibits CCT (50), 4) ArAc release and metabolism by cyclooxygenases/ lipoxygenases (COX/LOX) (Figure 1). Mitochondrial PLA2 is a sPLA2 that acts on PC, PE, and cardiolipin (51, 52). Induction of PLA2 has been shown to result in damage to the mitochondrial respiratory chain (53).
4.3.2. PC-PLC
The mammalian enzyme has a strong preference for PC and hydrolyzes it to form phosphocholine and DAG. The primary intracellular target for DAG is activation of protein kinase C (PKC) which stimulates cell proliferation (54). In addition to acting as a second messenger, hydrolysis of DAG by DAG lipases releases FFA including ArAc. Our studies show that the PC-PLC inhibitor (55) tricyclodecan-9-yl potassium xanthate (D609) attenuated infarction after tMCAO, evidence for a role of PC-PLC in stroke injury (Figure 5). Our results demonstrated that PC-PLC activity increased after focal cerebral ischemia; D609 blocked the activity in the assay confirming this as PC-PLC activity (41). However non-specific actions of D609 should be taken into consideration while interpreting data (43, 203).
Figure 5.
D609 (50 mg/kg in saline; i.p at the onset of reperfusion) attenuated ischemic injury volume (infarction) by 35 ± 5% after 1 hr MCAO/24 hr reperfusion (saline 270 ± 38 mm3).
4.3.3. PLD
PLD catalyzes the hydrolysis of PC to choline and PA. PA may act directly as a signaling molecule and can be further converted to other messenger molecules such as DAG and lyso-PA. PLD catalyzes a unique transphosphatidylation reaction in the presence of a primary alcohol to form a phosphatidylalcohol instead of PA (56). This reaction has been used to measure PLD activity and assess the role of PA in cell signaling. Two mammalian forms (56) have been identified, PLD1 (∼125 kDa) and PLD2 (∼105 kDa). PLD1 is expressed in neurons and glial cells (56) and PLD2 is expressed in astrocytes (56). Expression of PLD in forebrain ischemia (56) and other CNS injuries and disorders (57) has been demonstrated. Interestingly there are no reports examining the role of PLD in stroke (focal cerebral ischemia) injury, which may be due to lack of specific inhibitors (primary alcohols block PA formation but should not be considered as PLD inhibitors). Our data showed increased protein expression of PLD2 after 1 hr MCAO and reperfusion over 7 day (41).
4.4. SM, SM synthases and SMases
PC also serves as the phosphocholine donor in SM synthesis, another phospholipid important for maintaining the membrane integrity. SM synthase (SMS) transfers the phosphocholine group from PC to ceramide to generate SM and DAG. SMS serves as a bridge between metabolism of glycerolipids (PC and DAG) and sphingolipids (SM and ceramide) since it regulates not only cellular SM and ceramide levels but also contributes to formation of DAG (Figure 6). Recent literature suggests there are three forms of SMS: SMS1 and SMS2. SMS1 is predominantly in Golgi while SMS2 is localized to plasma membrane (197-199). Another alternative SM synthesis pathway has been proposed in which ceramide is converted to ethanolamine phosphorylceramide (EPC) via transfer of the head group from PE, a third SMS-related enzyme (SMSr). EPC later converted to SM by methylation in a reaction one similar to the S-adenosyl methionine (AdoMet or SAM) mediated conversion of PE to PC (197). SMS is closely related to PC-PLC in that both enzymes use PC as a substrate and generate DAG as a product. There has been some debate actually whether PC-PLC and SM synthase are distinct enzymes or one and the same (58). Mammalian PC-PLC has not yet been cloned and characterized. Commercial antibodies of this enzyme are presently unavailable.
Figure. 6.
Cytokine mediated generation of lipid mediators DAG, ArAc and ceramide. This scheme also shows the relationship between glycerophospholipids and sphingolipids (148). TNF-α and IL-1 activate N-SMase and A-SMase through stimulation of PLA2 and PC-PLC and release of ArAc and DAG, respectively. N-SMase and A-SMase hydrolyze SM to liberate ceramide. SM synthase transfers the phosphocholine head group of PC to ceramide to form SM and DAG.
SMases are C-type phospholipases with specificity for SM. SMase hydrolyzes SM to form ceramide, which inhibits cell proliferation (59) and induces apoptosis (60). The role of ceramide in cell signaling has been studied extensively (61). Two distinct SMases have been described: both acidic SMase (A-SMase) and neutral SMase (N-SMase) are expressed in brain tissue. Expression of A-SMase has been shown in astrocytes (62), but the cellular localization (neurons, astrocytes, etc.) of these two forms is not well characterized. These SMases exhibit distinct sub-cellular distribution: A-SMase is lysosomal while N-SMase is localized to the outer leaflet of the plasma membrane (63, 64). Since ceramide released by these enzymes is hydrophobic and does not diffuse from its site of release, A-SMase and N-SMase have different signaling functions. A-SMase deficient mice showed reduction in infarct volume compared to wild type (65). However one should bear in mind that these mice have Niemann-Pick related disease symptoms (148). Niemann-Pick disease is a lysosomal storage disorder that lacks A-SMase activity leads to lysosomal accumulation of SM.
The externalization of PS (an exclusive inner plasma membrane anionic phospholipid) to outer plasma membrane initiates an apoptotic death signal. The PS relocation also causes flipping of SM from outer to inner leaflet and hydrolysis by a cytosolic SMase (66-68) to generate ceramide with subsequent activation of death cascade mechanisms.
5. CYTOKINES
5.1. Cytokines are pleiotropic, multifunctional, and redundant with dual nature (pro- and anti-inflammatory functions)
Inflammation is the first response of the immune system to infection or irritation and may be referred to as the innate cascade (69, 70). Inflammation is characterized by the following quintet: redness (rubor), heat (calor), swelling (tumor), pain (dolor) and dysfunction of the organs involved (functio laesa). The first four characteristics were defined by Celsus around AD 40 (71); functio laesa was added to the definition of inflammation by Rudolf Virchow in 1858. Cytokines are low molecular weight, soluble proteins that are produced in response to an antigen and were originally described as mediators for regulating the innate and adaptive immune systems. Cytokines thus serve as chemical messengers and include tumor necrosis factors, interleukins, interferons, chemokines, and growth factors. One cytokine can act on a number of different cell types rather than one type (pleiotropic); the same cytokine regulates a number of different functions (multifunctional); and a number of different cytokines can carry out the same function (redundant) (69, 70). The redundancy is due to the utilization of shared key components of intracellular signaling pathways.
A regulated inflammatory response is characterized by an interplay between pro-inflammatory (go signals) and anti-inflammatory effects (stop signals) mediated by various cytokines. Cytokines are commonly classified as either pro-inflammatory or anti-inflammatory: TNF-α, IL-1, interferon-γ (IFN-γ), IL-12, IL-18 and granulocyte-macrophage colony stimulating factor (GM-CSF) are well characterized as pro-inflammatory cytokines whereas IL-4, IL-10, IL-13, IFN-α and transforming growth factor-ß (TGF-ß) are recognized as anti-inflammatory cytokines. TGF-ß neuroprotectant action in brain lesions in stroke are based on neutralization experiments (72). Application of soluble TGF ß type II receptor to rats in stroke aggravated the infarction volume (73). Realistically, classification of cytokines as either pro- or anti-inflammatory is too simplistic: some cytokines, for example IL-6, are considered to have both pro- and anti-inflammatory actions (74, 75). Furthermore, some cytokines may behave as pro- as well as anti-inflammatory agents depending on the amount of the cytokine, the target cell, the activating signal, the timing and sequence of action, and the experimental model (76).
5.2. Induction of cytokines after CNS injury is complementary and reciprocal
Interestingly, expression of different cytokines is often related. TNF-α induces expression of IL-1 and IL-6, while IL-1 can induce both TNF-α and IL-6. Specifically, IL-1ß is a significantly more potent stimulator of IL-6 release compared to IL-1α (77). Thus after a CNS insult, the initial upregulation of cytokines will lead to infiltration of other inflammatory mediators to the site of injury and secondary cytokine signaling is subsequently initiated (78). Cytokines can also act in concert in regulating various pathways: for example, TNF-α, IL-1α, and IFN-γ were shown to act synergistically in induction of cell death in mouse primary cortical neuron/glia co-cultures (79).
5.3. TNF-α, IL-1 and IL-6 in the CNS
Cytokines affecting CNS: a) originate from peripheral immune organs, cross blood-brain barrier or initiate signaling by binding to receptors on the vascular endothelium and b) produced by glial cells (astrocytes and microglia). Within the CNS, cytokines increase in response to a variety of injuries including cerebral ischemia. The inflammatory responses often include early and significant glia activation, presumably with intent of suppressing or removing the pathogenic initiation to inhibit the injury process (80). However an unchecked inflammatory response may promote or aggravate the injury process. Among the cytokines produced in the CNS in response to injury, TNF-α and IL-1 are the most extensively studied (78, 81). Both cytokines are produced as precursor proteins (pro-TNF-α and pro-IL-1) and must be cleaved to release the active forms. TNF-α is initially synthesized as a 26 kDa membrane bound protein which is cleaved by the Zn-metalloprotease TNF-α converting enzyme (TACE) to form 17 kDa mature, soluble TNF-α (82). IL-1 exists in α and ß forms, both having a molecular weight of 17 kDa. Both IL-1α and IL-1ß are synthesized as 32 kDa precursor proteins. Pro-IL-1α is biologically active and can be cleaved to the 17 kDa mature form by membrane-bound calpain. Pro-IL-1ß is inactive and requires cleavage by interleukin-1 converting enzyme (ICE, also known as caspase 1) (83, 84). The majority of IL-1α remains membrane bound, while IL-1ß is released from the cells and is the main form expressed in the brain (85, 86).
5.3.1. TNF-α and IL-1 are increased in cerebral ischemia
In normal brain, expression of most cytokines, including TNF-α and IL-1, is very low (85). TNF-α and IL-1, major pro-inflammatory cytokines, are rapidly up-regulated in brain after cerebral ischemia (84, 87-89). TNF-α levels increased in CSF and sera of patients and correlated with stroke severity (90-92). Some of the effects caused by TNF-α in stroke are due to inhibition of glutamate uptake by astrocytes; induction of ICAM-1, increased MMP expression that leads to disruption of blood-brain-barrier and invasion of inflammatory leukocytes (82). TNF-α mediates its effects by binding to two receptors (p55 and p75), both of which are up-regulated in the ischemic brain (84, 93). The majority of biological effects of TNF-α, including cytotoxicity, ArAc release, and inflammatory responses are mediated through the p55 receptor (84, 93) that contains the death domain. The function of p75 remains obscure, although studies have indicated it may participate in protective signaling following injury (94). TNF-α receptors are differentially up-regulated after cerebral ischemia: p55 (contains the death domain) is expressed within 6 hr, whereas p75 increases at 24 hr (93). Increased expression of TNF-α p75 receptor was shown following permanent MCAO; it was suggested that the expression of this receptor was predominantly localized to microglia and macrophages over 1−10 day period, reaching a peak at day-5 (95). Nevertheless no definitive conclusions regarding the role of this receptor were provided (95) and still the function of this receptor remains elusive.
5.3.2. TNF-α has a dual role in mediating both beneficial and detrimental effects in brain injury
Studies on TNF-α neurotoxicity are considerable but controversial (81, 96-99). A number of studies have supported the deleterious effects of TNF-α in experimental stroke studies. TNF-α binding protein is a soluble protein produced by cleavage of the TNF-α extracellular binding domain of its membrane-bound receptor and is an endogenously produced inhibitor of TNF-α signaling (100). The actions of TNF-α can be blocked using specific TNF-α neutralizing antibody (TNF-α ab) or TNF-α binding protein to bind TNF-α and prevent it from interacting with its receptors (96). Administration of TNF-α ab (101) or TNF-α binding protein (81, 101, 102) that attenuates the acute phase of TNF-α following cerebral ischemia have consistently demonstrated beneficial effects (81, 84, 96). Transgenic mice deficient in TNF-α (99) showed dramatic reduction in infarction compared to wild-type mice, and infusion of TNF-α exacerbated infarct volume in focal cerebral ischemia (102). However, transgenic mice with targeted deletion of TNF-α receptors (p55 and p75) showed increased infarction after tMCAO (98), providing conflicting results obtained with TNF-α null mice. A follow-up study showed that mice lacking p55 receptors are more susceptible for ischemic injury compared to wild type or p75 receptor deficient mice (103). Possible explanations for this discrepancy are a compensatory increase in other cytokines such as IL-1 in TNF-α p55 receptor deficient mice, or, although speculative, that another as yet unidentified TNF-α receptor exists in the CNS (104).
