Both type 1 and type 2 diabetes can occur in children and adolescents. Of the 230 million people affected by diabetes worldwide, 4.9 million have type 1 diabetes. Type 1 diabetes is the most common chronic disease in children in the developed countries. Classification of childhood diabetes has become increasingly difficult as better measures of genetic testing have identified different forms of monogenetic diabetes masquerading as type 1 diabetes. Also the distinction between type 1 and 2 is not always clear‐cut, particularly in overweight adolescents. Every year approximately 70 000 children under the age of 15 develop type 1 diabetes worldwide. The paediatric incidence of type 1 diabetes is growing by 3–5% each year. “Diabetes is different in children” is the motto of World Diabetes Day announced by the International Diabetes Federation following the United Nations resolution on diabetes in December 2006 and dedicating the next triennium to diabetes in children and adolescents. Recent developments indicate that the efforts of diabetes teams implementing the new approaches in paediatric diabetes care are successful.
Goals of treatment: reducing complication risk
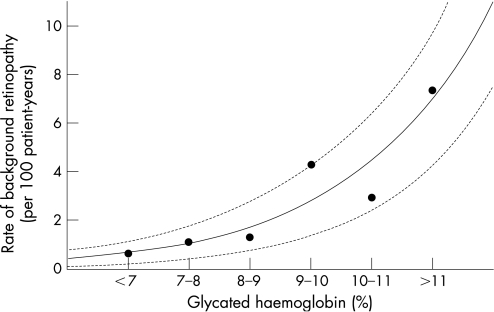
The Diabetes Control and Complications Trial (DCCT) and its follow‐up Epidemiology of Diabetes Interventions and Complications (EDIC) study confirmed that an improvement in long‐term glucose control, as obtained with intensified insulin therapy, can also reduce the incidence of complications and delay the progression of existing complications in type 1 diabetes in paediatric patients.1,2 Although only a subgroup of adolescents participated in the DCCT, longitudinal studies in the paediatric population such as the Berlin Retinopathy Study have revealed comparable results (fig 1).3 Reductions in HbA1c lead to the most dramatic fall in the rate of retinopathy when HbA1c is above 9%, a level which is deemed unacceptable in most guidelines. In addition, lower rates of retinopathy are achieved with every further drop in HbA1c. In particular, adolescents with suboptimal control should understand that a reduction in HbA1c, even though it is significantly above the target of 7.5%, still is likely to have a major impact on long‐term prognosis when maintained over time.
Figure 1 Relationship of median annual HbA1c from onset of diabetes in 346 children with type 1 diabetes (190 males and 156 females with an average age at onset of 9 years) studied prospectively with repeated retinal fluorescein angiographies, at intervals of 1–2 years in the Berlin Retinopathy Study (median (range) age 19.8 (8.8–35.4) years; diabetes duration 10.4 (1.1–27.4) years at last eye examination). (Data taken from Danne et al3 with permission.)
It is now believed that there is a metabolic memory, so that poor glycaemic control early during the disease course has long‐lasting effects despite better control later on. This is important both for microvascular complications such as retinopathy or nephropathy and for macrovascular disease due to accelerated development of atherosclerosis.4 These observations increase the importance of good paediatric diabetes care even more. For each individual the target should be the lowest achievable HbA1c without inducing severe hypoglycaemia.5,6 An HbA1c of less than 7.5% for children of all ages, which is slightly above the target for adults, has been adopted by many paediatric diabetes centres.
