Abstract
The two consecutive activities of the cobalamin biosynthetic pathway that catalyze the conversion of cobinamide to cobinamide phosphate (cobinamide kinase) and of cobinamide phosphate to GDP-cobinamide (cobinamide phosphate guanylytransferase) were shown to be carried by the same protein in Pseudomonas denitrificans. This bifunctional protein was purified to homogeneity by high-performance liquid chromatography of extracts of a recombinant strain of this microorganism, and the sequence of the first 10 amino acid residues at the N terminus was determined. Both activities were specific to the coenzyme forms of the corrinoid substrates and exhibited an optimum pH at 8.8. Both ATP and GTP were shown to be in vitro gamma-phosphate donors for cobinamide kinase. However, competition experiments demonstrated that ATP was the preferred substrate, a result that can be explained in terms of the kinetic properties of the enzyme. Labeling experiments established that the phosphate group of cobinamide phosphate is quantitatively retained as the inner phosphate of GDP-cobinamide during the guanylyltransferase reaction. The native protein had an apparent molecular weight of 40,000, as estimated by gel filtration, and consisted of two identical subunits of Mr 20,000, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. This protein had an isoelectric point of 5.35 and contained a high-affinity GTP-binding site (Kaff.(GTP) = 0.22 microM). Binding of GTP onto this site resulted in a marked increase of the affinity of cobinamide kinase for cobinamide. This property and other kinetic properties may regulate the enzyme and prevent the accumulation of cobinamide phosphate.
Full text
PDF
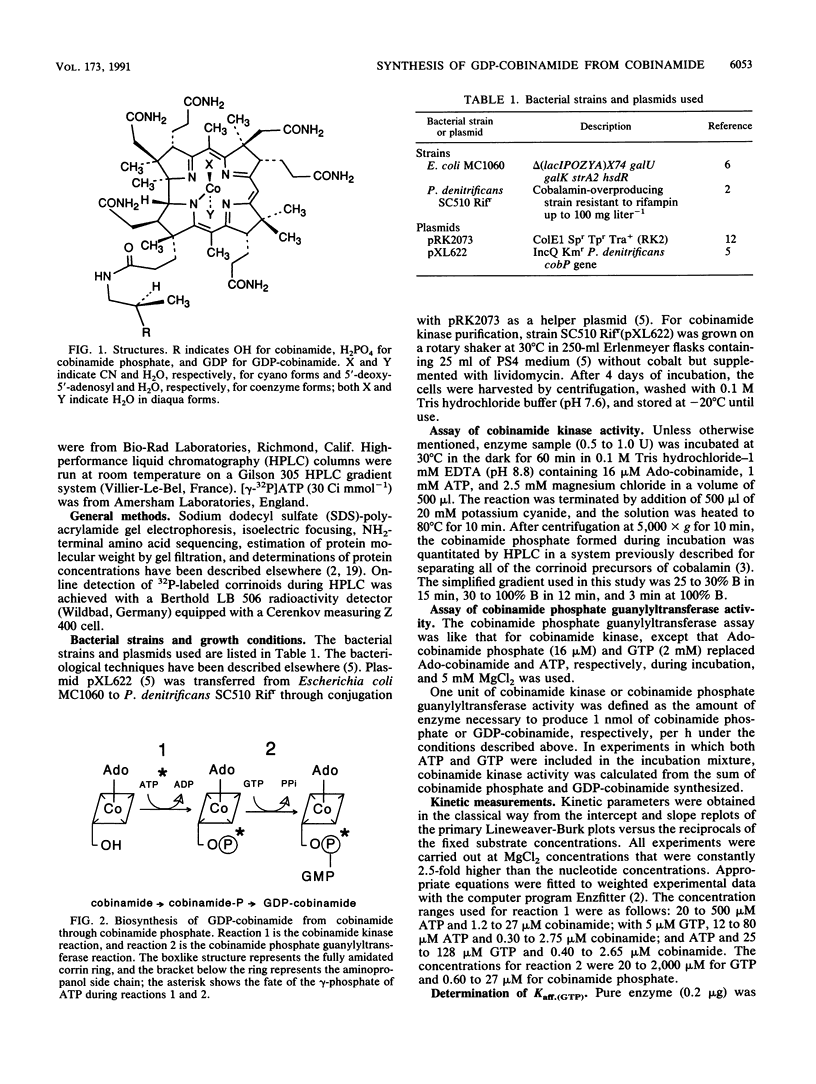




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORETTI G., DI MARCO A., FUOCO L., MARNATI M. P., MIGLIACCI A., SPALLA C. Intermediates in the biosynthesis of vitamin B12. Biochim Biophys Acta. 1960 Jan 15;37:379–380. doi: 10.1016/0006-3002(60)90260-2. [DOI] [PubMed] [Google Scholar]
- Berhauer K., Wagner F., Michna H., Rapp P., Vogelmann H. Zur Chemie und Biochemie der Corrinoide, XXIX. Biogenesewege von der Cobyrinsäure zur Cobysäure und zum Cobinamid bei Propionibacterium shermanii. Hoppe Seylers Z Physiol Chem. 1968 Oct;349(10):1297–1309. [PubMed] [Google Scholar]
- Blanche F., Debussche L., Thibaut D., Crouzet J., Cameron B. Purification and characterization of S-adenosyl-L-methionine: uroporphyrinogen III methyltransferase from Pseudomonas denitrificans. J Bacteriol. 1989 Aug;171(8):4222–4231. doi: 10.1128/jb.171.8.4222-4231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche F., Thibaut D., Couder M., Muller J. C. Identification and quantitation of corrinoid precursors of cobalamin from Pseudomonas denitrificans by high-performance liquid chromatography. Anal Biochem. 1990 Aug 15;189(1):24–29. doi: 10.1016/0003-2697(90)90038-b. [DOI] [PubMed] [Google Scholar]