Ischemic preconditioning is a phenomenon wherein an ischemic insult of short, sub-lethal duration confers protection against a subsequent severe ischemic injury, which has been attributed to altered gene expression by the initial ischemic event. Pretreatment of mice with TNF-α 48 hr before experimental stroke conferred neuroprotection (105) while another study demonstrated that TNF-α pretreatment 24 hr before MCAO aggravated the infarction (102). These studies suggest that exposure to TNF-α in the absence of injury (pretreatment) alters gene expression or similar effects that confers protection against subsequent stroke and that this phenomenon requires 48 hr. In vitro studies have also shown that pretreatment with TNF-α attenuated neuronal damage induced by oxygen-glucose deprivation (OGD) in organotypic hippocampal cultures, whereas TNF-α exacerbated neuronal damage when added after the injury (97). Thus the actions of TNF-α are dependent on whether it is present before or after an ischemic event. One school of thought is that TNF-α stimulates generation of reactive oxygen species (ROS), activation of the transcription factor NFκB, and up-regulation of manganese superoxide dismutase (Mn-SOD), which would provide defense against subsequent ROS generation. In cells stressed by an injury, addition of TNF-α during the injury stimulates ROS generation and aggravates the damage. The actions of TNF-α may be dependent on concentration and time of treatment (97), vary with the cell type, expression of its receptors, and the specific injury.
The over-expression of TNF-α in the early stage after brain injury may be deleterious, whereas lower levels in later phases may facilitate recovery (84, 96). In support of this proof-of-concept are studies showing that TNF-α null mice subjected to controlled cortical impact injury showed fewer neurological deficits compared to wild-type mice at 7 d post-injury. However, wild type mice had better recovery from brain injury by 2−3 weeks post-injury, whereas TNF-α null mice had poor recovery and exhibited greater neurological deficits compared to wild type mice at 4 weeks post-injury (106). This phenomenon apparently has not been investigated in stroke models. TNF-α null mice had smaller infarcts after tMCAO and 1 day reperfusion compared to wild type mice, evidence that elevated levels of TNF-α during the early reperfusion time are deleterious. However, studies have not examined longer reperfusion times (beyond day 1) to determine if TNF-α expression is needed during the later, recovery phase after tMCAO.
5.3.3. IL-1 and stroke
IL-1 is present in two forms in the brain, IL-1α and ß, which interact with two IL-1 receptors (107). IL-1 signaling is mediated by IL-1 receptor type I, while receptor type II is believed to be a non-signaling or “decoy” receptor (85). IL-1ß is the primary form expressed in the brain and up-regulated in response to stroke (85). Since both forms of IL-1 interact with the same receptor, they are believed to mediate much of the same signaling; however their functions may not completely overlap (77). A third member of the interleukin family is IL-1 receptor antagonist (IL-1ra), an endogenous protein that binds to the type I receptor without agonistic signaling and thus blocks effects of IL-1α/ß. IL-1ra knockout worsened the infarction after tMCAO and also exacerbated NMDA or AMPA induced neuronal death in mixed glial-neuronal co-cultures (108). Also administration of neutralizing antibody specific for IL-1ra to rats undergoing MCAO increased the neuronal injury. Expression of endogenous IL-1ra thus limits infarction after tMCAO and this neuroprotection is mediated through glia (108, 109). Treatment with exogenous IL-1ra reduced neuronal death in all forms of experimental cerebral ischemia (permanent and transient focal, global, and hypoxic) in a variety of species (rat, mouse, and gerbil) (110, 111). Individual knockout of IL-1α or IL-1ß did not significantly alter ischemic damage after tMCAO, which may have been due to compensatory responses. However transgenic mice deficient in both IL-1α and ß showed dramatic reduction in infarcts compared to wild-type (112).
Enzymatic cleavage of the extracellular binding domains of IL-1 type I and type II receptors produces soluble IL-1 receptors. Soluble type II IL-1 receptors bind IL-1ß with much higher affinity than IL-1ra and may function as inhibitors of IL-1 in vivo. In contrast, soluble type I IL-1 receptors bind IL-1ra selectively and thus may block or neutralize the natural anti-inflammatory actions of IL-lra (113).
Similar to TNF-α, the evidence that IL-1 is neurotoxic is also considerable but controversial. A few studies have indicated a neuroprotective role of IL-1 (114, 115). Pretreatment of cultured mouse primary cortical neurons with IL-1α or IL-1ß attenuated neurotoxicity induced by NMDA. IL-1 mediated neuroprotection was inhibited by addition of a neutralizing antibody to neuronal growth factor (NGF) (114). In the other study (115), treatment of cultured rat primary cortical neurons with IL-1ß attenuated neuronal death induced by exposure to excitotoxic amino acids (glutamate, NMDA, AMPA, and kainate). The neuroprotective effects of IL-1ß appeared to be mediated, at least in part, by induction of NGF. In these studies (115), IL-1ß was added to culture media both before (pretreatment) and after (post-treatment) exposure to excitatory amino acids. It was not determined whether pretreatment alone would have conferred greater neuroprotection nor if post-treatment alone would have exacerbated neurotoxicity. The common factor in both studies (114, 115) appears to be that the neuroprotection afforded by IL-1 depends on exposure of cultures to IL-1 prior to injury (pretreatment).
5.3.4. IL-6: A complex cytokine and does it play a role in maintaining body temperature?
Unlike TNF-α and IL-1, IL-6 does not up-regulate major inflammatory mediators such as prostaglandins, nitric oxide, MMPs and synthesis of intercellular adhesion molecule ICAM-1 (74). Several reports have indicated that IL-6 induces PLA2 (a pro-inflammatory response) while other studies revealed that IL-6 may inhibit PLA2 induction. IL-6 exhibits several anti-inflammatory characteristics: it inhibits synthesis of TNF-α and IL-1 and promotes synthesis of TNF-α binding protein and IL-1 receptor antagonist (IL-1ra), which further inhibit TNF-α and IL-1 signaling (74).
IL-6 (89) is up-regulated following cerebral ischemia. Elevated levels of IL-6 in serum and CSF of stroke patients correlated with infarct volume and early worsening of neurological score (116, 117). IL-6 deficient mice showed either no reduction in infarction (118) or increased infarction (119) after MCAO compared to wild-type mice. Cytokines can affect body temperature through activation of the hypothalamic-pituitary-adrenal axis (120, 121). IL-6 deficient mice undergo severe prolonged hypothermia (26.7°C) with a survival rate of 90% when body temperature is unregulated following MCAO (119), which by itself attenuates infarction (122). A recent review on hypothermic neuroprotection discussed the role of cytokines and inflammation in stroke (123). When normal body temperature was maintained, IL-6 deficient mice showed increased infarction and very high mortality (20% of mice die within the first hour; ∼90% die within 10 hr) after MCAO. These studies are difficult to interpret since both permanent (up to 24 hr) and transient (1 or 2 hr occlusion) MCAO models were used (119), and it was unclear in one study whether body temperature was monitored during reperfusion (118).
These studies suggest that hypothermia in IL-6-null mice compensated for the detrimental effects of IL-6 deficiency, that at least two pathways are involved in the overall outcome and that endogenous IL-6 in wild-type mice limits infarction after tMCAO. This also highlights the need for careful monitoring of physiological parameters, particularly body temperature, to rule out a contribution of hypothermic effects to infarct reduction in transgenic animals or with drug interventions after cerebral ischemia (122). In contrast to the results from IL-6 null mice, other studies demonstrated that mice over-expressing IL-6 in astrocytes developed severe neurological deficits, suggesting that IL-6 could have a pathogenic role in CNS injury (124). These contrasting studies may highlight the differential effects resulting from the extent and duration of cytokine expression
6. CYTOKINES AND LIPID METABOLISM IN STROKE
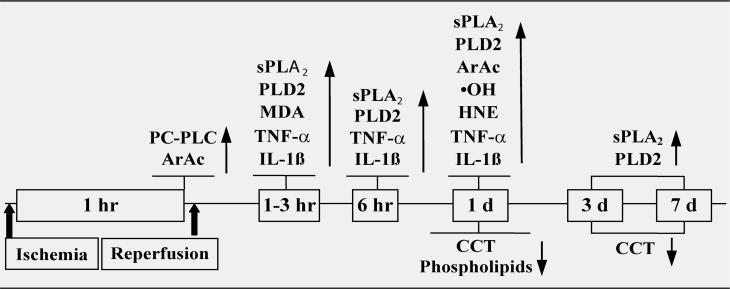
The time course of changes related to cytokines, lipid metabolism and oxidative stress after transient focal cerebral ischemia are shown in Figure 7 (next page).
Figure 7.
Time course of changes related to cytokines, lipid metabolism and oxidative stress after stroke (148). ↑=increase, ↓=decrease, compared to control. HNE: 4-hydroxynonenal; MDA: malondialdehyde; •OH: hydroxyl radical. PLA2 enzyme activity, sPLA2 mRNA and protein expression, PC-PLC activity and PLD2 protein expression were increased after stroke. CCT catalyzes the rate-limiting step in the biosynthesis of PC. CCT activity and protein expression decreased following stroke. Activation of phospholipases and loss of CCT collectively resulted in the loss of PC (41).
6.1. Cytokine up-regulation stimulates PC and SM hydrolysis, inhibits PC and SM synthesis and disrupts membrane integrity in stroke
Our studies have shown increases in TNF-α and IL-1ß in the ipsilateral cortex after tMCAO (Table 1), consistent with other studies in the literature (84, 87-89). A number of studies suggest that up-regulation of these cytokines alter phospholipid metabolism after stroke. CDP-choline, an intermediate in PC synthesis, significantly attenuated TNF-α and IL-1ß levels and may differentially affect phospholipases and phospholipid synthesis in restoring the lost phospholipid levels after stroke.
Table 1.
TNF-α and IL-1ß levels after 1 hr tMCAO and 1 hr reperfusion in rat, pg/mg protein. Quantification of TNF-α and IL-1ß were done by using R&D Systems Quantikine M ELISA kits.
| Cortex | Striatum | ||||
|---|---|---|---|---|---|
| Treatment | Contra- | Ipsi- | Contra- | Ipsi- | |
| TNF-alpha | Saline | 5 ± 2 | 25 ± 5 * | 6 ± 3 | 27 ± 5 * |
| CDP-choline | 4 ± 2 | 15 ± 2 * * | 5 ± 2 | 17 ± 5 * * | |
| IL-1beta | Saline | 3 ± 2 | 17 ± 2 * | 5 ± 3 | 14 ± 1 * |
| CDP-choline | 4 ± 3 | 9 ± 2 * * | 5 ± 2 | 10 ± 2 * * | |
* p < 0.05 ipsi- vs contra-; * * p < 0.05 CDP-choline vs saline (n = 5).
6.1.1. Do TNF-α and IL-1 up-regulate cPLA2 in stroke?
Increased cPLA2 mRNA (125) and immunoreactivity (126, 127) have been demonstrated after both focal and global cerebral ischemia. cPLA2 immunoreactivity was present in reactive glia cells where neuron loss occurred, but was absent where neurons are protected or did not degenerate (126-128). Human brain also expressed cPLA2 immunoreactivity that was associated with astrocytes (128). These studies demonstrate that cPLA2 is up-regulated after stroke and expression is largely confined to astrocytes. However, other studies did not observe any obvious change in the cPLA2 mRNA expression in the penumbral area of the ischemic cortex at 3 days after tMCAO (129), which may be due to the selection of time points. To assess the role of cPLA2 in stroke, transgenic 129/Sv mice (derived from C57BL/6) deficient in cPLA2 were developed. cPLA2 null mice showed smaller infarcts after tMCAO compared to wild-type counterparts (130), demonstrating the role of cPLA2 in brain injury after stroke. It should be noted that 129/Sv are naturally deficient in sPLA2 IIA due to a point mutation in the gene (131, 132) while other mouse strains such as BALB/c and DBA/1 have normal sPLA2 II genotype (131, 132). Inhibition of PLA2 attenuated TNF-α cytotoxicity (133, 134), indicating that induction of PLA2 is one of the major pathways mediating TNF-α cytotoxic signaling.
In vitro studies have demonstrated that exposure of normal mouse primary astrocyte cultures to IL-1ß resulted in 4-fold increase in cPLA2 protein expression as demonstrated by Western blotting (135). Simultaneous exposure of primary rat cultured astrocytes to TNF-α, IL-1ß, and IFN-γ resulted in increased phosphorylation of cPLA2 without a net change in overall cPLA2 protein levels (136), however, the effects of individual cytokines on cPLA2 were not shown. In our tMCAO studies in spontaneously hypertensive rat (SHR) we did not observe any significant change in cPLA2 protein expression over 24 hr reperfusion. However the phosphorylated vs non-phosphorylated forms of cPLA2 might not have been resolved to observe the changes (41). In an astrocytoma cell line, TNF-α stimulated phosphorylation of cPLA2 which was preceded by activation of c-Jun N-terminal kinase (JNK) and p38 MAP kinase (137). Since cPLA2 has been shown to be phosphorylated by MAP kinase (138), TNF-α likely stimulates cPLA2 phosphorylation through activation of the MAP kinase pathway. TNF-α also activated the transcription factor NF-κB and up-regulated COX-2 mRNA and protein expression (137). Thus activation of cPLA2 by MAP kinases and NF-κB-dependent COX-2 induction are major steps in the ArAc cascade regulated by TNF-α in astrocytoma cells.
These studies strongly suggest that the increases in TNF-α and IL-1 initiate the ArAc cascade through activation of cPLA2 and COX-2 in astrocytes after stroke, although this connection has not yet been demonstrated in vivo.
