Abstract
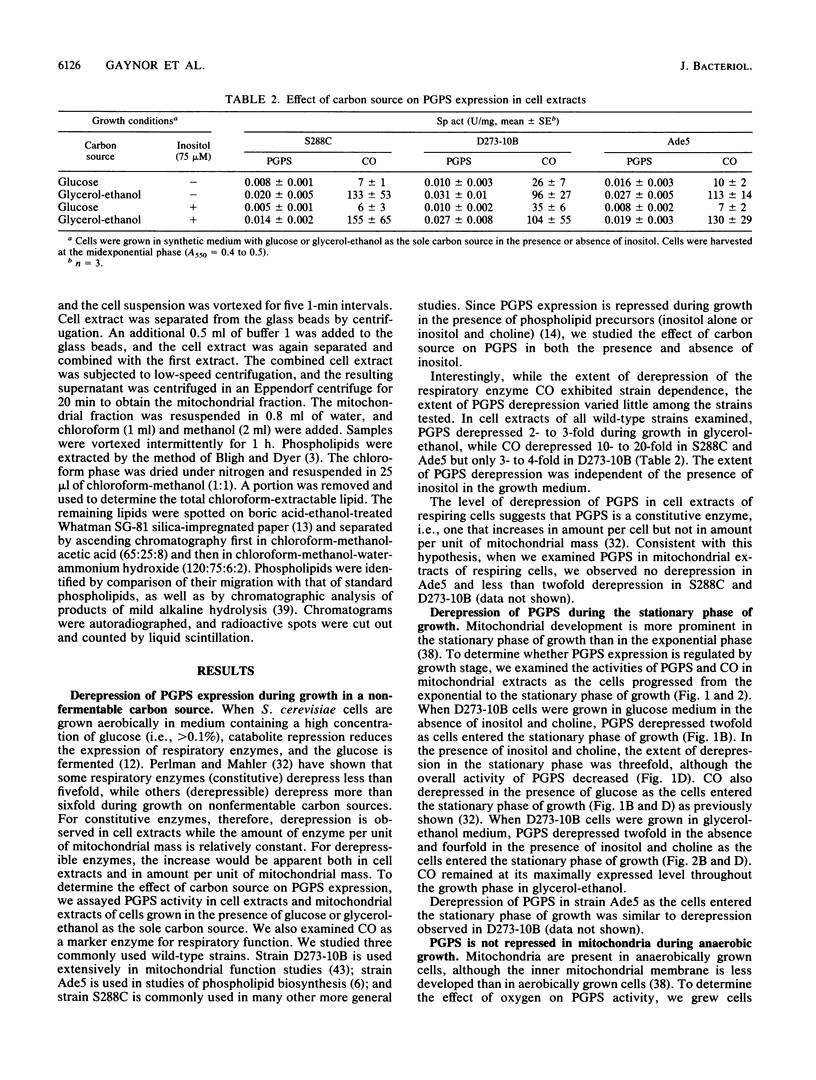
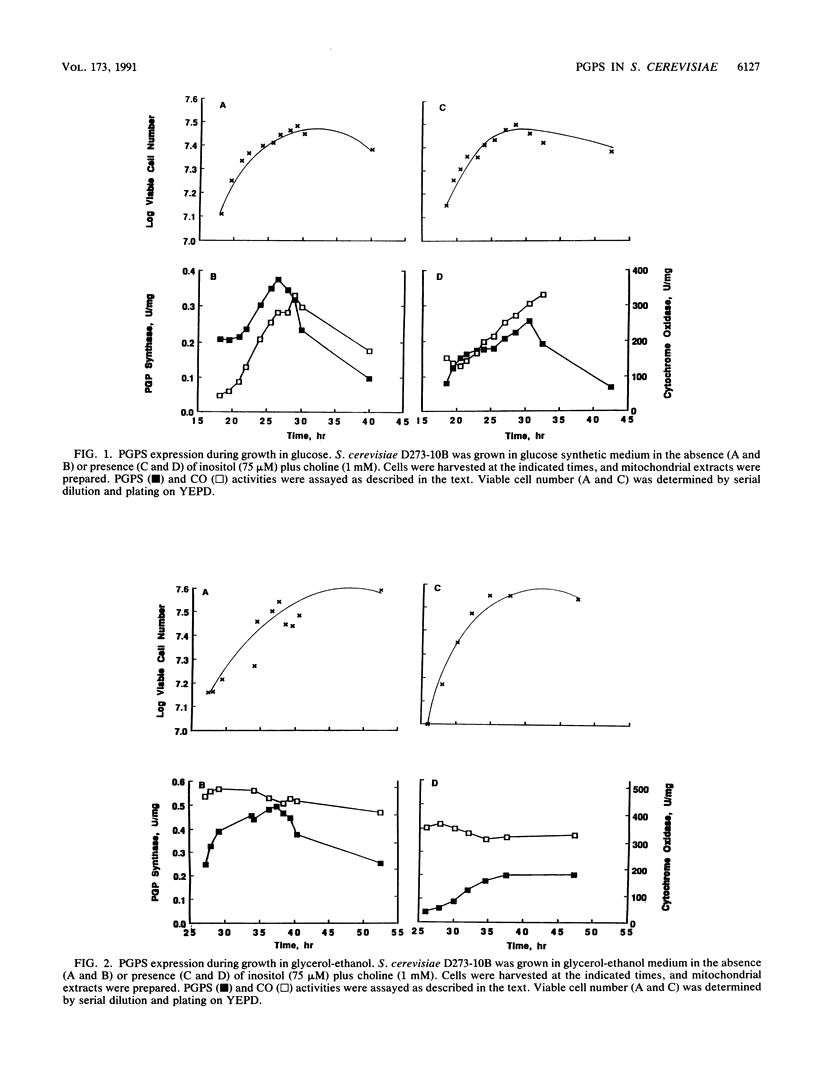
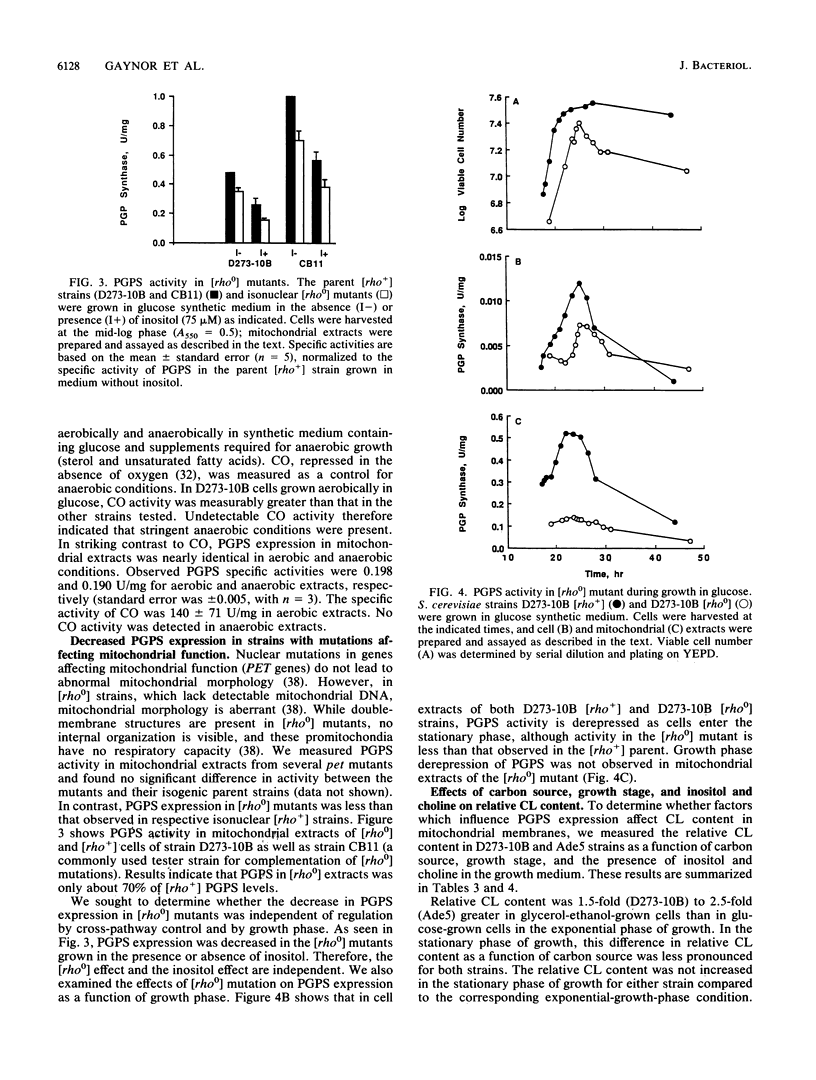
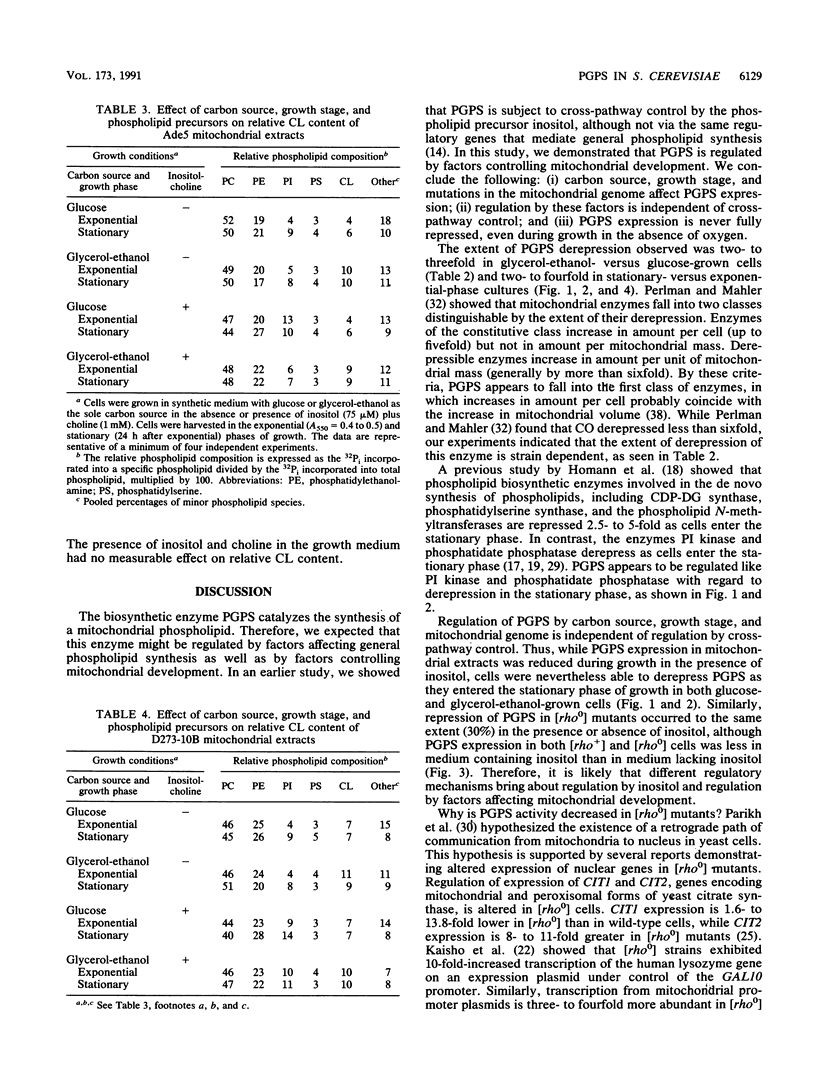
Phosphatidylglycerolphosphate synthase (PGPS; CDP-diacylglycerol glycerol 3-phosphate 3-phosphatidyltransferase; EC 2.7.8.5) catalyzes the first step in the synthesis of cardiolipin, an acidic phospholipid found in the mitochondrial inner membrane. In the yeast Saccharomyces cerevisiae, PGPS expression is coordinately regulated with general phospholipid synthesis and is repressed when cells are grown in the presence of the phospholipid precursor inositol (M. L. Greenberg, S. Hubbell, and C. Lam, Mol. Cell. Biol. 8:4773-4779, 1988). In this study, we examined the regulation of PGPS in growth conditions affecting mitochondrial development (carbon source, growth stage, and oxygen availability) and in strains with genetic lesions affecting mitochondrial function. PGPS derepressed two- to threefold when cells were grown in a nonfermentable carbon source (glycerol-ethanol), and this derepression was independent of the presence of inositol. PGPS derepressed two- to fourfold as cells entered the stationary phase of growth. Stationary-phase derepression occurred in both glucose- and glycerol-ethanol-grown cells and was slightly greater in cells grown in the presence of inositol and choline. PGPS expression in mitochondria was not affected when cells were grown in the absence of oxygen. In mutants lacking mitochondrial DNA [( rho0] mutants), PGPS activity was 30 to 70% less than in isogenic [rho+] strains. PGPS activity in [rho0] strains was subject to inositol-mediated repression. PGPS activity in [rho0] cell extracts was derepressed twofold as the [rho0] cells entered the stationary phase of growth. No growth phase derepression was