Abstract
The cobalamin biosynthetic pathway enzyme that catalyzes amidation of 5'-deoxy-5'-adenosyl-cobyrinic acid a,c-diamide was purified to homogeneity from extracts of a recombinant strain of Pseudomonas denitrificans by a four-column procedure. The purified protein had an isoelectric point of 5.6 and molecular weights of 97,300 as estimated by gel filtration and 57,000 as estimated by gel electrophoresis under denaturing conditions, suggesting that the active enzyme is a homodimer. Stepwise Edman degradation provided the sequence of the first 16 amino acid residues at the N terminus. The enzyme catalyzed the four-step amidation sequence from cobyrinic acid a,c-diamide to cobyric acid via the formation of cobyrinic acid triamide, tetraamide, and pentaamide intermediates. The amidations are carried out in a specific order; this order was not determined. The enzyme was specific to coenzyme forms of substrates and did not carry out amidation of the carboxyl group at position f. The amidation reactions were ATP/Mg2+ dependent and exhibited a broad optimum around pH 7.5. L-Glutamine was shown to be the preferred amide group donor (Km congruent to 45 microM) but could be replaced by ammonia (Km = 20 mM). For all of the four partially amidated substrates, the Km values were in the micromolar range and the Vmax values were about 7,000 nmol h-1 mg-1.
Full text
PDF
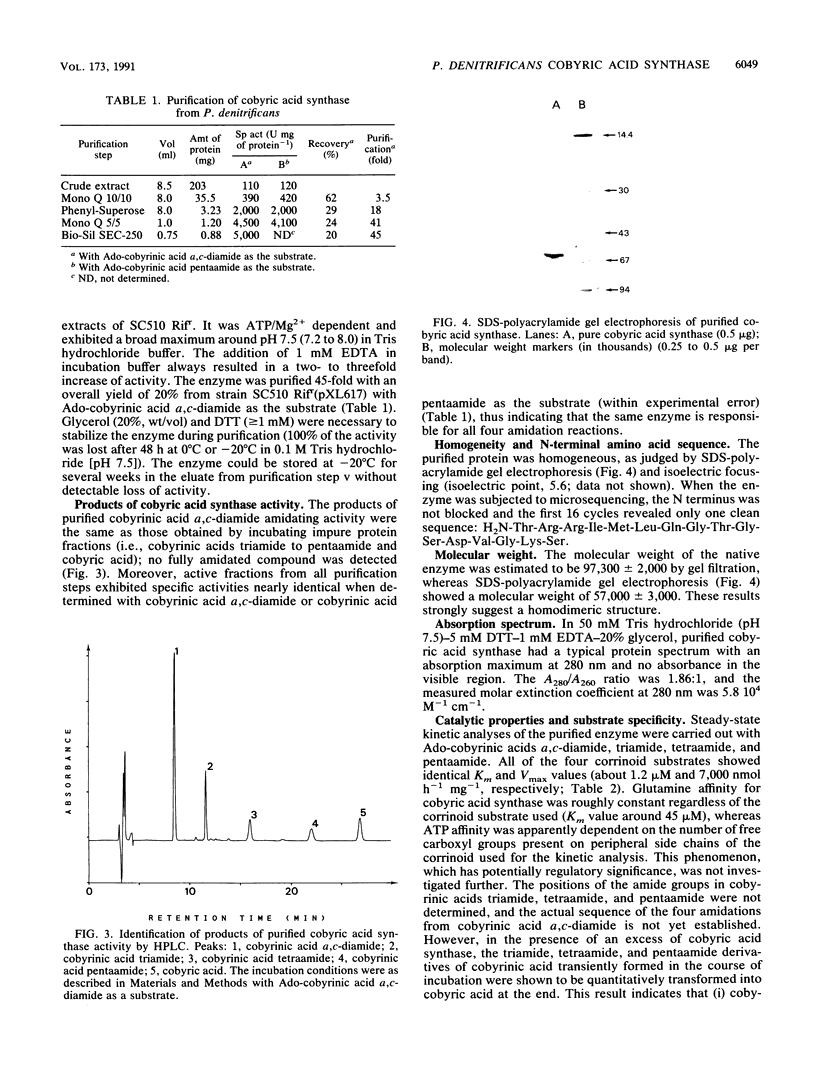


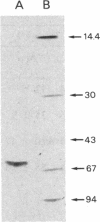
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartosiński B., Zagalak B., Pawelkiewicz J. The route of vitamin B 12 biosynthesis in Propionibacterium shermanii. Biochim Biophys Acta. 1967 Apr 25;136(3):581–584. doi: 10.1016/0304-4165(67)90023-2. [DOI] [PubMed] [Google Scholar]
- Berhauer K., Wagner F., Michna H., Rapp P., Vogelmann H. Zur Chemie und Biochemie der Corrinoide, XXIX. Biogenesewege von der Cobyrinsäure zur Cobysäure und zum Cobinamid bei Propionibacterium shermanii. Hoppe Seylers Z Physiol Chem. 1968 Oct;349(10):1297–1309. [PubMed] [Google Scholar]
- Blanche F., Debussche L., Thibaut D., Crouzet J., Cameron B. Purification and characterization of S-adenosyl-L-methionine: uroporphyrinogen III methyltransferase from Pseudomonas denitrificans. J Bacteriol. 1989 Aug;171(8):4222–4231. doi: 10.1128/jb.171.8.4222-4231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche F., Thibaut D., Couder M., Muller J. C. Identification and quantitation of corrinoid precursors of cobalamin from Pseudomonas denitrificans by high-performance liquid chromatography. Anal Biochem. 1990 Aug 15;189(1):24–29. doi: 10.1016/0003-2697(90)90038-b. [DOI] [PubMed] [Google Scholar]
- Cameron B., Briggs K., Pridmore S., Brefort G., Crouzet J. Cloning and analysis of genes involved in coenzyme B12 biosynthesis in Pseudomonas denitrificans. J Bacteriol. 1989 Jan;171(1):547–557. doi: 10.1128/jb.171.1.547-557.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Crouzet J., Cauchois L., Blanche F., Debussche L., Thibaut D., Rouyez M. C., Rigault S., Mayaux J. F., Cameron B. Nucleotide sequence of a Pseudomonas denitrificans 5.4-kilobase DNA fragment containing five cob genes and identification of structural genes encoding S-adenosyl-L-methionine: uroporphyrinogen III methyltransferase and cobyrinic acid a,c-diamide synthase. J Bacteriol. 