Insulin regimens for children
Treatment of diabetes in infants and toddlers is associated with additional, unique challenges specifically related to the physiological and developmental characteristics of children as compared with adolescents and adults. The incidence of hypoglycaemia is particularly high in young children.7 This is probably caused by a combination of physical and behavioural factors such as a lack of or lower awareness regarding hypoglycaemic episodes, unpredictable food intake and unpredictable physical activity.8 There has been a recent paradigm shift in the treatment of paediatric diabetes. Previously it was thought that the best way to overcome these barriers to treating children was to spare them from an insulin regimen consisting of many daily injections. Consequently, treatment consisted of two daily injections of premixed insulins. This was accompanied by the need to follow a strict diet and daily schedule in order to match the insulin intake. Indeed, some centres are reporting good results with this approach.9
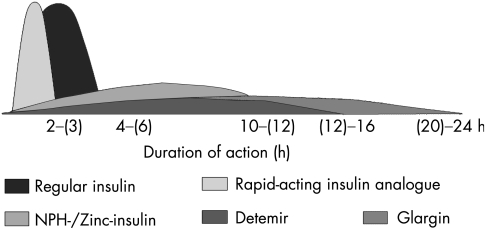
However, most paediatric diabetologists now believe that the gold standard treatment for children with diabetes is intensified insulin therapy, which aims to mimic as closely as possible the physiological insulin profile seen in non‐diabetic individuals. This kind of regimen is also believed to allow the flexibility required by the lifestyle needs of children with diabetes. To match these challenges, the availability of rapid‐acting, short‐acting, intermediate‐acting and long‐acting insulins and insulin analogues (fig 2), as well as devices such as insulin pumps and glucose sensors, has led to many recent new developments in the treatment options for children with diabetes.
Figure 2 Action curves for the main human insulins and insulin analogues available for paediatric diabetes treatment. The duration of action of most insulins is dose dependent in that a smaller dose has a shorter duration of effect and earlier peak.5 Numbers in parentheses are average duration of action.
Premixed insulin, multiple daily injections or pumps for children?
Administration of short‐acting insulin before main meals and intermediate/long‐acting insulin one to three times daily to cover basal insulin requirements is a common intensified insulin therapy used in children (fig 3). Over the last decade, continuous subcutaneous insulin infusion (CSII) has become increasingly popular for paediatric patients with diabetes. Theoretically, CSII offers the most physiological method of insulin delivery due to its ability to more closely simulate the normal pattern of insulin secretion, namely, continuous 24‐h adjustable basal delivery of insulin superimposed with prandial‐related boluses. In addition, CSII offers more flexibility and more precise insulin delivery than multiple daily injections (MDI) (fig 2). Although randomised controlled trials in very young children have not yielded the same beneficial effects as non‐randomised paired comparison studies, paediatric pump therapy offers real advantages over MDI.10,11 The results of the large European Pedpump data survey indicate that pumps for all age groups are safe and document the flexibility of CSII with many children taking seven or more daily prandial or correction boluses.12 The low rate of hypoglycaemia makes pumps an attractive choice, particularly for preschool children.13 Pump therapy is clearly not ideal for all children and families. Indeed, the positive effects on glycaemic control and hypoglycaemia in non‐randomised studies have been demonstrated precisely because clinicians selected patients likely to succeed. Poor motivation and support leading to a low number of boluses or failure to follow the rules for diabetic ketoacidosis prevention in CSII may lead to adverse outcomes. The vast majority of the studies cited utilised a multidisciplinary trained team that is not usually available to the general paediatrician or non‐academic paediatric endocrinologist. This may be a caveat to prescribing CSII.10 It is particularly important to take family resources, developmental stage and physical activity into account when deciding on the best type of therapy.
Figure 3 Insulin action curves for the two types of intensive insulin therapy. Top panel: multiple daily injections (MDI); bottom panel: continuous subcutaneous insulin infusion (CSII) or insulin pump therapy.
