Abstract
Calcineurin is an important regulator of extracellular matrix (ECM) accumulation in the kidney but functions in a cell-specific manner. Previously, we identified a novel role for calcineurin in mesangial cells where calcineurin activity is required for TGFβ-mediated induction of fibronectin expression. In this study we examined the role of the calcineurin substrate NFATc in transcriptional regulation of fibronectin. First, inhibition of calcineurin blocks TGFβ induction of the fibronectin promoter. Moreover, expression of constitutively active calcineurin in mesangial cells is sufficient to increase fibronectin transcription. Next, inhibition of the calcineurin substrate NFATc1 blocked TGFβ-mediated activation of the fibronectin promoter. Finally, stable expression of a dominant negative NFATc protein reduced transcriptional activation of the promoter and inhibited TGFβ-mediated fibronectin expression. In conclusion, TGFß activation of calcineurin in mesangial cells results in regulation of ECM accumulation at least in part by direct transcriptional activity of NFATc on the fibronectin promoter.
Keywords: TGFβ, mesangial cells, extracelluar matrix, calcineurin, NFATc1
Introduction
The role of calcineurin in regulating extracellular matrix is complex. There is consistent data that inhibition of calcineurin activity results in upregulation of matrix proteins including fibronectin. In particular, both cultured fibroblasts and renal epithelial cells respond to addition of calcineurin inhibitors such as cyclosporin A (CsA) with increased extracellular matrix (ECM) production [1-3]. One potential mechanism for upregulation of matrix appears to be via the pleotropic cytokine TGFβ. Not only is TGFβ production increased along with matrix proteins in cultured cells after calcineurin inhibition [4], but CsA-mediated fibrosis can be reduced by neutralizing TGFβ antibody in vivo [5, 6]. From these experiments, it is reasonable to conclude that calcineurin activity negatively regulates TGFβ and, consequently, extracellular matrix. However, we have shown that the role of calcineurin in TGFβ regulation of matrix is cell-type dependent. For example, we found that CsA administration to diabetic rats was additive with regard to matrix production in the cortical tubulo-interstitium [7]. But in glomeruli, inhibition of calcineurin actually reduced fibronectin and collagen IV expression in the glomerulus [7]. Similarly, we found that both IGF-I and TGFβ signaling pathways involve activation of calcineurin in cultured mesangial cells [8, 9]. Inhibition of calcineurin in this cell type blocks TGFβ-mediated induction of matrix proteins including fibronectin.
Since fibrosis is a common and detrimental side-effect of clinical calcineurin inhibition, it is important to understand the mechanisms of calcineurin action. Moreover, investigation of cell-dependent mechanisms where calcineurin inhibition prevents matrix accumulation rather than induces it may be especially useful. Previously, we showed that TGFβ-mediated activation of calcineurin is required in the proximal signal, only minutes after addition of TGFβ. The importance of early calcineurin signaling to matrix regulation measured up to 72 hours later suggests a direct involvement of calcineurin at the level of fibronectin transcription. Consistent with that idea, we found that TGFβ activation of calcineurin in mesangial cells resulted in nuclear translocation of NFATc transcription factor [8].
NFATc proteins are known to be expressed in the kidney, and are regulated in mesangial cells in response to IGF-I [9], TGFβ [8], and endothelin [10, 11]. In vivo, NFATc has also been found to be regulated by calcineurin activation in the glomerulus [7]. Therefore, we hypothesized that there is a direct role for calcineurin/NFATc in transcriptional regulation of ECM in the kidney downstream of TGFβ. To test this, we first confirmed that fibronectin expression is transcriptionally regulated in response to TGFβ and that calcineurin activity is required for this regulation. Next, we examined the contribution of NFATc to calcineurin-mediated fibronectin upregulation. Finally, we overexpressed wildtype and dominant-negative NFATc in mesangial cells and examined the effect on fibronectin transcription and expression. These data demonstrate for the first time a direct role for NFATc proteins in transcriptional regulation of fibronectin.