observed in mitochondrial extracts of the [rho0] cells. Relative cardiolipin content increased in glycerol-ethanol-grown cells but was not affected by growth stage or by growth in the presence of the phospholipid precursors inositol and choline. These results demonstrate that (i) PGPS expression is regulated by factors affecting mitochondrial development; (ii) regulation of PGPS by these factors is independent of cross-pathway control; and (iii) PGPS expression is never fully repressed, even during anaerobic growth.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson K. D., Jensen B., Kolat A. I., Storm E. M., Henry S. A., Fogel S. Yeast mutants auxotrophic for choline or ethanolamine. J Bacteriol. 1980 Feb;141(2):558–564. doi: 10.1128/jb.141.2.558-564.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baker K. P., Schatz G. Mitochondrial proteins essential for viability mediate protein import into yeast mitochondria. Nature. 1991 Jan 17;349(6306):205–208. doi: 10.1038/349205a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carman G. M., Belunis C. J. Phosphatidylglycerophosphate synthase activity in Saccharomyces cerevisiae. Can J Microbiol. 1983 Oct;29(10):1452–1457. doi: 10.1139/m83-222. [DOI] [PubMed] [Google Scholar]
- Carman G. M., Henry S. A. Phospholipid biosynthesis in yeast. Annu Rev Biochem. 1989;58:635–669. doi: 10.1146/annurev.bi.58.070189.003223. [DOI] [PubMed] [Google Scholar]
- Cobon G. S., Crowfoot P. D., Linnane A. W. Biogenesis of mitchondria. Phospholipid synthesis in vitro by yeast mitochondrial and microsomal fractions. Biochem J. 1974 Nov;144(2):265–275. doi: 10.1042/bj1440265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson M. R., Henry S. A. Inositol-requiring mutants of Saccharomyces cerevisiae. Genetics. 1975 May;80(1):23–40. doi: 10.1093/genetics/80.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G. Lipids of mitochondria. Biochim Biophys Acta. 1985 Jun 12;822(1):1–42. doi: 10.1016/0304-4157(85)90002-4. [DOI] [PubMed] [Google Scholar]