6.1.2. TNF-α and IL-1 up-regulate sPLA2 IIA in stroke
To-date most of the studies in ischemia focused on cPLA2. Recent studies showed that sPLA2 IIA was up-regulated after tMCAO and (129). sPLA2 mRNA expression was maximum between 12 hr to 72 hr; in situ hybridization showed that sPLA2 IIA mRNA was expressed mainly in the penumbral area of the ischemic cortex. Immunohistochemical studies showed that sPLA2 IIA is localized to GFAP positive astrocytes. Our data also showed that sPLA2 IIA mRNA and protein levels were elevated after 1 hr tMCAO (41). Indoxam (139), a putative sPLA2 IIA inhibitor, reduced infarction in MCAO. However, one should be aware of non-specific actions of indoxam: it not only inhibits the enzymatic activity of sPLA2-IB, -IIA and -V, but also blocks the binding of sPLA2-IB to its receptor (140) (see next section on PLA2 receptors 6.1.3.). Transient forebrain ischemia for 20 min in rat also showed an increase in sPLA2 expression localized to hippocampal CA1, CA3, dentate gyrus and neocortex during 7 and 20 day reperfusion demonstrated by in situ hybridization (141). Pancreatic sPLA2 (group IB) was not expressed in ischemic brain. A large number of sPLA2 IIA inhibitors have been developed as anti-inflammatory agents (142-144) but to the best of our knowledge they have not been tested in stroke models.
In unstimulated cultured rat astrocytes, basal PLA2 activity and expression of sPLA2 II mRNA and protein is very low. Exposure of astrocyte cultures to TNF-α or IL-1ß resulted in a substantial increase in sPLA2 II mRNA and protein expression and increase in PLA2 activity in the culture medium (145). TNF-α stimulated PLA2 II expression was enhanced by forskolin, an agent commonly used to stimulate cellular levels of cyclic AMP. Forskolin alone did not directly activate PLA2 II gene expression in astrocytes, but up-regulated stimulation by TNF-α, suggesting the involvement of protein kinase A (PKA) (145). These studies also demonstrated that lipopolysaccharide (LPS) stimulated release of PLA2 activity from astrocytes, which was attenuated by H-7, a protein kinase C (PKC) inhibitor. H-7 had relatively little effect on TNF-α stimulated release of PLA2 activity, indicating that in astrocytes, PLA2 II expression is regulated by two distinct protein kinases, PKA and PKC, depending on the extracellular stimulus (145). It all likelihood the PLA2 form II mentioned in these studies is now referred to as type IIA since only IIA had been identified and cloned at that time (146). Later studies also showed that TNF-α in addition to inducing sPLA2 IIA also induced sPLA2 V in cultured rat astrocytes (147). These studies suggested that increased TNF-α and/or IL-1 levels after cerebral ischemia could be responsible for the up-regulation of sPLA2 IIA. To determine this aspect, rats were injected with TNF-α antibody (TNF-α ab, 0.36 mg/kg i.p.) or IL-1ra (20 micrograms by intracerebroventricular injection) at the onset of reperfusion after 1 hr MCAO. After 24 hr reperfusion, Western blots demonstrated a 3.7-fold increase in sPLA2 IIA protein expression in the ipsilateral cortex (IC) compared to the contralateral cortex (CC) (Figure 8). Administration of TNF-α ab or IL-1ra attenuated the ischemia-induced increase in sPLA2 IIA in the ipsi-cortex to contralateral levels, indicating the role of TNF-α and IL-1 in sPLA2 IIA induction after stroke. Administration of CDP-choline (500 mg/kg i.p.), the rate-limiting intermediate in PC synthesis, attenuated the increases in TNF-α and IL-1ß (Table 1), sPLA2 IIA protein expression (Figure 8) and PLA2 activity (Table 2, next page). These data suggested that anti-inflammatory effects of CDP-choline may be mediated through attenuation of cytokine expression and sPLA2 IIA activation (148).
Figure. 8.
sPLA2 protein expression increased in ipsi. cortex (IC) vs contra. cortex (CC); treatment with CDP-choline, TNF-α antibody (TNF-α ab), or IL-1 receptor antagonist (IL-1ra) attenuated this increase to CC levels. Relative sPLA2 protein expression is given as IC/CC ratio.
Table 2.
Effect of CDP-choline, TNF-α antibody (TNF-α ab) or IL-1 receptor antagonist (IL-1ra) on PLA2 activity (pmol/min/mg protein) after tMCAO.
| Reperfusion | Contra./Saline | Ipsi./saline | Ipsi./CDP | Ipsi/TNF-α ab | Ipsi/IL-1ra |
|---|---|---|---|---|---|
| 3 h | 38±7 | 65±5* | 47±7# | 45±5# | 48±3# |
| 6 h | 28±8 | 56±8* | 42±5# | 40±5# | 39±5# |
| 1 d | 40±8 | 92±4** | 50±6## | 50±8## | 54±8## |
*p<0.05 and **p<0.01 compared to contra; #p<0.05 and ##p<0.01 compared to ipsi/saline (n=4). Neither CDP-choline, TNF-α ab nor IL-1ra had any significant effect on contralateral PLA2 activity. PLA2 activity was determined as previously described (46).
6.1.3. sPLA2 receptors
In addition to their catalytic activity as enzymes, sPLA2s also know to function as ligands by binding to specific receptors (149). Two types of sPLA2 receptors have been identified: muscular (M-type) and neuronal (N-type) (146, 150). The M-type receptor is mainly expressed in skeletal muscle tissue and was originally characterized for its high affinity binding of snake venom sPLA2s. M-type receptors are also expressed in lung, liver, heart, and kidney, and have endocytic properties, rapidly internalizing sPLA2 (146). N-type receptors are present at high levels in rat brain, are structurally and pharmacologically distinct from M-type receptors and exhibit a different spectrum of affinities for various sPLA2s. High affinity “N-type-like” receptors, similar but not identical to brain N-type receptors, are present in other tissues such as lung, liver, heart, and kidney (146). Although ten different mammalian sPLA2s have been identified, binding studies to M-type and N-type receptors have been conducted only for group IB (pancreatic) and IIA (synovial or non-pancreatic) sPLA2. Both sPLA2 IB and IIA bind to M-type receptors but do not have high affinity for N-type receptors (151, 152). Surprisingly, human sPLA2 IB and IIA do not bind to either M-type or N-type human receptors (150). Possible mechanisms of actions of sPLA2s through binding to these membrane receptors have been hypothesized, but none have been clearly demonstrated (150). Elucidating the physiological roles of these sPLA2 receptors is hampered by the lack of specific antagonists that block receptor function without inhibiting sPLA2 enzymatic activity, allowing these two functions of sPLA2 to be differentiated.
6.1.4. Crosstalk between iPLA2, cPLA2 and sPLA2
Incubation of astrocytoma cells with sPLA2 IIA was shown to activate MAP kinases and phosphorylation of cPLA2 (153). Treatments with sPLA2 inhibitors did not prevent the effect of sPLA2 on cPLA2 activation, indicating that this is independent of its catalytic activity. In contrast, pre-incubation with an antagonist to the sPLA2 receptor prevented activation of cPLA2 by sPLA2. However, since the astrocytoma cell line in these studies is of human origin, the involvement of M-type or N-type sPLA2 receptors is not supported since these do not bind sPLA2 IIA. It was suggested that the action of sPLA2 involves interaction with a membrane receptor similar to the macrophage mannose receptor or engagement of heparan sulfate proteoglycans (153). Other studies showed that sPLA2 is an up-stream regulator of cPLA2 via 5-LOX metabolites in HaCaT cells (154). Functional coupling between cPLA2 and sPLA2 through COX-2 has been demonstrated in macrophages (155). In contrast, other studies have shown that products generated by the cPLA2-12/15-LOX pathway served as regulators of sPLA2 IIA expression in rat fibroblastic cells. These studies demonstrate important coupling between sPLA2 and cPLA2 expression, although the pathways linking them may be dependent on cell type. A novel role for iPLA2 in up-regulating IL-1ß that may drive the subsequent induction of sPLA2 (IIA and V) and cPLA2 (IV) during inflammation has been reported recently (156).
6.1.5. PLA2 metabolites in inflammatory resolution
Activation of PLA2s release ArAc, eicosapentaenoic acid, and DHA. Metabolism of ArAc to prostaglandins and leukotrienes is a major pathway in induction of inflammation. PLA2s also play an important role in the later stage of inflammation by releasing ArAc, eicosapentaenoic acid, and DHA for the formation of lipoxins, resolvins, and protectins that are important in resolution of inflammation (157, 204). Specialized chemical mediators such as aspirin can acetylate COX-2; prostaglandin synthesis is inhibited when COX-2 is acetylated and metabolism is shifted to generate pro-resolution lipoxins.
The DHA metabolite 10,17S-docosatriene (neuroprotectin D1, NPD1) was recently identified in cerebral ischemia/reperfusion in mouse. NPD1 was found to serve an endogenous neuroprotective role by inhibiting apoptotic DNA damage, up-regulating anti-apoptotic proteins Bcl-2 and BclxL, and down-regulating pro-apoptotic Bax and Bad expression. NPD1 also inhibited oxidative stress-induced caspase-3 activation and IL-1ß-stimulated COX-2 expression (158, 159). Administration of albumin causes systemic mobilization of n-3 polyunsaturated fatty acids (PUFA) including DHA, and provides substantial neuroprotection in models of brain ischemia and trauma. It was demonstrated that after DHA-albumin administration there was an increase in NPD1 in the ipsilateral brain after tMCAO in rat (160).
6.1.6. TNF-α and IL-1 activate PLD
A few studies have shown activation of PLD by TNF-α (161-163) or IL-1 (164). In some studies (161-163), PLD activation was determined as increased activity measured by its transphosphatidylation reaction and thus did not differentiate whether PLD1, PLD2, or both were induced. However, not all cell lines respond to TNF-α stimulation by up-regulating PLD (162). There are conflicting data as to whether activation of PLD mediates (161, 162) or protects against (163) TNF-α cytotoxicity. The role of PLD in cell survival vs death may depend on the specific cell line, such as tissue origin, normal vs neoplastic cells. Another factor could be whether cells have been sensitized to TNF-α cytotoxicity by inhibition of protein synthesis (163). Apoptotic signaling does not require protein synthesis and is potentiated by inhibition of protein synthesis; protein synthesis-dependent feedback processes may antagonize or modulate apoptotic signaling (81). The physiological role of PLD is not well understood at this time and whether activation of PLD is a cellular defense mechanism or part of the pathological cascade towards cell death remains an unresolved issue (56). n-Butanol, which blocks PA formation (transphosphatidylation reaction of PLD) (165) but does not as such inhibit PLD activity, increased cell death during hypoxia or oxygen-glucose deprivation (OGD) suggesting that PLD activation has beneficial effects (43, 166). In this context it is worth noting that beneficial effects of TNF-α in stroke may be partly mediated through PLD, but this needs further investigation.
6.1.7. Cytokine involvement in PC, SM homeostasis: Role of PC-PLC, SMases, CCT and SM synthase
CD95 (APO-1, Fas) receptor is one member of the TNF receptor superfamily that is involved in triggering apoptosis. CD95 and its endogenous agonist CD95 ligand (CD95L) are up-regulated in cerebral ischemia (99). Transgenic mice lacking functional CD95L showed significant reduction in infarct volume after tMCAO, demonstrating its contribution to ischemic brain injury (99). It was also demonstrated that mice lacking both TNF-α and functional CD95L showed a dramatic reduction in infarction, indicating that TNF-α and CD95 act synergistically in stroke injury (99). It has been shown that CD95 activates PC-PLC, which appeared to be a requirement for subsequent activation of A-SMase, since the PC-PLC inhibitor D609 blocked CD95-induced A-SMase activation (167). CD95 also induced PLA2 and N-SMase, which was independent of PC-PLC/A-SMase pathway since N-SMase activation was unaffected by D609 (167). Both TNF-α and IL-1 have been shown to activate PC-PLC and A-SMase (168). Activation of A-SMase is mediated by formation of the PC-PLC product DAG (168). Besides TNF-α and IL-1, PC-PLC has been shown to be involved in signaling pathways of a number of cytokines such as IL-3, colony-stimulating factor 1, platelet-derived growth factor, epidermal growth factor, and interferons (168). Independently of PC-PLC activation, TNF-α and IL-1 also stimulate N-SMase (168). Because of the rapid up-regulation of N-SMase by TNF-α, it is believed that activation of N-SMase couples directly to the p55 TNF-α receptor (169). Alternatively, ArAc was shown to be a potent stimulator of N-SMase but not A-SMase in a cell-free system (170), suggesting that TNF-α and IL-1 can stimulate N-SMase through activation of cPLA2 or sPLA2 and release of ArAc.