Methods
Materials
Recombinant TGFβ was purchased from R&D Systems, Inc. (Minneapolis, MN). Cyclosporin A and anti-fibronectin antibody were from Sigma (St. Louis, MO). Anti-fibronectin and anti-actin antibodies were from Sigma and anti-NFATc1 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). NFATc inhibitor peptide was obtained from CalbioChem (Temecula, CA). Fibronectin-luciferase promoter construct was a gift of RL Widom (Boston University School of Medicine) [12], and the 3X NFATc-luciferase construct was purchased from Promega (Madison, WI). A constitutively active calcineurin construct (CA-CnA) was obtained from P Camacho (University of Texas Health Science Center, San Antonio) and NFATc and dominant-negative NFATc (DN-NFATc) were from NA Clipsone (Northwestern University Medical School). Rat mesangial cells were obtained from HE Abboud (University of Texas Health Science Center, San Antonio). For these experiments, rat mesangial cells were used between passage 26 and 32. LLCPK-1 cells were from American Type Culture Collection.
Western blots
Cells were plated in 60mm dishes and allowed to grow to 80-90% confluence. Medium was changed to serum-free medium (SFM) for 24 hours and the cells were treated as indicated. Cells were harvested with trypsin-EDTA, pelleted, washed with 1X PBS, and lysed using TNESV lysis buffer (50mM Tris -HCl pH 7.4, 2 mM EDTA, 1% NP-40, 100mM NaCl, 100mM Na orthovanadate, 100μg/ml leupeptin, 20μg/ml aprotonin, and 10-7M phenylmethylsulfonyl (PMSF)). 25μg of protein was analyzed by 7.5% SDS-PAGE and proteins transferred to nitrocellulose. The membrane was incubated in 5% milk-TBST (20mM Tris-HCl, pH 7.6, 137mM NaCl, 0.1% Tween 20) and then immunoblotted with appropriate dilutions of primary antibodies followed by HRP-conjugated secondary antibodies, and proteins were visualized by enhanced chemiluminescence (Pierce, Rockford, IL).
TGFβ ELISA
Cells were grown to 80% confluence, and then media were changed to SFM for 24 hours. CsA was added as indicated for an additional 24 hours and supernatants collected. TGFβ levels were determined using a commercially available kit according to the manufacturer's instructions (Promega).
Stable transfections
NMS cells between passages 26 and 32 were transfected with fibronectinluciferase in PGL2basic, pBJ alone, pBJ-NFATc1, or pBJ-DN-NFATc1 using lipofectamine (Invitrogen, Carlsbad, CA). Cells were re-plated at a sparse density and cells resistant to G418 were selected for using media supplemented with 400ng/ml neomycin. After approximately 2 weeks, clonal populations were harvested by ring cloning and expanded. Resulting cell lines were screened and experiments performed within 4-8 passages.
Promoter experiments
Mesangial cells were plated into 6-well dishes and the next day transfected with the reporter constructs as indicated using lipofectamine. Cells were allowed to recover for 24 hours and then media were changed to SFM for 24 hours prior to stimulation as indicated. Cells were harvested using passive lysis buffer and luciferase was measured according to the manufacturer's instructions. For transient transfection experiments, cells were co-transfected with a renilla plasmid (Promega) as an internal control and was measured also according to the manufacturer's instructions. For experiments with stably transfected cells, total protein for each sample was determined and luciferase values were normalized by protein content.
Electromobility gel shift
Mesangial cells were grown in serum-free media and then stimulated with TGFβ for 15 minutes. Nuclear lysates were prepared by hypotonic lysis [13]. To assess NFATc binding, consensus NFATc oligos were purchased from Santa Cruz (Santa Cruz, CA) and were end-labeled with 32Pγ[ATP]. 40,000cpm of labeled probe, 1.5μg of nuclear lysate and 1X Gel Shift Binding Buffer (Promega) were incubated at room temperature for 30 minutes. 2ug of anti-NFATc antibody was then added and reactions incubated at room temperature for an additional 30 minutes. Protein/DNA complexes were resolved by non-denaturing polyacrylamide gel electrophoresis and exposed to film.