Rapid‐acting analogues
Three rapid‐acting insulin analogues are currently available for children (aspart, glulisine, lispro). They have a rapid onset and shorter duration of action than soluble insulin. As preprandial insulin treatment is often problematic in young children with unpredictable and irregular eating habits, postprandial injection of rapid‐acting insulin analogues means administration time and dosage can be adjusted according to meal time and meal size. The pharmacokinetics of insulin aspart has been investigated in 18 children and adolescents aged 6–17 years. In accordance with the pharmacokinetic results obtained in adults, insulin aspart was rapidly absorbed and eliminated.14 Interestingly, higher maximum insulin concentrations of both insulin aspart and human insulin were found in adolescents compared with children. The maximum concentration of insulin aspart was about twice that of human insulin, and the median time for maximum concentration after injection was 40 min for insulin aspart compared with 75 min for human insulin. These findings with regular insulin are in line with a separate study comparing another rapid‐acting insulin analogue, insulin glulisine, with human insulin. Again, treatment with regular insulin showed 65% higher maximum insulin concentrations and 50% higher overall concentrations of insulin for adolescents compared with children, with no differences in the case of insulin glulisine.15 In both studies, there were no discrepancies between adolescents and children in postprandial blood glucose excursions. These results are in line with the relatively impaired insulin sensitivity and higher insulin concentrations reported in healthy adolescents.16 Thus, adult data can not readily be transferred to paediatric patients, making it necessary to study the effects of these new insulins separately in all age groups.
Preprandial and postprandial injections of insulin aspart were compared in a 3‐month trial which included 76 children and adolescents with type 1 diabetes (6–18 years of age). It was concluded that postprandial administration of insulin aspart is a safe and effective alternative to preprandial administration.17 Similarly, in a trial with preschool children (2–6 years of age), data for 7‐point blood glucose profiles, HbA1c and fructosamine consistently indicated that the two treatment regimens are comparable.18 While the parents of young children preferred the postprandial injection, the parents of adolescents did not, possibly because of reduced parental control after meals and subsequent insulin omission.17 As a result of these and other studies, several indications for rapid‐acting insulin analogues in paediatric diabetology have been developed (table 1).5
Table 1 Potential indications for using short‐acting insulin analogues in paediatric diabetology.
| Insulin pump therapy |
| Postprandial hyperglycaemia is known or suspected |
| Nocturnal hypoglycaemia is known or suspected |
| Child or his/her carer finds injection of insulin 30 min before meals inconvenient or impossible |
| Child wants to reduce his/her dependence on snacks |
| Child eats large snacks that compromise preprandial control |
| Child eats variable or unpredictable amounts at each meal |
Long‐acting analogues
Insulin suspensions with neutral protamine Hagedorn (NPH) or zinc have been used for several years for delaying insulin action for basal insulin substitution. Two new long‐acting insulins have been developed. Firstly, insulin glargine, where pH change in the subcutaneous tissue with formation of microprecipitates leads to a nearly peakless insulin level for 22–24 h and secondly, insulin detemir, which has a 14‐C fatty acid that binds to human albumin leading to a 12–16‐h insulin duration of action with lower variability than NPH in children and adolescents.19 In most countries, the two basal analogues have not been formally approved for children below the age of 6 years. However, there are reports that glargine has been successfully used in children from under 1 to 5 years of age.20 Randomised and observational studies with insulin glargine as the basal insulin have also shown reductions in nocturnal hypoglycaemia.21,22 In a 6‐month multi‐centre trial, 347 children (aged 6–17 years) with type 1 diabetes received comparable doses of insulin detemir or NPH insulin plus premeal insulin aspart.23 At follow‐up, mean HbA1c had decreased by approximately 0.8% to 8.0% in both treatment groups, but the children in the insulin detemir group had a significant 26% reduction in nocturnal hypoglycaemia compared with those in the NPH insulin group. In another cross‐over study of 68 adolescents comparing the bedtime injection of semilente zinc‐insulin and insulin detemir, both insulins were equally effective regarding fasting plasma glucose levels. In spite of an average 1.7 times higher insulin dose to achieve the fasting blood glucose target, the number of mild and severe night‐time hypoglycaemic episodes was lower with detemir.24 Compared with NPH, insulin detemir is also associated with less excess weight gain in paediatric patients.23