6.1.7.1. SM hydrolysis by SMases generates pro-apoptotic and anti-proliferative ceramide
Due to their distinct subcellular distribution: A-SMase is lysosomal while N-SMase is localized to the plasma membrane (63, 64), ceramide generated by these two enzymes mediates different cellular signaling pathways. Ceramide produced by N-SMase triggers the MAP kinase cascade via ceramide activated protein kinase, eventually resulting in phosphorylation of cPLA2 (169). On the other hand, A-SMase appears to be imbedded in the pathway from PC-PLC to activation of the transcription factor NF-κB, whereas ceramide generated by N-SMase is apparently excluded from NF-κB activation pathway (169). In A-SMase null mice subjected to tMCAO, ceramide levels showed little or no increase in ischemic cortical tissue compared to a 3- to 5-fold increase in wild type mice (65). Levels of mRNAs encoding for TNF-α, IL-1α, IL-1ß, granulocyte-colony stimulating factor, and IL-2 were significantly increased in wild type mice after tMCAO, whereas there was no detectable increase in mRNAs for these cytokines in A-SMase null mice (65). These studies indicated that A-SMase is primarily responsible for increased ceramide levels after tMCAO and that ceramide released by A-SMase regulates expression of several cytokines, which likely involves activation of NF-κκB. Inhibition of PC-PLC with D609 significantly reduced infarction after tMCAO, which was ascribed to inhibition of PC-PLC/DAG/A-SMase/ceramide pathway. D609 largely prevented the increases in ceramide levels and mRNA expression for cytokines, suggesting that activation of A-SMase was attenuated (65). D609 was also shown to block pro-inflammatory actions of TNF-α, which was attributed to PC-PLC inhibition (171). We have also shown that D609 significantly attenuated infarction by 35±5% after 1 hr MCAO and 24 hr reperfusion in spontaneously hypertensive rat (Figure 5).
6.1.7.2. PC-PLC inhibitor D609 inhibited cell proliferation (5'-bromo-2-deoxyuridine (BrdU) incorporation)
The PC-PLC product DAG is necessary for protein kinase C activation and cell proliferation (54). D609 (100 μM, IC50=94 μM) (43) inhibited proliferation of cultured neuronal progenitor cells. After 3 days in culture with D609, neurospheres were significantly smaller (Figure 9B) compared to controls (Figure 9A). The number of BrdU-positive cells was reduced by 75±5% (Figure 9D) compared to controls (Figure 9C), indicating that D609 inhibited proliferation of the neurospheres. D609 also inhibited proliferation of primary astrocytes (59) as well as neural stem cells (172). This could also suggest that D609 beneficial effects could arise from inhibiting proliferating microglia/macrophages that are the primary source for TNF-α and IL-1 and other pro-inflammatory cytokines in stroke (173), however this needs further investigation.
Figure 9.
D609 inhibited proliferation (75 ± 5%) of neurospheres. (A, B) bright field images; (C, D) BrdU incorporation (red); Hoechst nuclear stain (blue). (A, C) controls; (B, D) 3 days after exposure to 100 μM D609.
6.1.7.3. TNF-α and IL-1 inhibit PC and SM synthesis by affecting CCT and SM synthase respectively
PC homeostasis is regulated by the opposing actions of hydrolysis by phospholipases (174) and synthesis through CCT (Figure 10). TNF-α can activate caspases (175) which in turn can down-regulate CCT (176) and SM synthase (177, 178) through proteolytic cleavage during apoptosis. Other studies have shown that TNF-α can stimulate degradation of CCT through the ubiquitin-proteasome and calpain-mediated proteolytic pathways (42). TNF-α and IL-1 may inhibit PC and SM synthesis through activation of SMases and release of ceramide. Ceramide and/or its metabolites such as sphingosine inhibit both CCT and SM synthase, and thus inhibit synthesis of both PC and SM (179). In conjunction with activation of phospholipases and SMases, this results in further loss of PC and SM, disruption of the phospholipid membrane, and contributes to cell death. We have shown CDP-choline counteracted these actions of cytokines on phospholipid metabolism (Tables 1, 2 and Figure 8) in restoring PC levels and provided benefit after stroke (41).
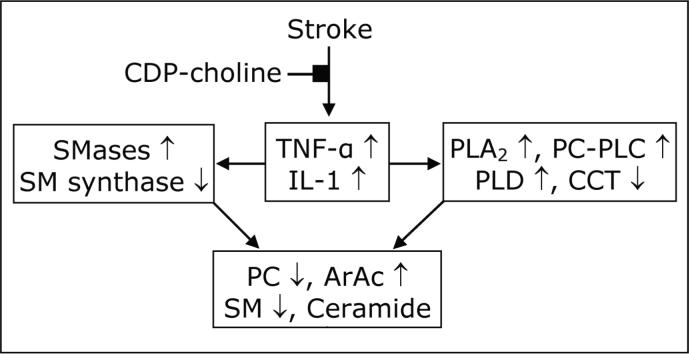
Figure. 10.
Integration of cytokine response and lipid metabolism after stroke (148). Up-regulation of TNF-α and IL-1 differentially affects phospholipases, SMases; CCT and SM synthase in collectively causing loss of PC and SM and release of ArAc and ceramide.
6.2. Arachidonic acid (ArAc): regulation of cytokines expression
Energy failure and increases in intracellular Ca++ during cerebral ischemia result in activation of phospholipases and large increases in FFA including ArAc (25, 180). There is a surge of ArAc release during ischemia (23, 25, 27, 181, 182) which may stimulate expression of TNF-α and IL-1ß (183). Thus the release of ArAc during ischemia (25, 182) may be one of the initial events that up-regulate cytokine expression. Activation of phospholipases by TNF-α and IL-1 may reinforce the feedback regulation, suggesting a potential self-re-enforcing cycle which could be interrupted by blocking either phospholipases or cytokines.
7. INFLAMMATION, CYTOKINES, LIPID METABOLISM, AND REACTIVE OXYGEN SPECIES (ROS)
Cessation of blood flow to the brain leads to energy loss and necrotic cell death. This initiates the immune response, activating inflammatory cells including microglia/macrophages and generating ROS (including hydroxyl radical, superoxide anion radical, and hydrogen peroxide). ROS can further stimulate the ischemic cells to release cytokines that cause up-regulation of adhesion molecules, mobilization and activation of leukocytes, platelets and endothelium that will result in additional ROS production (184). These activated inflammatory cells further release cytokines, MMPs, nitric oxide and more ROS in a feed-back fashion (80). ArAc metabolites, synthesized by and liberated from astrocytes, microglial cells and macrophages, are intimately involved in the inflammatory process by enhancing vascular permeability and modulating inflammatory cell activities and ROS generation.
The study of ROS and oxidative stress in inflammation is difficult due to the transient nature of ROS, the number and complexity of ongoing processes, the interactions between these processes due to direct and reverse relations, and the capacity of ROS to alter a large number of cellular components. ROS are produced by a number of cellular oxidative metabolic processes including oxidative phosphorylation by the mitochondrial respiratory chain, xanthine oxidase, NAD(P)H oxidases, metabolism of ArAc by LOX, and monoamine oxidases (25). However, recent studies suggest that COX does not directly produce ROS during ArAc oxidative metabolism, but does form free radicals (i.e., carbon-centered radicals on ArAc) (148, 200). ROS cause oxidative damage to nucleic acids, proteins, carbohydrates, and lipids. Beyond damage to membranes, lipid peroxides give rise to reactive α, ß-unsaturated aldehydes including malondialdehyde, 4-hydroxynonenal (HNE) and acrolein. These aldehydes covalently bind to proteins through reaction with thiol groups and alter their function. Although there are intracellular defenses against ROS, increased production of ROS or loss of antioxidant defenses leads to progressive cell damage and decline in physiological function. “Oxidative stress” results when generation of ROS exceeds the cell's capacity to detoxify them. The brain is believed to be particularly vulnerable to oxidative stress as it contains high concentrations of PUFAs that are susceptible to lipid peroxidation, consumes relatively large amounts of oxygen for energy production, and has lower antioxidant defenses compared to other organs. The time-course of alterations in lipid metabolism and formation of lipid metabolites and lipid peroxidation products after transient cerebral ischemia is presented in Figure 7. Generation of HNE has been demonstrated by us and others in brain tissue after stroke (25). Recently, elevated levels of an acrolein-protein conjugate were demonstrated in plasma of stroke patients (185).
8. SUMMARY AND PERSPECTIVE
There are substantial data from both animal models and clinical studies that cytokines including TNF-α, IL-1 and IL-6 are up-regulated after stroke. Although the roles of cytokines in stroke pathology remain controversial, the majority of studies support their deleterious effects, at least in the early phase of stroke injury. Whether cytokines mediate pro-survival or pro-apoptotic signaling appears to depend on their concentration, the target cell, the activating signal, and the timing and sequence of action (76). Although a great deal of information has been published individually on cytokines as well as lipid metabolism, the integration of these two areas in stroke is less explored and certainly needs more attention. A number of in vitro studies have shown that TNF-α and IL-1 activate phospholipases. However, most of these studies were conducted in cell lines not derived from brain origin, and their applicability to stroke must be interpreted with caution since response to cytokines is cell-specific. The increases in TNF-α and IL-1 together with activation of phospholipases in animal models of stroke suggest that these events are inter-related, although in vivo experimental data is limited. This is supported by our studies demonstrating that neutralization of TNF-α or IL-1 in animal model of stroke attenuated sPLA2 protein expression (Figure 8). It should be reminded that various sPLA2 inhibitors have been developed but not many have been tested in stroke models (142-144).
A systemic inflammatory response involving up-regulation of TNF-α and IL-1 is believed to be instrumental in the formation and destabilization of plaques, one of the greatest risk factors for ischemic stroke (3, 4). sPLA2 IIA (inflammatory PLA2) has been related to various inflammatory diseases such as rheumatoid arthritis may contribute to development of atherosclerosis. A variety of cells relevant to atherosclerosis such as macrophages and neutrophils can release sPLA2 in response to TNF-α and IL-1 (17). The inter-relationship of systemic inflammation- inflammatory PLA2 with stroke pathology has not been well studied, even though considerable clinical data indicate that it is associated with unfavorable outcome in stroke patients (9).
9. FUTURE TRENDS: INFLAMMATION, LIPIDOMICS AND RNA SILENCING
The emerging field of lipidomics tries to define the crucial role of lipids in the cell; the research is aimed at mapping the entire lipid population in a biological system, describing the composition and biological function (186-188). The main progress that has spurred recent advances in lipid analysis was the development of new mass spectroscopic techniques, particularly the “soft ionization” techniques electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI). Lipidomics seeks to provide a molecular signature to a certain pathway or a disease condition. These approaches have provided crucial information that was previously unachievable (189). Lipidomic approaches also hold the promise of elucidating how various signaling pathways such as cytokines interact in determining the net cellular outcome.
RNA interference (RNAi) (190-192) takes advantage of a naturally occurring process to degrade RNA, the intermediary translator between the DNA of genes and the protein molecules they encode. RNAi takes advantage of a naturally occurring process to degrade RNA. By degrading RNA, genes can be selectively and temporarily “turned off.”, RNAi provides a tool to analyze protein function and is a method of choice particularly for genes where transgenic knockouts are not possible due to lethality, for example CCT (36) and PLD (56). There have been few reports on the use of siRNA in animal models (193) and the development of appropriate vector systems is a prerequisite (194) before siRNA can be regarded as a suitable experimental tool for in vivo studies (195). Use of siRNA in cell culture has been achieved with striking potency at a relatively low concentration (190, 196, 199).
Lipidomic analyses together with RNAi may provide powerful tools to identify lipid mediators, characterize new lipid intermediates, and elucidate their roles in specific cell signaling pathways. In particular, by selectively silencing expression of specific cytokines and lipid metabolizing enzymes, lipidomics may elucidate the roles of cytokines during the acute (pro-inflammatory) and resolution (anti-inflammatory) phases of inflammation. The role of lipids in formation of cell membranes makes them both ligand and substrate for proteins, suggesting that advances in lipidomics could have far reaching implications in genomic, proteomic and metabolomic fields. Impressive achievements and advancements have surfaced during the last few years (157, 159). Lipidomics holds the promise of characterizing complex mixtures of lipids, identifying previously unknown changes in lipid metabolism (21). A deeper knowledge and understanding of the complexity of cytokine-lipid signaling will elevate our understanding of their role in stroke and various CNS disorders, opening new opportunities for drug development and therapies for neurological diseases.
10. ACKNOWLEDGEMENTS
This work was supported by grants from NIH/NINDS (NS42008), American Heart Association Greater Midwest Affiliate Grant-in-Aid (0655757Z), University of Wisconsin-School of Medicine and Public Health and University of Wisconsin-Graduate school (to RMA), and laboratory resources provided by William S. Middleton VA Hospital. We would like to thank Dr. Haviryaji Kalluri for providing Figure 9.
Glossary
Abbreviations:
- ArAc
Arachidonic acid
- CDP-choline
Cytidine-5'-diphosphocholine
- CNS
Central nervous system
- COX/LOX
Cyclooxygenase/ lipoxygenase
- D609
Tricyclodecan-9-yl potassium xanthate
- DAG
1,2-Diacylglycerol
- DHA
Docosahexaenoic acid
- FFA
Free fatty acid
- HNE
4-Hydroxynonenal
- IL-1ß
Interleukin 1ß
- IL-1ra
IL-1 receptor antagonist
- Lp-PLA2
Lipoprotein-PLA2
- MMP
Matrix metalloprotease
- PA
Phosphatidic acid
- PAF
Platelet activating factor
- PC
Phosphatidylcholine
- PI
Phosphatidylinositol
- PLA2
Phospholipase A2
- sPLA2
Secretory PLA2 or inflammatory PLA2
- PLC
Phospholipase C
- PS
Phosphatidylserine
- PUFA
Polyunsaturated fatty acid
- ROS
Reactive oxygen species
- SM
Sphingomyelin
- SMase
Sphingomyelinase
- SMS
SM Synthase
- TNF-α
Tumor necrosis factor-α
- TNF-α ab
TNF-α antibody
Footnotes
Dedicated to the memory of Ann E Kelley, a great educator and researcher of our neuroscience community who passed away on August 5, 2007.