- Cameron B., Briggs K., Pridmore S., Brefort G., Crouzet J. Cloning and analysis of genes involved in coenzyme B12 biosynthesis in Pseudomonas denitrificans. J Bacteriol. 1989 Jan;171(1):547–557. doi: 10.1128/jb.171.1.547-557.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Crouzet J., Levy-Schil S., Cameron B., Cauchois L., Rigault S., Rouyez M. C., Blanche F., Debussche L., Thibaut D. Nucleotide sequence and genetic analysis of a 13.1-kilobase-pair Pseudomonas denitrificans DNA fragment containing five cob genes and identification of structural genes encoding Cob(I)alamin adenosyltransferase, cobyric acid synthase, and bifunctional cobinamide kinase-cobinamide phosphate guanylyltransferase. J Bacteriol. 1991 Oct;173(19):6074–6087. doi: 10.1128/jb.173.19.6074-6087.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B. E., Hudson G. S. Chorismate mutase-prephenate dehydrogenase from Escherichia coli. Methods Enzymol. 1987;142:440–450. doi: 10.1016/s0076-6879(87)42055-7. [DOI] [PubMed] [Google Scholar]
- Friedmann H. C., Cagen L. M. Microbial biosynthesis of B12-like compounds. Annu Rev Microbiol. 1970;24:159–208. doi: 10.1146/annurev.mi.24.100170.001111. [DOI] [PubMed] [Google Scholar]
- Leong S. A., Ditta G. S., Helinski D. R. Heme biosynthesis in Rhizobium. Identification of a cloned gene coding for delta-aminolevulinic acid synthetase from Rhizobium meliloti. J Biol Chem. 1982 Aug 10;257(15):8724–8730. [PubMed] [Google Scholar]
- Rapp P. Amidierung von Corrinoidcarbonsäuren in Rohextrakten aus Propionibacterium shermanii. Hoppe Seylers Z Physiol Chem. 1973 Feb;354(2):136–140. [PubMed] [Google Scholar]
- Renz P. Enzymic synthesis of cobinamide phosphate from cobinamide by extracts of Propionibacterium shermanii. Biochem Biophys Res Commun. 1968 Feb 26;30(4):373–378. doi: 10.1016/0006-291x(68)90754-7. [DOI] [PubMed] [Google Scholar]
- Renz P. Reaktionsfolge der enzymatischen Synthese von Vitamin B12 aus Cobinamid bei Propionibacterium shermanii. Hoppe Seylers Z Physiol Chem. 1968 Aug;349(8):979–981. [PubMed] [Google Scholar]
- Ronzio R. A., Barker H. A. Enzymic synthesis of guanosine diphosphate cobinamide by extracts of propionic acid bacteria. Biochemistry. 1967 Aug;6(8):2344–2354. doi: 10.1021/bi00860a009. [DOI] [PubMed] [Google Scholar]
- Thibaut D., Couder M., Crouzet J., Debussche L., Cameron B., Blanche F. Assay and purification of S-adenosyl-L-methionine:precorrin-2 methyltransferase from Pseudomonas denitrificans. J Bacteriol. 1990 Nov;172(11):6245–6251. doi: 10.1128/jb.172.11.6245-6251.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSBACH H., LADD J. N., VOLCANI B. E., SMYTH R. D., BARKER H. A. Structure of the adenylcobamide coenzyme: degradation by cyanide, acid, and light. J Biol Chem. 1960 May;235:1462–1473. [PubMed] [Google Scholar]