Results and Discussion
Calcineurin-mediated regulation of fibronectin in mesangial cells
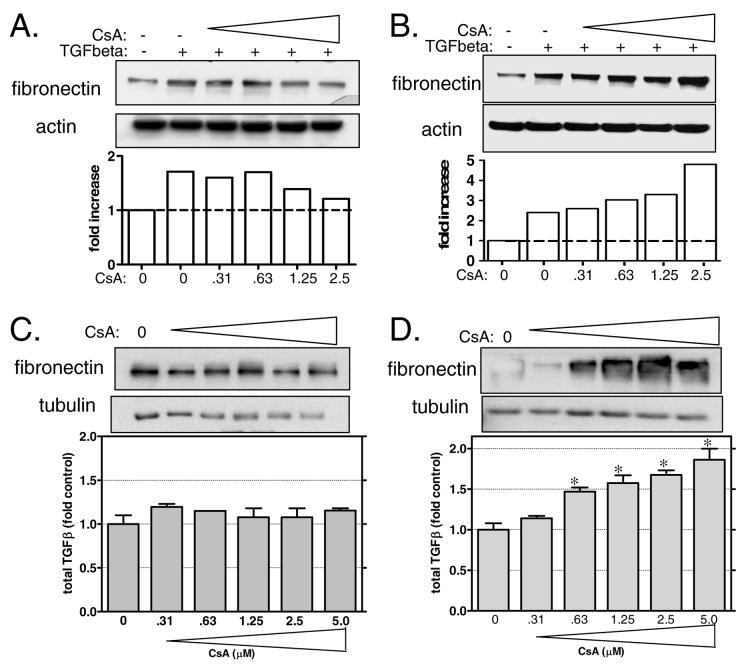
While there is considerable data to suggest that inhibition of calcineurin by cyclosporin (CsA) and FK506 up-regulates both matrix proteins and TGFβ, we have found that mesangial cells represent a distinct calcineurin signaling pathway. This point is illustrated in Figure 1. Panel A shows the effect of TGFβ on fibronectin expression in glomerular mesangial. TGFβ significantly increases fibronectin expression in mesangial cells and addition of CsA blocks the effect in a dose-responsive manner. This result is in contrast to the effect of CsA on a renal epithelial cell line (LLCPK-1) where TGFβ and CsA result in an additive effect on fibronectin expression (Fig 1B).
Figure 1. Differential effects of calcineurin inhibition on TGFβ and fibronectin in tubule epithelial versus mesangial cells.
A and B: Mesangial cells (A) and LLCKP1 epithelial cells (B) were cultured with increasing amounts of cyclosporin and then treated with TGFβ (2ng/ml). Cells were lysed and total proteins were separated by SDS-PAGE. Fibronectin was detected using a primary antibody specific to fibronectin. Actin was determined as an internal control. Results of a typical immunoblot were semi-quantitated and graphed. Data shown are representative of at least 3 independent experiments. C and D: Mesangial cells (C) and LLCPK-1 cells (D) were treated with increasing amounts of CsA and then fibronectin expression and TGFβ production were measured. Fibronectin expression was determined in cell lysates by direct immunoblotting. In identically treated cells, the amount of TGFβ in the supernatants was measured by ELISA using a commercially available kit. Data shown are the mean +/− SEM of triplicate samples.
Fibronectin up-regulation in response to CsA has been linked to stimulation of TGFβ in some cell types [6, 14]. Therefore, we determined what effect CsA treatment has on fibronectin expression and TGFβ production in mesangial cells and in LLCPK-1 cells. Cells were cultured in the presence of increasing amounts of CsA and lysates were harvested and supernatants collected for measurement fibronectin by western blotting and total TGFβ by ELISA. CsA resulted in a dose-dependent increase in both fibronectin and TGFβ in LLCPK-1 cells (Fig 1D). In contrast, neither fibronectin nor TGFβ is not up-regulated by CsA treatment in mesangial cells (Fig 1C).
Transcriptional regulation of fibronectin requires calcineurin
Consistent with previous data, Figure 1 demonstrates that calcineurin plays a unique role in TGFβ-mediated regulation of fibronectin in mesangial cells. The mechanism of this action is, however, unknown. Previous experiments suggested that the requirement for calcineurin is early in TGFβ signaling and involves upregulation of the calcineurin substrate NFATc [8]. Therefore, we examined whether regulation of fibronectin by TGFβ in mesangial cells is transcriptional. Mesangial cells were stably transfected with a fibronectin-promoter luciferase construct [12] (Fibro-luc), selected by antibiotic resistance, and then clonal cell populations were screened for luciferase activity. Multiple clones with high levels of luciferase activity were selected for further study and data from a representative clone is shown in Figure 2. Figure 2A shows that TGFβ regulates fibronectin transcription in a dose-dependent manner in Fibro-luc mesangial cells. Moreover, Figure 2B shows that TGFβ-mediated activation of fibronectin transcription is inhibited by increasing amounts of CsA. Next, a direct role for calcineurin in regulating fibronectin was tested by overexpressing constitutively active calcineurin (CA-CnA) and then examining fibronectin expression (Figure 2C) and promoter activity (Figure 2D). CA-CnA was sufficient to increase both fibronectin protein and transcription of the fibronectin promoter in a dose-dependent manner. This activity was blocked by pre-treatment with CsA.