New approaches in educational and psychosocial issues
Ideally, the child with diabetes should have access to a specialised multidisciplinary team of diabetes healthcare professionals including a paediatric diabetologist, a diabetes nurse educator and a dietician, as well as additional access to a psychologist and social workers. In many countries age‐appropriate educational programmes have been developed and evaluated for efficacy. The diabetes healthcare team will require special skills to deal with children of different ages, families and children with various levels of comprehension and education, and a mixture of languages and cultures in the community. Recently, a mobile diabetes education and care team was shown to be effective in improving the quality of care of children with type 1 diabetes who have limited access to specialised diabetes care in rural areas.25 Regardless of the insulin regimen prescribed, increased frequency of blood glucose self‐monitoring correlates with improved glucose control and better treatment adherence.26 The increased availability of continuous glucose sensors is likely to have a significant impact on paediatric diabetes therapy and education in the future.27 Historically, youths were encouraged towards independence in diabetes care, but recent studies indicate that premature withdrawal of parents from diabetes care is associated with adverse outcomes.28 Support from school and day care is also important in the management of diabetes in this age group because many children require insulin with lunch or at other times when they are away from home.29 In particular, the social and professional integration of mothers with younger children needs to be improved through support measures outside the family at diabetes onset. In a recent survey of 580 German families, 31% of mothers reduced their working time or stopped working and 33% reported adverse effects on their career development, especially those with a child below 6 years of age at diabetes onset (44%). There were negative financial consequences in 44% of families.30 Patient and family education and close contact with the diabetes team are associated with reduced hospitalisations and emergency room visits, and improved glycaemic control.31 Additional telephone contacts may be beneficial.32
Will the prognosis of children with diabetes improve?
In Hannover change from a conventional twice daily regimen of a pre‐mixed fixed mixture and later free mixing of soluble and isophane insulin, to multiple dose injections and, more recently, insulin pumps has been associated with continuous improvement in glycaemic control. This advance has to be seen in the context of improvements in patient education, self monitoring and the development of diabetes teams. The role of age‐appropriate education for children and adolescents with diabetes and their families with flexible, intensive insulin regimens with clear targets from the onset of type 1 diabetes is also very important (fig 4).33 Improvement with the introduction of multiple injection therapy has not been observed in all centres34 and it remains to be clarified in individual cases if daily administration of four injections is true intensive insulin management discriminating between the substitution of basal and prandial insulin needs or rather a conventional insulin therapy with four insulin injections daily. In our experience, reproduction of the physiological ratio between prandial and basal insulin is a prerequisite for near‐normoglycaemic metabolic control with MDI or CSII in children and adolescents.35 Basal insulin to cover hepatic gluconeogenesis should not be more than 30% to 40% of total daily insulin, while the prandial insulin usually should be more than 50% of the total dose. Evidence for a slight improvement in average HbA1c also comes from the international multicentre Hvidore collaboration for paediatric diabetes outcome quality studies: from 19937 to 199834 the average HbA1c remained unchanged at 8.6%, while the most recent survey found in more than 2000 children that the average had dropped to 8.2%. Although there are still striking differences in HbA1c between centres, diabetes management issues such as access to a diabetes team and particularly the setting of clear targets, were identified as playing a major role in outcome.36
Figure 4 (A) Change from conventional two‐injection therapy to intensified insulin therapy (multiple daily injections (MDI) and continuous subcutaneous insulin infusion (CSII)) over the last two decades at the Kinderkrankenhaus auf der Bult in Hannover, Germany and (B) changes in the level of glycaemic control in the last 10 years.