11. REFERENCES
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y, S. for the American Heart Association statistics committee and stroke statistics Heart disease and stroke statistics--2007 update: A report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- 3.Emsley HCA, Tyrrell PJ. Inflammation and infection in clinical stroke. J Cereb Blood Flow Metab. 2002;22:1399–1419. doi: 10.1097/01.WCB.0000037880.62590.28. [DOI] [PubMed] [Google Scholar]
- 4.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 5.Young JL, Libby P, Schönbeck U. Cytokines in the pathogenesis of atherosclerosis. Thrombosis and Haemostasis. 2002;88:554–567. [PubMed] [Google Scholar]
- 6.Elkind MS. Inflammation, atherosclerosis, and stroke. Neurologist. 2006;12:140–148. doi: 10.1097/01.nrl.0000215789.70804.b0. [DOI] [PubMed] [Google Scholar]
- 7.Stoll G, Bendszus M. Inflammation and atherosclerosis: Novel insights into plaque formation and destabilization. Stroke. 2006;37:1923–1932. doi: 10.1161/01.STR.0000226901.34927.10. [DOI] [PubMed] [Google Scholar]
- 8.Chapman MJ. From pathophysiology to targeted therapy for atherothrombosis: A role for the combination of statin and aspirin in secondary prevention. Pharmacol Therap. 2007;113:184–196. doi: 10.1016/j.pharmthera.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 9.McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Six DA, Dennis EA. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 11.Lavi S, McConnell JP, Rihal CS, Prasad A, Mathew V, Lerman LO, Lerman A. Local production of lipoprotein-associated phospholipase A2 and lyso-phosphatidylcholine in the coronary circulation: Association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation. 2007;115:2715–2721. doi: 10.1161/CIRCULATIONAHA.106.671420. [DOI] [PubMed] [Google Scholar]
- 12.Oei H-HS, van der Meer IM, Hofman A, Koudstaal PJ, Stijnen T, Breteler MMB, Witteman JCM. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: The Rotterdam study. Circulation. 2005;111:570–575. doi: 10.1161/01.CIR.0000154553.12214.CD. [DOI] [PubMed] [Google Scholar]
- 13.Zalewski A, Nelson JJ, Hegg L, Macphee C. Lp-PLA2: A new kid on the block. Clin Chem. 2006;52:1645–1650. doi: 10.1373/clinchem.2006.070672. [DOI] [PubMed] [Google Scholar]
- 14.Kinnunen PKJ, Holopainen JM. Sphingomyelinase activity of LDL - A link between atherosclerosis, ceramide, and apoptosis? Trends Cardiovasc Med. 2002;12:37–42. doi: 10.1016/s1050-1738(01)00143-8. [DOI] [PubMed] [Google Scholar]
- 15.Hurt-Camejo E, Andersen S, Standal R, Rosengren B, Sartipy P, Stadberg E, Johansen B. Localization of non-pancreatic secretory phospholipase A2 in normal and atherosclerotic arteries: Activity of the isolated enzyme on low-density lipoproteins. Arterioscler Thromb Vasc Biol. 1997;17:300–309. doi: 10.1161/01.atv.17.2.300. [DOI] [PubMed] [Google Scholar]
- 16.Menschikowski M, Kasper M, Lattke P, Schiering A, Schiefer S, Stockinger H, Jaross W. Secretory group II phospholipase A2 in human atherosclerotic plaques. Atherosclerosis. 1995;118:173–181. doi: 10.1016/0021-9150(95)05604-1. [DOI] [PubMed] [Google Scholar]
- 17.Ivandic B, Castellani LW, Wang X-P, Qiao J-H, Mehrabian M, Navab M, Fogelman AM, Grass DS, Swanson ME, de Beer MC, de Beer F, Lusis AJ. Role of group II secretory phospholipase A2 in atherosclerosis: 1. Increased atherogenesis and altered lipoproteins in transgenic mice expressing group IIA phospholipase A2. Arterioscler Thromb Vasc Biol. 1999;19:1284–1290. doi: 10.1161/01.atv.19.5.1284. [DOI] [PubMed] [Google Scholar]
- 18.Ghesquiere SAI, Gijbels MJJ, Anthonsen MW, van Gorp PJJ, van der Made I, Johansen B, Hofker MH, de Winther MPJ. Macrophage-specific over-expression of group IIA sPLA2 increases atherosclerosis and enhances collagen deposition. J Lipid Res. 2005;46:201–210. doi: 10.1194/jlr.M400253-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Kougias P, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C. Lysophosphatidylcholine and secretory phospholipase A2 in vascular disease: mediators of endothelial dysfunction and atherosclerosis. Med Sci Monit. 2006;12:RA5–16. [PubMed] [Google Scholar]
- 20.Ter Horst GJ, Korf J. Humana. Totowa; New Jersey: 1997. Clinical Pharmacology of Cerebral Ischemia. [Google Scholar]
- 21.Adibhatla RM, Hatcher JF. Cytidine 5′-diphosphocholine (CDP-choline) in stroke and other CNS disorders. Neurochem Res. 2005;30:15–23. doi: 10.1007/s11064-004-9681-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 23.Rao AM, Hatcher JF, Dempsey RJ. Lipid metabolism in ischemic neuronal death. Recent Res Develop Neurochem. 1999;2:533–549. [Google Scholar]
- 24.Phillis JW, O'Regan MH. The role of phospholipases, cyclooxygenases, and lipoxygenases in cerebral ischemic/traumatic injuries. Crit Rev Neurobiol. 2003;15:61–90. doi: 10.1615/critrevneurobiol.v15.i1.30. [DOI] [PubMed] [Google Scholar]
- 25.Adibhatla RM, Hatcher JF. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med. 2006;40:376–387. doi: 10.1016/j.freeradbiomed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 26.Adibhatla RM, Hatcher JF, Dempsey RJ. Lipids and lipidomics in brain injury and diseases. AAPS J. 2006;8:E314–E321. doi: 10.1007/BF02854902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao AM, Hatcher JF, Kindy MS, Dempsey RJ. Arachidonic acid and leukotriene C4: Role in transient cerebral ischemia of gerbils. Neurochem Res. 1999;24:1225–1232. doi: 10.1023/a:1020916905312. [DOI] [PubMed] [Google Scholar]
- 28.Mattson MP, Culmsee C, Yu ZF. Apoptotic and anti-apoptotic mechanisms in stroke. Cell Tissue Res. 2000;301:173–87. doi: 10.1007/s004419900154. [DOI] [PubMed] [Google Scholar]
- 29.Traystman RJ. Animal models of focal and global cerebral ischemia. ILAR J. 2003;44:85–95. doi: 10.1093/ilar.44.2.85. [DOI] [PubMed] [Google Scholar]
- 30.Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Prog Lipid Res. 2000;39:257–288. doi: 10.1016/s0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- 31.Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- 32.Cui Z, Houweling M. Phosphatidylcholine and cell death. Biochim Biophys Acta. 2002;1585:87–96. doi: 10.1016/s1388-1981(02)00328-1. [DOI] [PubMed] [Google Scholar]
- 33.Exton JH. Phosphatidylcholine breakdown and signal transduction. Biochim Biophys Acta. 1994;1212:26–42. doi: 10.1016/0005-2760(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 34.Klein J. Membrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipids. J Neural Transm. 2000;107:1027–1063. doi: 10.1007/s007020070051. [DOI] [PubMed] [Google Scholar]
- 35.Li Z, Agellon LB, Vance DE. Phosphatidylcholine homeostasis and liver failure. J Biol Chem. 2005;280:37798–37802. doi: 10.1074/jbc.M508575200. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Magdaleno S, Tabas I, Jackowski S. Early embryonic lethality in mice with targeted deletion of the CTP:phosphocholine cytidylyltransferase α gene (Pcyt1a). Mol Cell Biol. 2005;25:3357–3363. doi: 10.1128/MCB.25.8.3357-3363.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kent C. CTP-phosphocholine cytidylyltransferase. Biochim Biophys Acta. 1997;1348:79–90. doi: 10.1016/s0005-2760(97)00112-4. [DOI] [PubMed] [Google Scholar]
- 38.Araki W, Wurtman RJ. Control of membrane phosphatidylcholine biosynthesis by diacylglycerol levels in neuronal cells undergoing neurite outgrowth. Proc Natl Acad Sci USA. 1997;94:11946–11950. doi: 10.1073/pnas.94.22.11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright MM, Howe AG, Zaremberg V. Cell membranes and apoptosis: role of cardiolipin, phosphatidylcholine, and anticancer lipid analogues. Biochem Cell Biol. 2004;82:18–26. doi: 10.1139/o03-092. [DOI] [PubMed] [Google Scholar]
- 40.Adibhatla RM, Hatcher JF, Dempsey RJ. Cytidine-5′-diphosphocholine (CDP-choline) affects CTP:phosphocholine cytidylyltransferase and lyso-phosphatidylcholine after transient brain ischemia. J Neurosci Res. 2004;76:390–396. doi: 10.1002/jnr.20078. [DOI] [PubMed] [Google Scholar]
- 41.Adibhatla RM, Hatcher JF, Larsen EC, Chen X, Sun D, Tsao F. CDP-choline significantly restores the phosphatidylcholine levels by differentially affecting phospholipase A2 and CTP-phosphocholine cytidylyltransferase after stroke. J Biol Chem. 2006;281:6718–6725. doi: 10.1074/jbc.M512112200. [DOI] [PubMed] [Google Scholar]
- 42.Mallampalli RK, Ryan AJ, Salome RG, Jackowski S. TNF-α inhibits expression of CTP:phosphocholine cytidylyltransferase. J Biol Chem. 2000;275:9699–9708. doi: 10.1074/jbc.275.13.9699. [DOI] [PubMed] [Google Scholar]
- 43.Larsen EC, Hatcher JF, Adibhatla RM. Effect of tricyclodecan-9-yl potassium xanthate (D609) on phospholipid metabolism and cell death during oxygen-glucose deprivation in PC12 cells. Neuroscience. 2007;146:946–961. doi: 10.1016/j.neuroscience.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murakami M, Kudo I. Phospholipase A2. J Biochem. 2002;131:285–292. doi: 10.1093/oxfordjournals.jbchem.a003101. [DOI] [PubMed] [Google Scholar]
- 45.Adibhatla RM, Hatcher JF. Citicoline decreases phospholipase A2 stimulation and hydroxyl radical generation in transient cerebral ischemia. J Neurosci Res. 2003;73:308–315. doi: 10.1002/jnr.10672. [DOI] [PubMed] [Google Scholar]
- 46.Adibhatla RM, Hatcher JF, Dempsey RJ. Phospholipase A2, hydroxyl radicals and lipid peroxidation in transient cerebral ischemia. Antioxid Redox Signal. 2003;5:647–654. doi: 10.1089/152308603770310329. [DOI] [PubMed] [Google Scholar]
- 47.Akiba S, Sato T. Cellular function of calcium-independent phospholipase A2. Biol Pharm Bull. 2004;27:1174–1178. doi: 10.1248/bpb.27.1174. [DOI] [PubMed] [Google Scholar]
- 48.Kinsey GR, Cummings BS, Beckett CS, Saavedra G, Zhang W, McHowat J, Schnellmann RG. Identification and distribution of endoplasmic reticulum iPLA2. Biochem Biophys Res Comm. 2005;327:287–293. doi: 10.1016/j.bbrc.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 49.Hirabayashi T, Murayama T, Shimizu T. Regulatory mechanism and physiological role of cytosolic phospholipase A2. Biol Pharm Bull. 2004;27:1168–1173. doi: 10.1248/bpb.27.1168. [DOI] [PubMed] [Google Scholar]
- 50.Boggs KP, Rock CO, Jackowski S. Lyso-phosphatidylcholine and 1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine inhibit the CDP-choline pathway of phosphatidylcholine synthesis at the CTP-phosphocholine cytidylyltransferase step. J Biol Chem. 1995;270:7757–7764. doi: 10.1074/jbc.270.13.7757. [DOI] [PubMed] [Google Scholar]
- 51.Nakahara I, Kikuchi H, Taki W, Nishi S, Kito M, Yonekawa Y, Goto Y, Ogata N. Changes in major phospholipids of mitochondria during postischemic reperfusion in rat brain. J Neurosurg. 1992;76:244–250. doi: 10.3171/jns.1992.76.2.0244. [DOI] [PubMed] [Google Scholar]
- 52.Zhang YP, Lemasters J, Herman B. Secretory group IIA PLA2 generates anti-apoptotic survival signals in kidney fibroblasts. J Biol Chem. 1999;274:27726–27733. doi: 10.1074/jbc.274.39.27726. [DOI] [PubMed] [Google Scholar]
- 53.Higuchi M, Shirotani K, Higashi N, Toyoshima S, Osawa T. Damage to mitochondrial respiration chain is related to PLA2 activation caused by TNF. J Immunother. 1992;12:41–49. doi: 10.1097/00002371-199207000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Cerbon J, del Carmen Lopez-Sanchez R. Diacylglycerol generated during sphingomyelin synthesis is involved in protein kinase C activation and cell proliferation in Madin-Darby canine kidney cells. Biochem J. 2003;373:917–924. doi: 10.1042/BJ20021732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramoni C, Spadaro F, Barletta B, Dupuis ML, Podo F. Phosphatidylcholine-specific phospholipase C in mitogen-stimulated fibroblasts. Exp Cell Res. 2004;299:370–382. doi: 10.1016/j.yexcr.2004.05.037. [DOI] [PubMed] [Google Scholar]