Figure 2. Transcriptional regulation of fibronectin by TGFβ in mesangial cells.
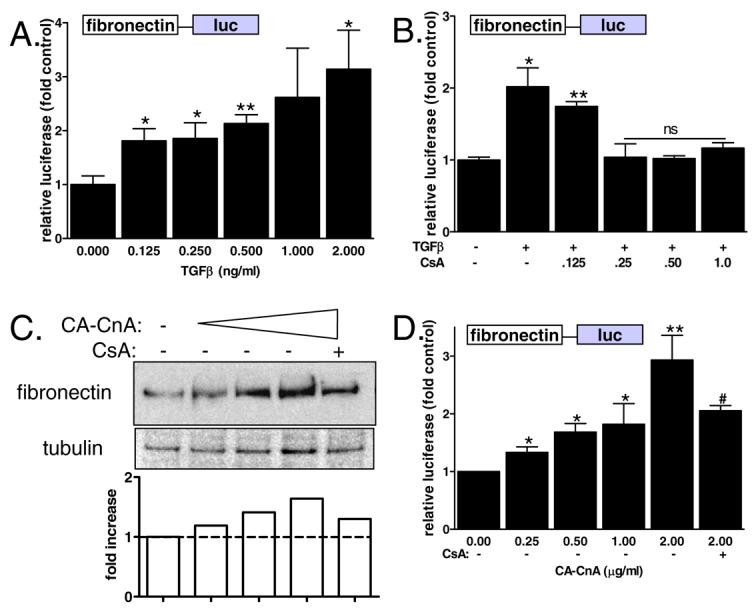
A) Mesangial cells stably transfected with a fibronectin-luciferase (fibro-luc) reporter construct were treated with increasing amounts of TGFβ. Fibronectin promoter activity was determined by luciferase measurement and normalized by protein concentration. Data shown are the mean +/− SEM of triplicate samples. * p<0.05 ** p<0.01 B) Fibro-luc mesangial cells were treated with increasing amounts of CsA prior to addition of TGFβ (2ng/ml). Fibronectin promoter activity was determined by measurement of luciferase normalized by protein concentration. Data shown are the mean +/− SEM of triplicate samples. *p<0.05 **p<0.01 C) Mesangial cells were transiently transfected with increasing amounts of constitutively active calcineurin for 72 hours. Cells were lysed, total protein collected and separated by SDS-PAGE. Fibronectin was detected using a primary antibody specific for fibronectin. Tubulin expression was also determined as an internal control. Results of a typical experiment were semi-quantitated and graphed. Data shown are representative of 3 independent experiments. D) Fibro-luc mesangial cells were transiently transfected with increasing amounts of constitutively active calcineurin. Fibronectin promoter activity was determined by measurement of luciferase normalized by protein concentration. Data shown are the mean +/− SEM of triplicate samples. *p<0.05, ** p<0.01, #
NFATc DNA binding and transcriptional activity is regulated by TGFβ
Previously, we showed that TGFβ-mediated nuclear localization of the calcineurin substrate NFATc [8]. We next wanted to examine DNA binding of NFATc1 in response to TGFβ by electromobility shift assay (EMSA) using a consensus NFATc sequence (Fig. 3A). Addition of TGFβ produced an increase in a DNA-protein complex (arrow). Inclusion of NFATc1 antibody in the reaction produced a super-shifted complex (arrowhead), confirming the binding of NFATc1 to the consensus site in response to TGFβ. Specificity of the protein/DNA complex was verified by competition with excess cold probe and by incubation of samples with a mutated NFATc site. Both methods blocked formation of the NFATc/DNA complex.
Figure 3. Inhibition of NFATc blocks TGFβ induction of fibronectin transcription.