- Eilers M., Endo T., Schatz G. Adriamycin, a drug interacting with acidic phospholipids, blocks import of precursor proteins by isolated yeast mitochondria. J Biol Chem. 1989 Feb 15;264(5):2945–2950. [PubMed] [Google Scholar]
- Endo T., Eilers M., Schatz G. Binding of a tightly folded artificial mitochondrial precursor protein to the mitochondrial outer membrane involves a lipid-mediated conformational change. J Biol Chem. 1989 Feb 15;264(5):2951–2956. [PubMed] [Google Scholar]
- Fine J. B., Sprecher H. Unidimensional thin-layer chromatography of phospholipids on boric acid-impregnated plates. J Lipid Res. 1982 May;23(4):660–663. [PubMed] [Google Scholar]
- Greenberg M. L., Hubbell S., Lam C. Inositol regulates phosphatidylglycerolphosphate synthase expression in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Nov;8(11):4773–4779. doi: 10.1128/mcb.8.11.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra A. K. Biosynthesis of acyl dihydroxyacetone phosphate in guinea pig liver mitochondria. J Biol Chem. 1968 Jun 25;243(12):3458–3465. [PubMed] [Google Scholar]
- Holland K. M., Homann M. J., Belunis C. J., Carman G. M. Regulation of phosphatidylinositol kinase activity in Saccharomyces cerevisiae. J Bacteriol. 1988 Feb;170(2):828–833. doi: 10.1128/jb.170.2.828-833.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann M. J., Poole M. A., Gaynor P. M., Ho C. T., Carman G. M. Effect of growth phase on phospholipid biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1987 Feb;169(2):533–539. doi: 10.1128/jb.169.2.533-539.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka K., Yamashita S. Regulatory role of phosphatidate phosphatase in triacylglycerol synthesis of Saccharomyces cerevisiae. Biochim Biophys Acta. 1984 Oct 24;796(1):110–117. [PubMed] [Google Scholar]