- 56.Klein J. Functions and pathophysiological roles of phospholipase D in the brain. J Neurochem. 2005;94:1473–1487. doi: 10.1111/j.1471-4159.2005.03315.x. [DOI] [PubMed] [Google Scholar]
- 57.Jin J-K, Kim N-H, Min DS, Kim J-I, Choi J-K, Jeong B-H, Choi S-I, Choi E-K, Carp RI, Kim Y-S. Increased expression of phospholipase D1 in the brains of scrapie-infected mice. J Neurochem. 2005;92:452–461. doi: 10.1111/j.1471-4159.2004.02881.x. [DOI] [PubMed] [Google Scholar]
- 58.Luberto C, Hannun YA. Sphingomyelin synthase, a potential regulator of intracellular levels of ceramide and diacylglycerol during SV40 transformation. Does sphingomyelin synthase account for the putative phosphatidylcholine-specific phospholipase C? J Biol Chem. 1998;273:14550–14559. doi: 10.1074/jbc.273.23.14550. [DOI] [PubMed] [Google Scholar]
- 59.Riboni L, Viani P, Bassi R, Giussani P, Tettamanti G. Basic fibroblast growth factor-induced proliferation of primary astrocytes. Evidence for the involvement of sphingomyelin biosynthesis. J Biol Chem. 2001;276:12797–12804. doi: 10.1074/jbc.M011570200. [DOI] [PubMed] [Google Scholar]
- 60.Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta. 2002;1585:114–125. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- 61.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nature Reviews Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 62.Riboni L, Tettamanti G, Viani P. Ceramide in primary astrocytes from cerebellum: metabolism and role in cell proliferation. The Cerebellum. 2002;1:129–135. doi: 10.1080/147342202753671268. [DOI] [PubMed] [Google Scholar]
- 63.Marchesini N, Hannun YA. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol. 2004;82:27–44. doi: 10.1139/o03-091. [DOI] [PubMed] [Google Scholar]
- 64.Futerman AH, van Meer G. The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol. 2004;5:554–565. doi: 10.1038/nrm1423. [DOI] [PubMed] [Google Scholar]
- 65.Yu ZF, Nikolova-Karakashian M, Zhou DH, Cheng GJ, Schuchman EH, Mattson MP. Pivotal role for acidic sphingomyelinase in cerebral ischemia-induced ceramide and cytokine production, and neuronal apoptosis. J Mol Neurosci. 2000;15:85–97. doi: 10.1385/JMN:15:2:85. [DOI] [PubMed] [Google Scholar]
- 66.Hofmann K, Tomiuk S, Wolff G, Stoffel W. Cloning and characterization of the mammalian brain-specific, Mg2+-dependent neutral sphingomyelinase. Proc Natl Acad Sci USA. 2000;97:5895–5900. doi: 10.1073/pnas.97.11.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tepper AD, Ruurs P, Wiedmer T, Sims PJ, Borst J, van Blitterswijk WJ. Sphingomyelin hydrolysis to ceramide during the execution phase of apoptosis results from phospholipid scrambling and alters cell-surface morphology. J Cell Biol. 2000;150:155–164. doi: 10.1083/jcb.150.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fadeel B, Xue D. PS externalization: from corpse clearance to drug delivery. Cell Death Differ. 2005;13:360–362. doi: 10.1038/sj.cdd.4401836. [DOI] [PubMed] [Google Scholar]
- 69.Viviani B, Bartesaghi S, Corsini E, Galli CL, Marinovich M. Cytokines role in neurodegenerative events. Toxicol Lett. 2004;149:85–89. doi: 10.1016/j.toxlet.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 70.Huang J, Upadhyay UM, Tamargo R. l. J. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66:232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 71.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 72.Stoll G. Inflammatory cytokines in the nervous system: multifunctional mediators in autoimmunity and cerebral ischemia. Rev Neurol (Paris) 2002;158:887–891. [PubMed] [Google Scholar]
- 73.Ruocco A, Nicole O, Docagne F, Ali C, Chazalviel L, Komesli S, Yablonsky F, Roussel S, MacKenzie ET, Vivien D, Buisson A. A transforming growth factor-ß antagonist unmasks the neuroprotective role of this endogenous cytokine in excitotoxic and ischemic brain Injury. J Cereb Blood Flow Metab. 1999;19:1345–1353. doi: 10.1097/00004647-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 74.Barton BE. IL-6: Insights into novel biological activities. Clinical Immunology and Immunopathology. 1997;85:16–20. doi: 10.1006/clin.1997.4420. [DOI] [PubMed] [Google Scholar]
- 75.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 76.Cavaillon JM. Pro- versus anti-inflammatory cytokines: myth or reality. Cell Mol Biol (Noisy-le-grand) 2001;47:695–702. [PubMed] [Google Scholar]
- 77.Andre R, Pinteaux E, Kimber I, Rothwell NJ. Differential actions of IL-1 α and IL-1ß in glial cells share common IL-1 signaling pathways. Neuroreport. 2005;16:153–157. doi: 10.1097/00001756-200502080-00017. [DOI] [PubMed] [Google Scholar]
- 78.Lucas S-M, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147:S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jeohn GH, Kong LY, Wilson B, Hudson P, Hong JS. Synergistic neurotoxic effects of combined treatments with cytokines in murine primary mixed neuron/glia cultures. J Neuroimmunol. 1998;85:1–10. doi: 10.1016/s0165-5728(97)00204-x. [DOI] [PubMed] [Google Scholar]
- 80.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hallenbeck JM. The many faces of TNF in stroke. Nature Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- 82.Lovering F, Zhang Y. Therapeutic potential of TACE inhibitors in stroke. Curr Drug Targets CNS Neurol Disord. 2005;4:161–168. doi: 10.2174/1568007053544147. [DOI] [PubMed] [Google Scholar]
- 83.Boutin H, Kimber I, Rothwell NJ, Pinteaux E. The expanding interleukin-1 family and its receptors: do alternative IL-1 receptor/signaling pathways exist in the brain? Mol Neurobiol. 2003;27:239–248. doi: 10.1385/MN:27:3:239. [DOI] [PubMed] [Google Scholar]
- 84.Wang CX, Shuaib A. Involvement of inflammatory cytokines in central nervous system injury. Prog Neurobiol. 2002;67:161–172. doi: 10.1016/s0301-0082(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 85.Rothwell NJ. Cytokines - killers in the brain? J Physiol (Lond) 1999;514:3–17. doi: 10.1111/j.1469-7793.1999.003af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Touzani O, Boutin H, Chuquet J, Rothwell N. Potential mechanisms of IL-1 involvement in cerebral ischaemia. J Neuroimmunol. 1999;100:203–215. doi: 10.1016/s0165-5728(99)00202-7. [DOI] [PubMed] [Google Scholar]
- 87.Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, Feuerstein GZ. TNF-α expression in ischemic neurons. Stroke. 1994;25:1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- 88.Loddick SA, Rothwell NJ. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1996;16:932–940. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- 89.Wang X, Yue TL, Barone FC, White RF, Gagnon RC, Feuerstein GZ. Concomitant cortical expression of TNF-α and IL-1ß mRNAs follows early response gene expression in transient focal ischemia. Mol Chem Neuropath. 1994;23:103–114. doi: 10.1007/BF02815404. [DOI] [PubMed] [Google Scholar]
- 90.Dziewulska D, Mossakowski MJ. Cellular expression of TNF-α and its receptors in human ischemic stroke. Clin Neuropathol. 2003;22:35–40. [PubMed] [Google Scholar]
- 91.Sairanen T, Carpen O, Karjalainen-Lindsberg ML, Paetau A, Turpeinen U, Kaste M, Lindsberg PJ. Evolution of cerebral TNF-α production during human ischemic stroke. Stroke. 2001;32:1750–1758. doi: 10.1161/01.str.32.8.1750. [DOI] [PubMed] [Google Scholar]
- 92.Zaremba J, Losy J. Early TNF-α levels correlate with ischaemic stroke severity. Acta Neurol Scand. 2001;104:288–295. doi: 10.1034/j.1600-0404.2001.00053.x. [DOI] [PubMed] [Google Scholar]
- 93.Botchkina GI, Meistrell ME, Botchkina IL, Tracey KJ. Expression of TNF and TNF receptors (p55 and p75) in the rat brain after focal cerebral ischemia. Mol Med. 1997;3:765–781. [PMC free article] [PubMed] [Google Scholar]
- 94.Shen Y, Li R, Shiosaki K. Inhibition of p75 tumor necrosis factor receptor by antisense oligonucleotides increases hypoxic injury and ß-amyloid toxicity in human neuronal cell line. J Biol Chem. 1997;272:3550–3553. [PubMed] [Google Scholar]
- 95.Lambertsen KL, Clausen BH, Fenger C, Wulf H, Owens T, Dagnaes-Hansen F, Meldgaard M, Finsen B. Microglia and macrophages express tumor necrosis factor receptor p75 following middle cerebral artery occlusion in mice. Neuroscience. 2007;144:934–949. doi: 10.1016/j.neuroscience.2006.10.046. [DOI] [PubMed] [Google Scholar]
- 96.Shohami E, Ginis I, Hallenbeck JM. Dual role of TNF-α in brain injury. Cytokine Growth Fact Rev. 1999;10:119–130. doi: 10.1016/s1359-6101(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 97.Wilde GJ, Pringle AK, Sundstrom LE, Mann DA, Iannotti F. Attenuation and augmentation of ischaemia-related neuronal death by TNF-α in vitro. Eur J Neurosci. 2000;12:3863–3870. doi: 10.1046/j.1460-9568.2000.00273.x. [DOI] [PubMed] [Google Scholar]
- 98.Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, Holtsberg FW, Mattson MP. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nature Med. 1996;2:788–94. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- 99.Martin-Villalba A, Hahne M, Kleber S, Vogel J, Falk W, Schenkel J, Krammer PH. Therapeutic neutralization of CD95-ligand and TNF attenuates brain damage in stroke. Cell Death Differ. 2001;8:676–686. doi: 10.1038/sj.cdd.4400882. [DOI] [PubMed] [Google Scholar]
- 100.Dawson DA, Martin D, Hallenbeck JM. Inhibition of TNF-α reduced focal cerebral ischemic injury in the spontaneously hypertensive rat (SHR). Neurosci Lett. 1996;218:41–44. doi: 10.1016/0304-3940(96)13116-5. [DOI] [PubMed] [Google Scholar]
- 101.Lavine SD, Hofman FM, Zlokovic BV. Circulating antibody against TNF-α protects rat brain from reperfusion injury. J Cereb Blood Flow Metab. 1998;18:52–58. doi: 10.1097/00004647-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 102.Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette R, Lysko PG, Feuerstein GZ. TNF-α. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 103.Gary DS, Bruce-Keller AJ, Kindy MS, Mattson MP. Ischemic and excitotoxic brain injury is enhanced in mice lacking the p55 TNF receptor. J Cereb Blood Flow Metab. 1998;18:1283–1287. doi: 10.1097/00004647-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 104.Rothwell NJ, Luheshi GN. Brain TNF: damage limitation or damaged reputation? Nature Med. 1996;2:746–747. doi: 10.1038/nm0796-746. [DOI] [PubMed] [Google Scholar]
- 105.Nawashiro H, Tasaki K, Ruetzler CA, Hallenbeck JM. TNF-α pretreatment induces protective effects against focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 1997;17:483–490. doi: 10.1097/00004647-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 106.Scherbel U, Raghupathi R, Nakamura M, Saatman KE, Trojanowski JQ, Neugebauer E, Marino MW, McIntosh TK. Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. PNAS. 1999;96:8721–8726. doi: 10.1073/pnas.96.15.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nature Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- 108.Pinteaux E, Rothwell NJ, Boutin H. Neuroprotective actions of endogenous interleukin-1 receptor antagonist (IL-1ra) are mediated by glia. Glia. 2006;53:551–556. doi: 10.1002/glia.20308. [DOI] [PubMed] [Google Scholar]
- 109.Andre R, Lerouet D, Kimber I, Pinteaux, Rothwell NJ. Regulation of expression of the novel IL-1 receptor family members in the mouse brain. J Neurochem. 2005;94:324–330. doi: 10.1111/j.1471-4159.2005.03364.x. [DOI] [PubMed] [Google Scholar]