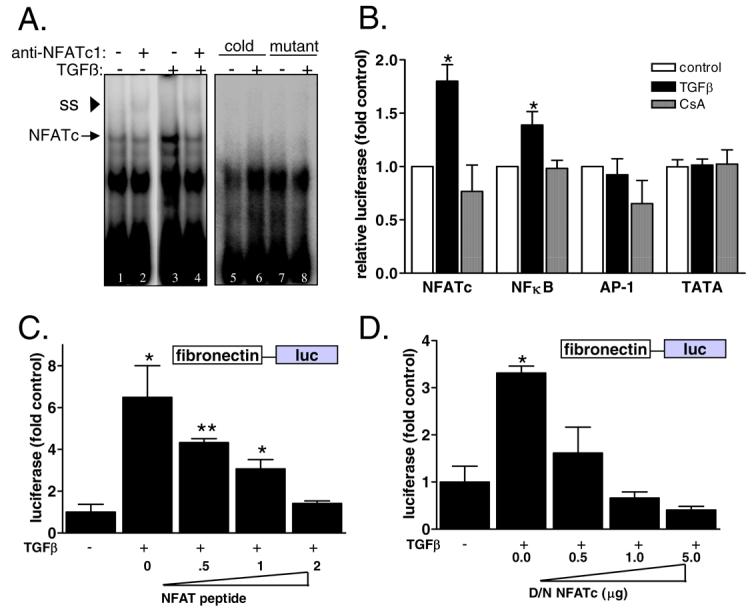
A) NFATc DNA binding in response to TGFβ was determined by electromobility shift assay. Mesangial cells were treated with SFM or TGFβ and then nuclear proteins were extracted and incubated with γ32P-labeled consensus NFATc probe or a mutant oligo. Finally, an antibody specific for NFATc1 was added. DNA/protein complexes were resolved by nondenaturing PAGE and results were visualized by autoradiography. Antibody binding was evident as a super-shifted (SS) band (asterisk). B) Mesangial cells were transiently transfected with an NFATc-responsive promoter sequence upstream of luciferase (NFATc-luc), AP1-luc, NFkB-luc, and TATA-luc along with a CMV-promoter driven renilla construct as a control for transfection. Cells were treated with SFM, TGFβ, or CsA+TGFβ and then activity of the target promoters were assessed by luciferase activity normalized by renilla. Data shown are the mean +/− SEM of triplicate samples. *p<0.05 C) Mesangial cells that stably express Fibro-luc were transiently transfected with increasing amounts of an NFATc inhibitory peptide and then fibronectin promoter activity in response to TGFβ was determined by luciferase activity normalized by protein concentration. Data shown are the mean +/− SEM of triplicate samples. * p<0.05 **p<0.01 D) Fibro-luc mesangial cells were transiently transfected with increasing amounts of a dominant negative NFATc cDNA and then fibronectin promoter activity in response to TGFβ was determined by luciferase activity normalized by protein concentration. Data shown are the mean +/− SEM of triplicate samples. * p<0.05.
We have shown that calcineurin is required for TGFβ-mediated upregulation of fibronectin in mesangial cells and that TGFβ leads to nuclear localization and DNA binding of the calcineurin-regulated transcription factor NFATc1. Therefore, we next examined whether NFATc1 was implicated in TGFβ-mediated regulation of fibronectin. First, we examined transcriptional activity of NFATc1 in mesangial cells using a promoter containing three consensus NFATc binding sites upstream of luciferase (NFATc-luc). Cells were transiently transfected with the NFATc-luc construct along with a plasmid regulating renilla expression as an internal control for transfection. Luciferase results were then normalized by renilla. Figure 3B shows that NFATc transcriptional activity is stimulated by TGFβ and blocked by CsA. In contrast, we find only mild activation of an NFκB consensus promoter in response to TGFβ and no significant increase of an AP-1 consensus promoter. As a control, cells were also transfected with a construct containing only a minimal TATA promoter upstream of luciferase. As expected, there was no increase observed with TGFβ and no inhibition by CsA.
Previous studies have shown multiple mechanisms whereby TGFβ controls matrix expression including SMAD proteins [15] and immediate early proteins c-jun and fos which form the transcription complex AP-1 [16]. Cross-talk between SMAD signaling and calcineurin has been suggested by data showing that SMAD proteins can interact with the calcineurin-binding protein calmodulin in a phosphorylation-dependent manner [17-19]. In addition, previous reports have shown that NFATc frequently co-operates with other transcriptional co-factors on target genes [20-23]. One common partner is AP-1 [21, 22]. It is therefore possible that NFATc cooperates with these mechanisms in TGFβ-mediated regulation of fibronectin.