1990 Oct;172(10):5968–5979. doi: 10.1128/jb.172.10.5968-5979.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzet J., Levy-Schil S., Cameron B., Cauchois L., Rigault S., Rouyez M. C., Blanche F., Debussche L., Thibaut D. Nucleotide sequence and genetic analysis of a 13.1-kilobase-pair Pseudomonas denitrificans DNA fragment containing five cob genes and identification of structural genes encoding Cob(I)alamin adenosyltransferase, cobyric acid synthase, and bifunctional cobinamide kinase-cobinamide phosphate guanylyltransferase. J Bacteriol. 1991 Oct;173(19):6074–6087. doi: 10.1128/jb.173.19.6074-6087.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debussche L., Couder M., Thibaut D., Cameron B., Crouzet J., Blanche F. Purification and partial characterization of Cob(I)alamin adenosyltransferase from Pseudomonas denitrificans. J Bacteriol. 1991 Oct;173(19):6300–6302. doi: 10.1128/jb.173.19.6300-6302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debussche L., Thibaut D., Cameron B., Crouzet J., Blanche F. Purification and characterization of cobyrinic acid a,c-diamide synthase from Pseudomonas denitrificans. J Bacteriol. 1990 Nov;172(11):6239–6244. doi: 10.1128/jb.172.11.6239-6244.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDRICH W., SANDECK W. ZUR KENNTNIS DER AMIDIERUNG NUCLEOTID- UND ISOPROPANOLAMINFREIER B12-POLYCARBONSAEUREN DURCH PROPIONIBACTERIUM SHERMANUE. Z Naturforsch B. 1965 Jan;20:79–80. [PubMed] [Google Scholar]
- Ford S. H. Amidation of, and (R)-1-amino-2-propanol attachment to, the corrin ring during vitamin B-12 biosynthesis by Clostridium tetanomorphum extracts. Biochim Biophys Acta. 1985 Sep 6;841(3):306–317. doi: 10.1016/0304-4165(85)90073-x. [DOI] [PubMed] [Google Scholar]
- Friedmann H. C., Cagen L. M. Microbial biosynthesis of B12-like compounds. Annu Rev Microbiol. 1970;24:159–208. doi: 10.1146/annurev.mi.24.100170.001111. [DOI] [PubMed] [Google Scholar]
- Friedrich W. Zur Biochemie der nucleotidfreien Vitamin B12-Carbonsäuren. Biochem Z. 1965 Jul 22;342(2):143–160. [PubMed] [Google Scholar]
- Hayward G. C., Hill H. A., Pratt J. M., Vanston N. J., Williams R. J. The chemistry of vitamin B 12. IV. The thermodynamic trans-effect. J Chem Soc Perkin 1. 1965 Sep;:6485–6493. [PubMed] [Google Scholar]
- Irion E., Ljungdahl L. Isolation of factor 3m coenzyme and cobyric acid coenzyme plus other B12 factors from Clostridium thermoaceticum. Biochemistry. 1965 Dec;4(12):2780–2790. doi: 10.1021/bi00888a031. [DOI] [PubMed] [Google Scholar]
- Leong S. A., Ditta G. S., Helinski D. R. Heme biosynthesis in Rhizobium. Identification of a cloned gene coding for delta-aminolevulinic acid synthetase from Rhizobium meliloti. J Biol Chem. 1982 Aug 10;257(15):8724–8730. [PubMed] [Google Scholar]
- Rapp P. Amidierung von Corrinoidcarbonsäuren in Rohextrakten aus Propionibacterium shermanii. Hoppe Seylers Z Physiol Chem. 1973 Feb;354(2):136–140. [PubMed] [Google Scholar]
- WEISSBACH H., LADD J. N., VOLCANI B. E., SMYTH R. D., BARKER H. A. Structure of the adenylcobamide coenzyme: degradation by cyanide, acid, and light. J Biol Chem. 1960 May;235:1462–1473. [PubMed] [Google Scholar]
- Zalkin H. Glutamine amidotransferases. Methods Enzymol. 1985;113:263–264. doi: 10.1016/s0076-6879(85)13035-1. [DOI] [PubMed] [Google Scholar]