- Kadenbach B., Mende P., Kolbe H. V., Stipani I., Palmieri F. The mitochondrial phosphate carrier has an essential requirement for cardiolipin. FEBS Lett. 1982 Mar 8;139(1):109–112. doi: 10.1016/0014-5793(82)80498-5. [DOI] [PubMed] [Google Scholar]
- Kaisho Y., Yoshimura K., Nakahama K. Increase in gene expression by respiratory-deficient mutation. Yeast. 1989 Mar-Apr;5(2):91–98. doi: 10.1002/yea.320050204. [DOI] [PubMed] [Google Scholar]
- Kelly B. L., Greenberg M. L. Characterization and regulation of phosphatidylglycerolphosphate phosphatase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1990 Sep 18;1046(2):144–150. doi: 10.1016/0005-2760(90)90181-v. [DOI] [PubMed] [Google Scholar]
- Liao X. S., Small W. C., Srere P. A., Butow R. A. Intramitochondrial functions regulate nonmitochondrial citrate synthase (CIT2) expression in Saccharomyces cerevisiae. Mol Cell Biol. 1991 Jan;11(1):38–46. doi: 10.1128/mcb.11.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- Marczynski G. T., Schultz P. W., Jaehning J. A. Use of yeast nuclear DNA sequences to define the mitochondrial RNA polymerase promoter in vitro. Mol Cell Biol. 1989 Aug;9(8):3193–3202. doi: 10.1128/mcb.9.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlock K. R., Lin Y. P., Carman G. M. Regulation of phosphatidate phosphatase activity by inositol in Saccharomyces cerevisiae. J Bacteriol. 1988 Aug;170(8):3561–3566. doi: 10.1128/jb.170.8.3561-3566.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V. S., Morgan M. M., Scott R., Clements L. S., Butow R. A. The mitochondrial genotype can influence nuclear gene expression in yeast. Science. 1987 Jan 30;235(4788):576–580. doi: 10.1126/science.3027892. [DOI] [PubMed] [Google Scholar]