NFATc is required for fibronectin regulation by TGFβ
To further examine the role of NFATc on fibronectin regulation, mesangial cells were co-transfected with the fibro-luc reporter construct along with increasing amounts of an NFATc competitive peptide (Fig. 3C) or a dominant-negative NFATc cDNA (Fig. 3D). Cells were stimulated with TGFβ and activity of the fibronectin promoter was determined. Inhibition of NFATc with either the competitive peptide or by expression of a dominant-negative protein blocked TGFβ-mediated induction of the fibronectin promoter in a dose-dependent manner.
Next, mesangial cells were stably transfected with either wildtype NFATc or a dominant-negative NFATc (DN-NFATc) and selected by antibiotic resistance to obtain clonal populations. As a control, parental mesangial cells were transfected with empty vector alone (pBJ) and subjected to identical antibiotic selection. Clones were then examined for functional differences in NFATc promoter activation and clones with the greatest degree of enhanced activity and inhibition of activity were selected for further study. Mesangial cells overexpressing wildtype NFATc1 had higher basal and serum-stimulated NFATc-mediated transcription compared to vector-transfected cells while cells overexpressing DN-NFATc1 had no response to serum (Fig 4A). Next, positive lines were tested for TGFβ-mediated transcriptional activity of the NFATc-luc promoter. Fig. 4B shows that mesangial cells overexpressing NFATc have higher basal activity and are activated further by TGFβ. Overexpression of DN-NFATc1, however, abolished TGFβ-mediated NFATc-luc activity. TGFβ activation of the fibronectin promoter was then determined in each cell type. Figure 4C shows that parental cells respond to TGFβ with approximately a 2-fold increase in promoter activity whereas both the basal and stimulated responses of cells overexpressing NFATc are significantly higher. In contrast, cells expressing DN-NFATc1 do not significantly increase fibronectin transcription in response to TGFβ. Finally, fibronectin protein levels were examined in rmesangial cells with altered NFATc1 expression. Figure 4D shows that TGFβ induced a two-fold increase in fibronectin in control cells and the increase was blocked by CsA. Overexpression of NFATc, however, resulted in enhanced basal and TGFβ-stimulated levels of fibronectin that was sensitive to CsA. Cells constitutively expressing DN-NFATc, however, had lower basal levels of fibronectin and an attenuated response to TGFβ.
Figure 4. NFATc can regulate fibronectin expression in mesangial cells.
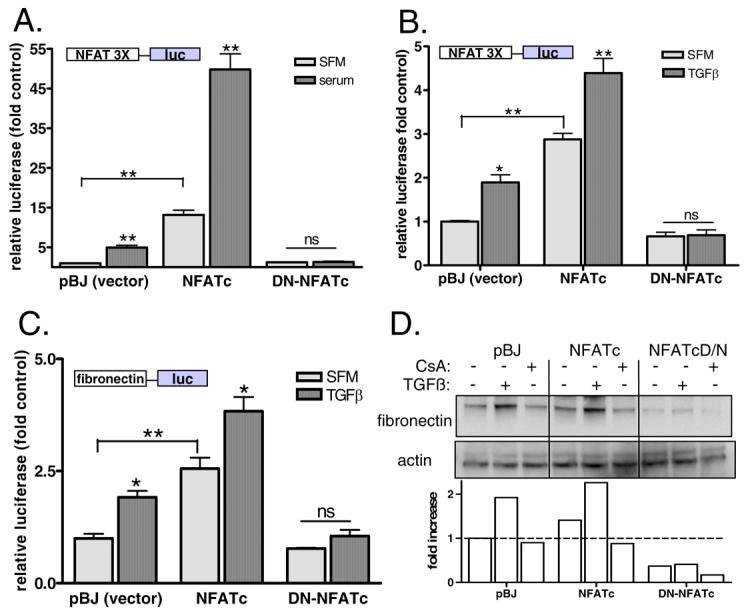
A) Mesangial cells were stably transfected with NFATc, a dominant-negative NFATc (DN-NFATc) or empty vector (pBJ). Clones that demonstrated antibiotic resistance were further screened for altered activity of a 3X NFATc promoter-luciferase construct. Data shown are the mean +/− SEM of triplicate samples. ** p<0.01 compared to control B) Parental, NFATc, and DN-NFATc mesangial cells were transiently transfected with a 3X NFATc-luciferase reporter construct and were then treated with either serum-free media or TGFβ (2ng/ml). NFATc transcriptional activity was determined by measurement of luciferase and normalized by renilla. Data shown are the mean +/− SEM of triplicate samples. * p<0.05, ** p<0.01 C) Parental, NFATc, and DNNFATc mesangial cells were transiently transfected with a fibro-luc reporter construct along with a renilla plasmid as an internal control. Cells were treated with SFM or TGFβ (2ng/ml) and then promoter activity was measured by luciferase and normalized by renilla. Data shown are the mean +/− SEM of triplicate samples. * p<0.05 ** p<0.01 D) Parental, NFATc, and DNNFATc mesangial cells were treated with TGFβ or CsA+TGFβ for 72 hours and then cells were lysed. Total proteins were isolated and then separated by SDS-PAGE. Fibronectin expression was determined by immunoblotting with a specific antibody and actin was detected as a control. Results from a representative immunoblot were semi-quantitated and graphed. Data shown are typical of at least 3 independent experiments.