- 110.Rothwell NJ, Loddick SA. Interleukin-1 and IL-1 receptor antagonist in stroke: mechanisms and potential therapeutics. In: Feuerstein GZ, editor. Inflammation and Stroke. Birkhauser Verlag; Basel: 2001. [Google Scholar]
- 111.Emsley HCA, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ, Tyrrell PJ, Investigators AS. A randomized phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76:1366–1372. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boutin H, LeFeuvre RA, Horai R, Asano M, Iwakura Y, Rothwell NJ. Role of IL-1α and IL-1ß in ischemic brain damage. J Neurosci. 2001;21:5528–5534. doi: 10.1523/JNEUROSCI.21-15-05528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: Role in biology. Ann Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 114.Carlson NG, Wieggel WA, Chen J, Bacchi A, Rogers SW, Gahring LC. Inflammatory cytokines IL-1 α, IL-1 ß, IL-6, and TNF-α impart neuroprotection to an excitotoxin through distinct pathways. J Immunol. 1999;163:3963–3968. [PubMed] [Google Scholar]
- 115.Strijbos PJ, Rothwell NJ. Interleukin-1ß attenuates excitatory amino acid-induced neurodegeneration in vitro: involvement of nerve growth factor. J Neurosci. 1995;15:3468–3474. doi: 10.1523/JNEUROSCI.15-05-03468.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vila N, Castillo J, Davalos A, Chamorro A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke. 2000;31:2325–2329. doi: 10.1161/01.str.31.10.2325. [DOI] [PubMed] [Google Scholar]
- 117.Smith CJ, Emsley HC, Gavin CM, Georgiou RF, Vail A, Barberan EM, del Zoppo GJ, Hallenbeck JM, Rothwell NJ, Hopkins SJ, Tyrrell PJ. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004;4 doi: 10.1186/1471-2377-4-2. doi: 10.1186/1471−2377−4−2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Clark WM, Rinker LG, Lessov NS, Hazel K, Hill JK, Stenzel-Poore M, Eckenstein F. Lack of IL-6 expression is not protective against focal central nervous system ischemia. Stroke. 2000;31:1715–1720. doi: 10.1161/01.str.31.7.1715. [DOI] [PubMed] [Google Scholar]
- 119.Herrmann O, Tarabin V, Suzuki S, Attigah N, Coserea I, Schneider A, Vogel J, Prinz S, Schwab S, Monyer H, Brombacher F, Schwaninger M. Regulation of body temperature and neuroprotection by endogenous interleukin-6 in cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:406–415. doi: 10.1097/01.WCB.0000055177.50448.FA. [DOI] [PubMed] [Google Scholar]
- 120.Spangelo BL, Judd AM, Call GB, Zumwalt JW, Gorospe WC. Role of the cytokines in the hypothalamic-pituitary-adrenal and gonadal axes. Neuroimmunomodulation. 1995;2:299–312. doi: 10.1159/000097209. [DOI] [PubMed] [Google Scholar]
- 121.Leon LR. Molecular biology of thermoregulation: Invited review: Cytokine regulation of fever: studies using gene knockout mice. J Appl Physiol. 2002;92:2648–2655. doi: 10.1152/japplphysiol.01005.2001. [DOI] [PubMed] [Google Scholar]
- 122.Thornhill J, Corbett D. Therapeutic implications of hypothermic and hyperthermic temperature conditions in stroke patients. Can J Physiol Pharmacol. 2001;79:254–261. [PubMed] [Google Scholar]
- 123.Liu L, Yenari MA. Therapeutic hypothermia: neuroprotective mechanisms. Front Biosci. 2007;12:816–825. doi: 10.2741/2104. [DOI] [PubMed] [Google Scholar]
- 124.Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, Mucke L. Neurologic disease induced in transgenic mice by cerebral over-expression of interleukin 6. Proc Natl Acad Sci USA. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Owada Y, Tominaga T, Yoshimoto T, Kondo H. Molecular cloning of rat cDNA for cytosolic phospholipase A2 and the increased gene expression in the dentate gyrus following transient forebrain ischemia. Mol Brain Res. 1994;25:364–368. doi: 10.1016/0169-328x(94)90174-0. published erratum appears in Brain Res Mol Brain Res 1994 Dec 27 (2):355. [DOI] [PubMed] [Google Scholar]
- 126.Clemens JA, Stephenson DT, Smalstig EB, Roberts EF, Johnstone EM, Sharp JD, Little SP, Kramer RM. Reactive glia express cytosolic phospholipase A2 after transient global forebrain ischemia in the rat. Stroke. 1996;27:527–535. doi: 10.1161/01.str.27.3.527. [DOI] [PubMed] [Google Scholar]
- 127.Stephenson D, Rash K, Smalstig B, Roberts E, Johnstone E, Sharp J, Panetta J, Little S, Kramer R, Clemens J. Cytosolic PLA2 is induced in reactive glia following different forms of neurodegeneration. Glia. 1999;27:110–128. doi: 10.1002/(sici)1098-1136(199908)27:2<110::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 128.Stephenson DT, Manetta JV, White DL, Chiou XG, Cox L, Gitter B, May PC, Sharp JD, Kramer RM, Clemens JA. Calcium-sensitive cPLA2 is expressed in human brain astrocytes. Brain Res. 1994;637:97–105. doi: 10.1016/0006-8993(94)91221-1. [DOI] [PubMed] [Google Scholar]
- 129.Lin T-N, Wang Q, Simonyi A, Chen J-J, Cheung W-M, He YY, Xu J, Sun AY, Hsu CY, Sun GY. Induction of secretory phospholipase A2 in reactive astrocytes in response to transient focal cerebral ischemia in the rat brain. J Neurochem. 2004;90:637–645. doi: 10.1111/j.1471-4159.2004.02540.x. [DOI] [PubMed] [Google Scholar]
- 130.Bonventre JV, Huang ZH, Taheri MR, Oleary E, Li E, Moskowitz MA, Sapirstein A. Reduced fertility and post-ischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 131.Kennedy BP, Payette P, Mudgett J, Vadas P, Pruzanski W, Kwan M, Tang C, Rancourt DE, Cromlish WA. A natural disruption of the secretory group II PLA2 gene in inbred mouse strains. J Biol Chem. 1995;270:22378–22385. doi: 10.1074/jbc.270.38.22378. [DOI] [PubMed] [Google Scholar]
- 132.MacPhee M, Chepenik KP, Liddell RA, Nelson KK, Siracusa LD, Buchberg AM. The secretory phospholipase A2 gene is a candidate for the Mom1 locus, a major modifier of ApcMin-induced intestinal neoplasia. Cell. 1995;81:957–966. doi: 10.1016/0092-8674(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 133.Hayakawa M, Ishida N, Takeuchi K, Shibamoto S, Hori T, Oku N, Ito F, Tsujimoto M. Arachidonic acid-selective cytosolic PLA2 is crucial in the cytotoxic action of TNF. J Biol Chem. 1993;268:11290–11295. [PubMed] [Google Scholar]
- 134.Rath PC, Aggarwal BB. TNF-induced signaling in apoptosis. J Clin Immunol. 1999;19:350–364. doi: 10.1023/a:1020546615229. [DOI] [PubMed] [Google Scholar]
- 135.Stella N, Estelles A, Sicilliano J, Tence M, Desagher S, Piomelli D, Glowinski J, Premont J. Interleukin-1 enhances the ATP-evoked release of arachidonic acid from mouse astrocytes. J Neurosci. 1997;17:2939–2946. doi: 10.1523/JNEUROSCI.17-09-02939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun GY, Xu J, Jensen MD, Yu S, Wood WG, Gonzalez FA, Simonyi A, Sun AY, Weisman GA. Phospholipase A2 in astrocytes: responses to oxidative stress, inflammation, and g protein-coupled receptor agonists. Mol Neurobiol. 2005;31:27–42. doi: 10.1385/MN:31:1-3:027. [DOI] [PubMed] [Google Scholar]
- 137.Hernandez M, Bayon Y, Crespo MS, Nieto ML. Signaling mechanisms involved in the activation of arachidonic acid metabolism in human astrocytoma cells by tumor necrosis factor-α. Phosphorylation of c PLA2 and transactivation of COX-2. J Neurochem. 1999;73:1641–1649. doi: 10.1046/j.1471-4159.1999.0731641.x. [DOI] [PubMed] [Google Scholar]
- 138.Lin L-L, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 139.Yagami T, Ueda K, Asakura K, Hata S, Kuroda T, Sakaeda T, Takasu N, Tanaka K, Gemba T, Hori Y. Human group IIA secretory phospholipase A2 induces neuronal cell death via apoptosis. Mol Pharmacol. 2002;61:114–126. doi: 10.1124/mol.61.1.114. [DOI] [PubMed] [Google Scholar]
- 140.Yagami T, Ueda K, Asakura K, Hayasaki-Kajiwara Y, Nakazato H, Sakaeda T, Hata S, Kuroda T, Takasu N, Hori Y. Group IB secretory phospholipase A2 induces neuronal cell death via apoptosis. J Neurochem. 2002;81:449–461. doi: 10.1046/j.1471-4159.2002.00800.x. [DOI] [PubMed] [Google Scholar]
- 141.Lauritzen I, Heurteaux C, Lazdunski M. Expression of group II PLA2 in rat brain after severe forebrain ischemia and in endotoxic shock. Brain Res. 1994;651:353–356. doi: 10.1016/0006-8993(94)90719-6. [DOI] [PubMed] [Google Scholar]
- 142.Reid RC. Inhibitors of secretory phospholipase A2 group IIA. Curr Med Chem. 2005;12:3011–3026. doi: 10.2174/092986705774462860. [DOI] [PubMed] [Google Scholar]
- 143.Cunningham TJ, Maciejewski J, Yao L. Inhibition of secreted phospholipase A2 by neuron survival and anti-inflammatory peptide CHEC-9. J Neuroinflammation. 2006;3 doi: 10.1186/1742-2094-3-25. doi:10.1186/1742−2094−3−25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rastogi P, Beckett CS, McHowat J. Prostaglandin production in human coronary artery endothelial cells is modulated differentially by selective phospholipase A (2) inhibitors. Prostaglandins Leukot Essent Fatty Acids. 2007;76:205–212. doi: 10.1016/j.plefa.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 145.Oka S, Arita H. Inflammatory factors stimulate expression of group II phospholipase A2 in rat cultured astrocytes. Two distinct pathways of the gene expression. J Biol Chem. 1991;266:9956–9960. [PubMed] [Google Scholar]
- 146.Valentin E, Lambeau G. Increasing molecular diversity of secreted phospholipases A2 and their receptors and binding proteins. Biochim Biophys Acta. 2000;1488:59–70. doi: 10.1016/s1388-1981(00)00110-4. [DOI] [PubMed] [Google Scholar]
- 147.Thomas G, Bertrand F, Saunier B. The differential regulation of group IIA and group V low molecular weight phospholipases A2 in cultured rat astrocytes. J Biol Chem. 2000;275:10876–10886. doi: 10.1074/jbc.275.15.10876. [DOI] [PubMed] [Google Scholar]
- 148.Adibhatla RM, Hatcher JF. Role of lipids in brain injury and diseases. Future Lipidology. 2007;2:403–422. doi: 10.2217/17460875.2.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Triggiani M, Granata F, Frattini A, Marone G. Activation of human inflammatory cells by secreted phospholipases A2. Biochim Biophys Acta. 2006;1761:1289–1300. doi: 10.1016/j.bbalip.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 150.Lambeau G, Lazdunski M. Receptors for a growing family of secreted phospholipases A2. Trends Pharmacol Sci. 1999;20:162–170. doi: 10.1016/s0165-6147(99)01300-0. [DOI] [PubMed] [Google Scholar]
- 151.Lambeau G, Barhanin J, Schweitz H, Qar J, Lazdunski M. Identification and properties of very high affinity brain membrane- binding sites for a neurotoxic phospholipase from the taipan venom. J Biol Chem. 1989;264:11503–11510. [PubMed] [Google Scholar]
- 152.Nicolas J-P, Lin Y, Lambeau G, Ghomashchi F, Lazdunski M, Gelb MH. Localization of structural elements of bee venom phospholipase A2 involved in N-type receptor binding and neurotoxicity. J Biol Chem. 1997;272:7173–7181. doi: 10.1074/jbc.272.11.7173. [DOI] [PubMed] [Google Scholar]
- 153.Hernandez M, Burillo SL, Crespo MS, Nieto ML. Secretory phospholipase A2 activates the cascade of mitogen-activated protein kinases and cytosolic phospholipase A2 in the human astrocytoma cell line 1321N1. J Biol Chem. 1998;273:606–612. doi: 10.1074/jbc.273.1.606. [DOI] [PubMed] [Google Scholar]
- 154.Anthonsen MW, Solhaug A, Johansen B. Functional coupling between secretory and cytosolic PLA2 modulates TNF-α and IL-1ß-induced NF-κB activation. J Biol Chem. 2001;276:30527–30536. doi: 10.1074/jbc.M008481200. [DOI] [PubMed] [Google Scholar]