New pharmacological approaches and transplantation
Although initial studies with inhaled insulin have been carried out in the paediatric population, the clinical significance of increased insulin antibody formation and changes in lung function parameters need to be clarified. However, insulin is not the only hormone that regulates plasma glucose levels. Glucagon‐like peptide 1 (GLP‐1), produced in the small intestine, and amylin, produced by β cells in the pancreas, also have glucose‐lowering effects. Incretin‐based therapy, with GLP‐1 analogues such as exenatide or dipeptidyl peptidase‐4 inhibitors, is now increasingly being used to treat patients with type 2 diabetes and may also hold some potential for type 1 diabetes.37
While the long‐term success rate of islet cell transplantation, the need for repeated transplantation to achieve insulin independence (even with the new Edmonton protocol) and the paucity of donor organs are still discouraging and preclude any use of this approach in children, stem cell transplantation is gaining momentum as a potential avenue to insulin independence.38 However, high‐dose immunosuppression is necessary with islet and stem cell transplantation, long‐term data are lacking, and only a small number of patients with newly diagnosed type 1 diabetes have been studied so far. As preservation of residual β cell function is associated with better outcome in the DCCT study, approaches to modify the immune response at the onset of disease may become an integral part of disease management in addition to insulin therapy. Short‐term anti‐T cell antibody treatment is also able to preserve residual β cell function and may delay or limit chronic complications in these patients.39 Studies including adolescent patients are under way.
It is likely that with better understanding of the molecular, medical and psychosocial mechanisms involved, further advances in the treatment of children with all forms of diabetes are imminent. Meanwhile, every effort should be made to investigate the long‐term benefits of these recent developments in children with diabetes.
Abbreviations
CSII - continuous subcutaneous insulin infusion
DCCT - Diabetes Control and Complications Trial
EDIC - Epidemiology of Diabetes Interventions and Complications
GLP‐1 - glucagon‐like peptide 1
MDI - multiple daily injections
NPH - neutral protamine Hagedorn
Footnotes
Competing interests: Although all authors have previously received honoraria for speaking engagements or research support from various pharmaceutical and device companies in the diabetes field, we do not have any competing interests to declare regarding the present manuscript.
References
- 1.The DCCT Research Group The effect of intensive diabetes treatment on the development and progression of long‐term complications in adolescents with insulin‐dependent diabetes mellitus: the Diabetes Control and Complications Trial. J Pediatr 1994125(2)177–188. [DOI] [PubMed] [Google Scholar]
- 2.DCCT/EDIC Research Group Beneficial effects of intensive therapy of diabetes during adolescence: outcomes after the conclusion of the Diabetes Control and Complications Trial. J Pediatr 2001139(6)804–812. [DOI] [PubMed] [Google Scholar]
- 3.Danne T, Weber B, Hartmann R.et al Long‐term glycemic control has a nonlinear association to the frequency of background retinopathy in adolescents with diabetes. Follow‐up of the Berlin Retinopathy Study. Diabetes Care 1994171390–1396. [DOI] [PubMed] [Google Scholar]
- 4.Dahl‐Jorgensen K, Larsen J R, Hanssen K F. Atherosclerosis in childhood and adolescent type 1 diabetes: early disease, early treatment? Diabetologia 200548(8)1445–1453. [DOI] [PubMed] [Google Scholar]
- 5.International Society for Pediatric and Adolescent Diabetes (ISPAD) 2000 consensus guidelines for the management of insulin‐dependent diabetes in childhood and adolescence. Available from http://www.ispad.org/Links.html (accessed 6 June 2007)