The identification and characterization of a novel calcineurin role in regulation of extracellular matrix is particularly relevant considering that fibrosis is a significant part of cyclosporin toxicity. Our work shows that there are cell-specific actions of calcineurin and that at least one pathway in mesangial cells represents a mechanism where inhibition of calcineurin prevents rather than induces fibrosis. This finding suggests several important areas for future investigation. First, could different NFATc isoforms be responsible for cell-specific actions of calcineurin? In addition to, or alternatively, could cell-specific expression of different co-factors be the mechanism? Finally, are there different upstream signals that result in cell-specific activation of calcineurin? While it is possible that NFATc does not regulate fibronectin in other cell types, mesangial cells offer an opportunity to further explore a pathway that may yield novel therapeutic strategies that prevent or reduce fibrosis.
Acknowledgements
The authors wish to thank Rebecca Klein, Brian Roberts, Taylor Knotts, and Valerie Suniga for technical contributions. Funding for this work was provided by the NIH/NIDDK DK066422 (JLG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ghiggeri GM, Altieri P, Oleggini R, Valenti F, Ginevri F, Perfumo F, Gusmano R. Cyclosporine enhances the synthesis of selected extracellular matrix proteins by renal cells “in culture”. Different cell responses and phenotype characterization. Transplantation. 1994;57:1382–1388. doi: 10.1097/00007890-199405150-00017. [DOI] [PubMed] [Google Scholar]
- 2.Johnson DW, Saunders HJ, Johnson FJ, Huq SO, Field MJ, Pollock CA. Cyclosporin exerts a direct fibrogenic effect on human tubulointerstitial cells: Roles of insulin-like growth factor I, transforming growth factor beta1, and platelet-derived growth factor. J Pharm Exp Therap. 1999;289:535–542. [PubMed] [Google Scholar]
- 3.Wolf G, Killen PD, Neilson EG. Cyclosporin A stimulates transcription and procollagen secretion in tubulointersititial fibroblasts and proximal tubule cells. J Am Soc Neph. 1990;6:918–922. doi: 10.1681/ASN.V16918. [DOI] [PubMed] [Google Scholar]
- 4.Wolf G, Thaiss F, Stahl RA. Cyclosporine stimulates expression of transforming growth factor-beta in renal cells. Possible mechanism of cyclosporines antiproliferative effects. Transplantation. 1995;60:237–241. doi: 10.1097/00007890-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Islam M, Burke JF, Jr., McGowan TA, Zhu Y, Dunn SR, McCue P, Kanalas J, Sharma K. Effect of anti-transforming growth factor-beta antibodies in cyclosporine-induced renal dysfunction. Kidney Int. 2001;59:498–506. doi: 10.1046/j.1523-1755.2001.059002498.x. [DOI] [PubMed] [Google Scholar]
- 6.Sharma K, Yin Y, Guo J, Ziyadeh F. Neutralization of TGF-beta by anti-TGFbeta antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes. 1996;45:522–530. doi: 10.2337/diab.45.4.522. [DOI] [PubMed] [Google Scholar]
- 7.Gooch JL, Barnes JL, Garcia S, Abboud HE. Calcineurin is activated in diabetes and is required for glomerular hypertrophy and ECM accumulation. Am J Physiol Renal Physiol. 2003;284:F144–154. doi: 10.1152/ajprenal.00158.2002. [DOI] [PubMed] [Google Scholar]
- 8.Gooch JL, Gorin Y, Zhang BX, Abboud HE. Involvement of calcineurin in transforming growth factor-beta-mediated regulation of extracellular matrix accumulation. J Biol Chem. 2004;279:15561–15570. doi: 10.1074/jbc.M308759200. [DOI] [PubMed] [Google Scholar]