- 155.Balsinde J, Balboa MA, Dennis EA. Functional coupling between secretory phospholipase A2 and cyclooxygenase-2 and its regulation by cytosolic group IV phospholipase A2. Proc Natl Acad Sci USA. 1998;95:7951–7956. doi: 10.1073/pnas.95.14.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gilroy DW, Newson J, Sawmynaden P, Willoughby DA, Croxtall JD. A novel role for phospholipase A2 isoforms in the checkpoint control of acute inflammation. FASEB J. 2004;18:489–498. doi: 10.1096/fj.03-0837com. [DOI] [PubMed] [Google Scholar]
- 157.Serhan CN. Resolution phases of inflammation: Novel endogenous anti-inflammatory and pro-resolving lipid mediators and pathways. Ann Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 158.Bazan NG. Neuroprotectin D1 (NPD1): A DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005;15:159–166. doi: 10.1111/j.1750-3639.2005.tb00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 160.Belayev L, Marcheselli VL, Khoutorova L, Rodriguez de Turco EB, Busto R, Ginsberg MD, Bazan NG. Docosahexaenoic acid complexed to albumin elicits high-grade ischemic neuroprotection. Stroke. 2005;36:118–123. doi: 10.1161/01.STR.0000149620.74770.2e. [DOI] [PubMed] [Google Scholar]
- 161.De Valck D, Beyaert R, Von Roy F, Fiers W. Tumor necrosis factor cytotoxicity is associated with phospholipase D activation. Eur J Biochem. 1993;212:491–497. doi: 10.1111/j.1432-1033.1993.tb17686.x. [DOI] [PubMed] [Google Scholar]
- 162.De Valck D, Vercammen D, Fiers W, Beyaert R. Differential activation of phospholipases during necrosis or apoptosis - a comparative study using TNF and anti-fas antibodies. J Cell Biochem. 1998;71:392–399. [PubMed] [Google Scholar]
- 163.Birbes H, Zeiller C, Komati H, Nemoz G, Lagarde M, Prigent AF. Phospholipase D protects ECV304 cells against TNFα-induced apoptosis. FEBS Lett. 2006;580:6224–6232. doi: 10.1016/j.febslet.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 164.Chen M-C, Paez-Espinosa V, Welsh N, Eizirik DL. Interleukin-1ß regulates phospholipase D1 expression in rat pancreatic ß cells. Endocrinology. 2000;141:2822–2828. doi: 10.1210/endo.141.8.7608. [DOI] [PubMed] [Google Scholar]
- 165.McDermott M, Wakelam MJ, Morris AJ. Phospholipase D. Biochem Cell Biol. 2004;82:225–253. doi: 10.1139/o03-079. [DOI] [PubMed] [Google Scholar]
- 166.Yamakawa H, Banno Y, Nakashima S, Sawada M, Yamada J, Yoshimura S, Nishimura Y, Nozawa Y, Sakai N. Increased phospholipase D2 activity during hypoxia-induced death of PC12 cells: its possible anti-apoptotic role. Neuroreport. 2000;11:3647–3650. doi: 10.1097/00001756-200011090-00049. [DOI] [PubMed] [Google Scholar]
- 167.Cifone MG, Roncaioli P, De Maria R, Camarda G, Santoni A, Ruberti G, Testi R. Multiple pathways originate at the Fas/APO-1 (CD95) receptor: sequential involvement of phosphatidylcholine-specific phospholipase C and acidic sphingomyelinase in the propagation of the apoptotic signal. Embo J. 1995;14:5859–5868. doi: 10.1002/j.1460-2075.1995.tb00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schutze S, Machleidt T, Kronke M. The role of diacylglycerol and ceramide in TNF-α and IL-1 signal transduction. J Leukocyte Biol. 1994;56:533–541. doi: 10.1002/jlb.56.5.533. [DOI] [PubMed] [Google Scholar]
- 169.Wiegmann K, Schutze S, Machleidt T, Witte D, Kronke M. Functional dichotomy of neutral and acidic sphingomyelinases in TNF-α signaling. Cell. 1994;78:1005–1015. doi: 10.1016/0092-8674(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 170.Jayadev S, Linardic CM, Hannun YA. Identification of arachidonic acid as a mediator of sphingomyelin hydrolysis in response to TNF-α. J Biol Chem. 1994;269:5757–5763. [PubMed] [Google Scholar]
- 171.Machleidt T, Kramer B, Adam D, Neumann B, Schutze S, Wiegmann K, Kronke M. Function of the p55 TNF receptor “death domain” mediated by phosphatidylcholine-specific PLC. J Exp Med. 1996;184:725–733. doi: 10.1084/jem.184.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang N, Lv X, Su L, Zhao B, Zhang S, Miao J. D609 blocks cell survival and induces apoptosis in neural stem cells. Bioorg Med Chem Lett. 2006;16:4780–4783. doi: 10.1016/j.bmcl.2006.06.080. [DOI] [PubMed] [Google Scholar]
- 173.Lambertsen KL, Meldgaard M, Ladeby R, Finsen B. A quantitative study of microglial-macrophage synthesis of tumor necrosis factor during acute and late focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25:119–135. doi: 10.1038/sj.jcbfm.9600014. [DOI] [PubMed] [Google Scholar]
- 174.Baburina I, Jackowski S. Cellular responses to excess phospholipid. J Biol Chem. 1999;274:9400–9408. doi: 10.1074/jbc.274.14.9400. [DOI] [PubMed] [Google Scholar]
- 175.Saha RN, Pahan K. TNF-α at the crossroads of neuronal life and death during HIV-associated dementia. J Neurochem. 2003;86:1057–1071. doi: 10.1046/j.1471-4159.2003.01942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lagace TA, Miller JR, Ridgway ND. Caspase processing and nuclear export of CTP:phosphocholine cytidylyltransferase α during farnesol-induced apoptosis. Mol Cell Biol. 2002;22:4851–4862. doi: 10.1128/MCB.22.13.4851-4862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bourteele S, Horn-Muller J, Ropke C, Schwarzmann G, Pfizenmaier K, Muller G. Tumor necrosis factor induces ceramide oscillations and negatively controls sphingolipid synthases by caspases in apoptotic Kym-1 cells. J Biol Chem. 1998;273:31245–31251. doi: 10.1074/jbc.273.47.31245. [DOI] [PubMed] [Google Scholar]
- 178.Hannun YA, Luberto C, Argraves KM. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry. 2001;40:4893–4903. doi: 10.1021/bi002836k. [DOI] [PubMed] [Google Scholar]
- 179.Vivekananda J, Smith D, King RJ. Sphingomyelin metabolites inhibit sphingomyelin synthase and CTP:phosphocholine cytidylyltransferase. Am J Physiol. 2001;281:L98–L107. doi: 10.1152/ajplung.2001.281.1.L98. [DOI] [PubMed] [Google Scholar]
- 180.Katsuki H, Okuda S. Arachidonic acid as a neurotoxic and neurotrophic substance. Prog Neurobiol. 1995;46:607–636. doi: 10.1016/0301-0082(95)00016-o. [DOI] [PubMed] [Google Scholar]
- 181.Rao AM, Hatcher JF, Dempsey RJ. CDP-choline: neuroprotection in transient forebrain ischemia of gerbils. J Neurosci Res. 1999;58:697–705. [PubMed] [Google Scholar]
- 182.Narita K, Kubota M, Nakane M, Kitahara S, Nakagomi T, Tamura A, Hisaki H, Shimasaki H, Ueta N. Therapeutic time window in the penumbra during permanent focal ischemia in rats: Changes of free fatty acids and glycerophospholipids. Neurol Res. 2000;22:393–400. doi: 10.1080/01616412.2000.11740689. [DOI] [PubMed] [Google Scholar]
- 183.Hughes-Fulford M, Li C-F, Boonyaratanakornkit J, Sayyah S. Arachidonic acid activates phosphatidylinositol 3-kinase signaling and induces gene expression in prostate cancer. Cancer Res. 2006;66:1427–1433. doi: 10.1158/0008-5472.CAN-05-0914. [DOI] [PubMed] [Google Scholar]
- 184.Alexandrova ML, Bochev PG. Oxidative stress during the chronic phase after stroke. Free Rad Biol Med. 2005;39:297–316. doi: 10.1016/j.freeradbiomed.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 185.Tomitori H, Usui T, Saeki N, Ueda S, Kase H, Nishimura K, Kashiwagi K, Igarashi K. Polyamine oxidase and acrolein as novel biochemical markers for diagnosis of cerebral stroke. Stroke. 2005;36:2609–2613. doi: 10.1161/01.STR.0000190004.36793.2d. [DOI] [PubMed] [Google Scholar]
- 186.Balazy M. Eicosanomics: targeted lipidomics of eicosanoids in biological systems. Prostaglandins Other Lipid Mediat. 2004;73:173–180. doi: 10.1016/j.prostaglandins.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 187.Hillard CJ. Lipids and drugs of abuse. Life Sci. 2005;77:1531–1542. doi: 10.1016/j.lfs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 188.Wenk MR. The emerging field of lipidomics. Nat Rev Drug Discov. 2005;4:594–610. doi: 10.1038/nrd1776. [DOI] [PubMed] [Google Scholar]
- 189.Lukiw WJ, Cui J-G, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cejka D, Losert D, Wacheck V. Short interfering RNA (siRNA): tool or therapeutic? Clin Sci (Lond) 2006;110:47–58. doi: 10.1042/CS20050162. [DOI] [PubMed] [Google Scholar]
- 191.Aagaard L, Rossi JJ. RNAi therapeutics: Principles, prospects and challenges. Adv Drug Deliv Rev. 2007;59:75–86. doi: 10.1016/j.addr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dykxhoorn DM, Palliser D, Lieberman J. The silent treatment: siRNAs as small molecule drugs. Gene Ther. 2006;13:541–552. doi: 10.1038/sj.gt.3302703. [DOI] [PubMed] [Google Scholar]
- 193.Paroo Z, Corey DR. Challenges for RNAi in vivo. Trends Biotechnol. 2004;22:390–394. doi: 10.1016/j.tibtech.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 194.Xia H, Mao Q, Paulson HL, Davidson BL. siRNA-mediated gene silencing in vitro and in vivo. Nat Biotechnol. 2002;20:1006–1010. doi: 10.1038/nbt739. [DOI] [PubMed] [Google Scholar]
- 195.Senn C, Hangartner C, Moes S, Guerini D, Hofbauer KG. Central administration of small interfering RNAs in rats: A comparison with antisense oligonucleotides. Eur J Pharmacol. 2005;522:30–37. doi: 10.1016/j.ejphar.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 196.Masuda S, Murakami M, Takanezawa Y, Aoki J, Arai H, Ishikawa Y, Ishii T, Arioka M, Kudo I. Neuronal expression and neuritogenic action of group X secreted phospholipase A2. J Biol Chem. 2005;280:23203–23214. doi: 10.1074/jbc.M500985200. [DOI] [PubMed] [Google Scholar]
- 197.Tafesse F, Ternes GP, Holthuis JCM. The multigenic sphingomyelin synthase family. J Biol Chem. 2006;281:29421–29425. doi: 10.1074/jbc.R600021200. [DOI] [PubMed] [Google Scholar]
- 198.Tafesse F, Huitema GK, Hermansson M, van der Poel S, van den Dikkenberg J, Uphoff A, Somerharju P, Holthuis JCM. Both sphingomyelin synthases SMS1 and SMS2 are required for sphingomyelin homeostasis and growth in human HeLa cells. J Biol Chem. 2007;282:17537–17547. doi: 10.1074/jbc.M702423200. [DOI] [PubMed] [Google Scholar]
- 199.Li Z, Hailemariam TK, Zhou H, Li Y, Duckworth DC, Peake DA, Zhang Y, Kuo M-S, Cao G, Jiang X-C. Inhibition of sphingomyelin synthase (SMS) affects intracellular sphingomyelin accumulation and plasma membrane lipid organization. Biochim Biophys Acta. 2007 doi: 10.1016/j.bbalip.2007.05.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kunz A, Anrather J, Zhou P, Orio M, Iadecola C. Cyclooxygenase-2 does not contribute to postischemic production of reactive oxygen species. J Cereb Blood Flow Metab. 2007;27:545–551. doi: 10.1038/sj.jcbfm.9600369. [DOI] [PubMed] [Google Scholar]
- 201.Miller DT, Ridker PM, Libby P, Kwiatkowski DJ. Atherosclerosis: The path from genomics to therapeutics. J Am. Col. Cardiol. 2007;49:1589–1599. doi: 10.1016/j.jacc.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 202.Forrester JS, Libby P. The inflammation hypothesis and its potential relevance to statin therapy. Am J Cardiol. 2007;99:732–738. doi: 10.1016/j.amjcard.2006.09.125. [DOI] [PubMed] [Google Scholar]
- 203.Chen C, Hu Q, Yan J, Lei J, Qin L, Shi X, Luan L, Yang L, Wang K, Han J, Nanda A, Zhou C. Multiple effects of 2ME2 and D609 on the cortical expression of HIF-1α and apoptotic genes in a middle cerebral artery occlusion induced focal ischemia rat model. J Neurochem. 2007 doi: 10.1111/j.1471-4159.2007.04652.x. in press. [DOI] [PubMed] [Google Scholar]
- 204.Kim H-Y. Novel metabolism of docosahexaenoic acid in neural cells. J Biol Chem. 2007;282:18661–18665. doi: 10.1074/jbc.R700015200. [DOI] [PubMed] [Google Scholar]
- 205.Libby P. In: Atherosclerosis and inflammation in: C-Reactive Protein and Cardiovascular Disease. MediEdition. Ridker PM, Rifai N, editors. St-Laurent; Canada: 2006. [Google Scholar]