- 6.National Institute for Clinical Excellence Type 1 diabetes: diagnosis and management of type 1 diabetes in children, young people and adults. 2004. Available from http://www.nice.org.uk/pdf/CG015NICEguideline.pdf (accessed 6 June 2007)
- 7.Mortensen H B, Hougaard P. Comparison of metabolic control in a cross‐sectional study of 2.873 children and adolescents with IDDM from 18 countries. Diabetes Care 199720714–720. [DOI] [PubMed] [Google Scholar]
- 8.Silverstein J, Klingensmith G, Copeland K.et al Care of children and adolescents with type 1 diabetes. A statement of the American Diabetes Association. Diabetes Care 200528186–212. [DOI] [PubMed] [Google Scholar]
- 9.Dorchy H. Dietary management for children and adolescents with diabetes mellitus: personal experience and recommendations. J Pediatr Endocrinol Metab 200316131–148. [DOI] [PubMed] [Google Scholar]
- 10.Phillip M, Battelino T, Rodriguez H.et al Consensus statement. Use of insulin pump therapy in the pediatric age‐group. Diabetes Care. 2007 Mar 19; [Epub ahead of print] [DOI] [PubMed]
- 11.Nimri R, Weintrob N, Benzaquen H.et al Insulin pump therapy in youth with type 1 diabetes: a retrospective paired study. Pediatrics 2006117(6)2126–2131. [DOI] [PubMed] [Google Scholar]
- 12.Danne T, Battelino T, Kordonouri O.et al A cross‐sectional international survey of continuous subcutaneous insulin infusion in 377 children and adolescents with type 1 diabetes mellitus from 10 countries. Pediatr Diabetes 20056(4)193–198. [DOI] [PubMed] [Google Scholar]
- 13.Weinzimer S A, Ahern J H, Doyle E A.et al Persistence of benefits of continuous subcutaneous insulin infusion in very young children with type 1 diabetes: a follow‐up report. Pediatrics 2004114(6)1601–1605. [DOI] [PubMed] [Google Scholar]
- 14.Mortensen H, Lindholm A, Olsen B.et al Rapid appearance and onset of action of insulin aspart in paediatric subjects with type 1 diabetes. Eur J Pediatr 2000159483–488. [DOI] [PubMed] [Google Scholar]
- 15.Danne T, Becker R H, Heise T.et al Pharmacokinetics, prandial glucose control, and safety of insulin glulisine in children and adolescents with type 1 diabetes. Diabetes Care 200528(9)2100–2105. [DOI] [PubMed] [Google Scholar]
- 16.Acerini C L, Cheetham T D, Edge J A.et al Both insulin sensitivity and insulin clearance in children and young adults with type I (insulin‐dependent) diabetes vary with growth hormone concentrations and with age. Diabetologia 20004361–68. [DOI] [PubMed] [Google Scholar]
- 17.Danne T, Aman J, Schober E.et al A comparison of postprandial and pre‐prandial administration of insulin aspart in children and adolescents with type 1 diabetes. Diabetes Care 2003262359–2384. [DOI] [PubMed] [Google Scholar]
- 18.Danne T, Råstam J, Odendahl R.et al Parental preference of prandial insulin aspart compared with preprandial human insulin in a basal‐bolus scheme with NPH insulin in a twelve week crossover‐study of preschool children with type 1 diabetes. Pediatr Diabetes 2007 (in press) [DOI] [PubMed]
- 19.Danne T, Lupke K, Walte K.et al Insulin detemir is characterized by a consistent pharmacokinetic profile across age‐groups in children, adolescents, and adults with type 1 diabetes. Diabetes Care 2003263087–3092. [DOI] [PubMed] [Google Scholar]
- 20.Alemzadeh R, Berhe T, Wyatt D T. Flexible insulin therapy with glargine insulin improved glycemic control and reduced severe hypoglycemia among preschool‐aged children with type 1 diabetes mellitus. Pediatrics 2005115(5)1320–1324. [DOI] [PubMed] [Google Scholar]
- 21.Chase H P, Dixon B, Pearson J.et al Reduced hypoglycemic episodes and improved glycemic control in children with type 1 diabetes using insulin glargine and neutral protamine Hagedorn insulin. J Pediatr 2003143(6)737–740. [DOI] [PubMed] [Google Scholar]
- 22.Schrober E, Schoenle E, Can Dyk J.et al Comparative trial between insulin glargine and NPH insulin in children and adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 200215(4)369–376. [DOI] [PubMed] [Google Scholar]