- 9.Gooch JL, Tang Y, Ricono JM, Abboud HE. Insulin-like growth factor-I induces renal cell hypertrophy via a calcineurin-dependent mechanism. J Biol Chem. 2001;276:42492–42500. doi: 10.1074/jbc.M102994200. [DOI] [PubMed] [Google Scholar]
- 10.Sugimoto T, Haneda M, Sawano H, Isshiki K, Maeda S, Koya D, Inoki K, Yasuda H, Kashiwagi A, Kikkawa R. Endothelin-1 induces cyclooxygenase-2 expression via nuclear factor of activated T-cell transcription factor in glomerular mesangial cells. Journal of the American Society of Nephrology. 2001;7:1359–1368. doi: 10.1681/ASN.V1271359. [DOI] [PubMed] [Google Scholar]
- 11.Sugimoto T, Stewart S, Guan K-L. The calcium/calmodulin-dependent protein phosphatase calcineurin is the major elk-1 phosphatase. J Biol Chem. 1997;272:29415–29418. doi: 10.1074/jbc.272.47.29415. [DOI] [PubMed] [Google Scholar]
- 12.Widom RL, Culic I, Lee JY, Korn JH. Cloning and characterization of hcKrox, a transcriptional regulator of extracellular matrix gene expression. Gene. 1997;198:407–420. doi: 10.1016/s0378-1119(97)00360-0. [DOI] [PubMed] [Google Scholar]
- 13.Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Islam M, Burke JF, McGowan TA, Zhu Y, Dun SR, McCue P, Kanalas J, Sharma K. Effect of transforming growth factor beta antibodies in cyclosporine-induced renal dysfunction. Kidney Intl. 2001;59:498–506. doi: 10.1046/j.1523-1755.2001.059002498.x. [DOI] [PubMed] [Google Scholar]
- 15.Schnaper HW, Hayashida T, Hubchak SC, Poncelet AC. TGF-beta signal transduction and mesangial cell fibrogenesis. Am J Physiol Renal Physiol. 2003;284:F243–252. doi: 10.1152/ajprenal.00300.2002. [DOI] [PubMed] [Google Scholar]
- 16.Choi M. Mechanisms of transforming growth factor-beta1 signaling. Kidney Int. 2000;77:S53–S58. [PubMed] [Google Scholar]
- 17.Zimmerman CM, Kariapper MST, Mathews LS. Smad proteins physically interact with calmodulin. J Biol Chem. 1998;273:677–680. doi: 10.1074/jbc.273.2.677. [DOI] [PubMed] [Google Scholar]
- 18.Wicks S, Lui S, Abdel-Wahab N, Mason R, Chantry A. Inactivation of Smad-transforming growth factor beta signaling by Ca2+-calmodulin-dependent protein kinase II. Mol Cell Biol. 2000;20:8103–8111. doi: 10.1128/mcb.20.21.8103-8111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scherer A, Graff J. Calmodulin differentially modulates Smad1 and Smad2 signaling. J Biol Chem. 2000;275:41430–41438. doi: 10.1074/jbc.M005727200. [DOI] [PubMed] [Google Scholar]
- 20.Santini MP, Talora C, Seki T, Bolgan L, Dotto GP. Cross talk among calcineurin, Sp1/Sp3, and NFAT in control of p21(WAF1/CIP1) expression in keratinocyte differentiation. Proc Natl Acad Sci U S A. 2001;98:9575–9580. doi: 10.1073/pnas.161299698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macian F, Lopez-Rodriguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20:2476–2489. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Glover J, Hogan P, Roa A, Harrison S. Structure of the DNA-binding domains from NFAT, Fos and Jun bound specifcally to DNA. Nature. 1998;392:42–48. doi: 10.1038/32100. [DOI] [PubMed] [Google Scholar]
- 23.Bert AG, Burrows J, Hawwari A, Vadas MA, Cockerill PN. Reconstitution of T cell-specific transcription by composite NFAT/Oct elements. Journal of Immunology. 2000;165:5646–5655. doi: 10.4049/jimmunol.165.10.5646. [DOI] [PubMed] [Google Scholar]