- Pember S. O., Powell G. L., Lambeth J. D. Cytochrome P-450scc-phospholipid interactions. Evidence for a cardiolipin binding site and thermodynamics of enzyme interactions with cardiolipin, cholesterol, and adrenodoxin. J Biol Chem. 1983 Mar 10;258(5):3198–3206. [PubMed] [Google Scholar]
- Perlman P. S., Mahler H. R. Derepression of mitochondria and their enzymes in yeast: regulatory aspects. Arch Biochem Biophys. 1974 May;162(1):248–271. doi: 10.1016/0003-9861(74)90125-8. [DOI] [PubMed] [Google Scholar]
- Robinson N. C. Specificity and binding affinity of phospholipids to the high-affinity cardiolipin sites of beef heart cytochrome c oxidase. Biochemistry. 1982 Jan 5;21(1):184–188. doi: 10.1021/bi00530a031. [DOI] [PubMed] [Google Scholar]
- Rubin M. S., Tzagoloff A. Cytochrome oxidase of Saccharomyces cerevisiae. Methods Enzymol. 1978;53:73–79. doi: 10.1016/s0076-6879(78)53015-2. [DOI] [PubMed] [Google Scholar]
- SMITH L. Spectrophotometric assay of cytochrome c oxidase. Methods Biochem Anal. 1955;2:427–434. doi: 10.1002/9780470110188.ch13. [DOI] [PubMed] [Google Scholar]
- Steiner M. R., Lester R. L. In vitro studies of phospholipid biosynthesis in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Feb 21;260(2):222–243. doi: 10.1016/0005-2760(72)90035-5. [DOI] [PubMed] [Google Scholar]
- Tamai K. T., Greenberg M. L. Biochemical characterization and regulation of cardiolipin synthase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1990 Sep 18;1046(2):214–222. doi: 10.1016/0005-2760(90)90192-z. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Dieckmann C. L. PET genes of Saccharomyces cerevisiae. Microbiol Rev. 1990 Sep;54(3):211–225. doi: 10.1128/mr.54.3.211-225.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