- 23.Robertson K, Schönle E, Gucev Z.et al Benefits of insulin detemir over NPH insulin in children and adolescents with type 1 diabetes: lower and more predictable fasting plasma glucose and lower risk of nocturnal hypoglycaemia [Abstract P66]. Diabet Med 200522(Suppl 2)45 [Google Scholar]
- 24.Kordonouri O, Datz N, Hoeffe J.et al Efficacy and safety of bedtime insulin detemir vs. insulin semilente in children, adolescents, and young adults with type 1 diabetes. A randomized, open‐label, cross‐over study [abstract]. Pediatr Diabetes 20067(Suppl 5)68–69. [Google Scholar]
- 25.von Sengbusch S, Muller‐Godeffroy E, Hager S.et al Mobile diabetes education and care: intervention for children and young people with type 1 diabetes in rural areas of northern Germany. Diabet Med 200623122–127. [DOI] [PubMed] [Google Scholar]
- 26.Skinner T C, John M, Hampson S E. Social support and personal models of diabetes as predictors of self‐care and well‐being: a longitudinal study of adolescents with diabetes. J Pediatr Psychol 200025257–267. [DOI] [PubMed] [Google Scholar]
- 27.Shalitin S, Phillip M. Closing the loop: combining insulin pumps and glucose sensors in children with type 1 diabetes mellitus. Pediatr Diabetes 20067(Suppl 4)45–49. [DOI] [PubMed] [Google Scholar]
- 28.Anderson B, Ho J, Brackett J.et al Parental involvement in diabetes management tasks: relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin‐dependent diabetes mellitus. J Pediatr 1997130257–265. [DOI] [PubMed] [Google Scholar]
- 29.American Diabetes Association Diabetes care in the school and day care setting (position statement). Diabetes Care 200427(Suppl 1)S122–S128. [DOI] [PubMed] [Google Scholar]
- 30.Lange K, Danne T, Kordonouri O.et al Diabetes in childhood: everyday burden and professional consequences for parents. Dtsch Med Wochenschr 2004129(20)1130–1134. [DOI] [PubMed] [Google Scholar]
- 31.Svoren B M, Butler D, Levine B S.et al Reducing acute adverse outcomes in youths with type 1 diabetes: a randomized, controlled trial. Pediatrics 2003112914–922. [DOI] [PubMed] [Google Scholar]
- 32.Howells L, Wilson A C, Skinner T C.et al A randomized control trial of the effect of negotiated telephone support on glycaemic control in young people with type 1 diabetes. Diabet Med 200219643–648. [DOI] [PubMed] [Google Scholar]
- 33.Chase H P, Lockspeiser T, Peery B.et al The impact of the Diabetes Control and Complications Trial and humalog insulin on glycohemoglobin levels and severe hypoglycemia in type 1 diabetes. Diabetes Care 200124430–434. [DOI] [PubMed] [Google Scholar]
- 34.Danne T, Mortensen H B, Hougaard P.et al Persistent differences among centers over 3 years in glycemic control and hypoglycemia in a study of 3,805 children and adolescents with type 1 diabetes from the Hvidore Study Group. Diabetes Care 2001241342–1347. [DOI] [PubMed] [Google Scholar]
- 35.Danne T, von Schutz W, Lange K.et al Current practice of insulin pump therapy in children and adolescents – the Hannover recipe. Pediatr Diabetes 20067(Suppl 4)25–31. [DOI] [PubMed] [Google Scholar]
- 36.Swift P G F, de Beaufort C E, Skinner T C, on behalf of the Hvidore Study Group Services provided by the diabetes team: do they effect the glycemic outcome? [abstract]. Pediatr Diabetes 20067(Suppl 5)19 [Google Scholar]
- 37.Jeha G S, Heptulla R A. Newer therapeutic options for children with diabetes mellitus: theoretical and practical considerations. Pediatr Diabetes 20067(2)122–138. [DOI] [PubMed] [Google Scholar]
- 38.Voltarelli J C, Couri C E, Stracieri A B.et al Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 20072971568–1576. [DOI] [PubMed] [Google Scholar]
- 39.Keymeulen B, Vandemeulebroucke E, Ziegler A G.et al Insulin needs after CD3‐antibody therapy in new‐onset type 1 diabetes. N Engl J Med 20053522598–2608. [DOI] [PubMed] [Google Scholar]