Abstract
Objectives
To determine whether a community‐delivered intervention targeting infant sleep problems improves infant sleep and maternal well‐being and to report the costs of this approach to the healthcare system.
Design
Cluster randomised trial.
Setting
49 Maternal and Child Health (MCH) centres (clusters) in Melbourne, Australia.
Participants
328 mothers reporting an infant sleep problem at 7 months recruited during October–November 2003.
Intervention
Behavioural strategies delivered over individual structured MCH consultations versus usual care.
Main outcome measures
Maternal report of infant sleep problem, depression symptoms (Edinburgh Postnatal Depression Scale (EPDS)), and SF‐12 mental and physical health scores when infants were 10 and 12 months old. Costs included MCH sleep consultations, other healthcare services and intervention costs.
Results
Prevalence of infant sleep problems was lower in the intervention than control group at 10 months (56% vs 68%; adjusted OR 0.58 (95% CI: 0.36 to 0.94)) and 12 months (39% vs 55%; adjusted OR 0.50 (0.31 to 0.80)). EPDS scores indicated less depression at 10 months (adjusted mean difference −1.4 (−2.3 to −0.4) and 12 months (−1.7 (−2.6 to −0.7)). SF‐12 mental health scores indicated better health at 10 months (adjusted mean difference 3.7 (1.5 to 5.8)) and 12 months (3.9 (1.8 to 6.1)). Total mean costs including intervention design, delivery and use of non‐MCH nurse services were £96.93 and £116.79 per intervention and control family, respectively.
Conclusions
Implementing this sleep intervention may lead to health gains for infants and mothers and resource savings for the healthcare system.
Trial registration
Current Controlled Trial Registry, number ISRCTN48752250 (registered November 2004).
Maternal depression impacts adversely on maternal quality of life, mother–child relationships and child development.1,2 Despite a prevalence of 15% in the first year postpartum,3 depression often remains undiagnosed and, even if detected, many mothers reject the diagnosis, the treatment or both.4
Maternal depression is linked to poor infant sleep. Problems with frequent night waking and difficulties settling to sleep are reported by over a third of parents in the second 6 months of life5,6 and are consistently associated with poor maternal health.7,8,9
In a previous efficacy trial, we demonstrated that treating infant sleep problems (simple behavioural techniques delivered in local well‐child centres over two to three sessions) significantly reduced maternal reports of depression symptoms as well as infant sleep problems.10 However, efficacy and generalisability may be limited by the predominantly middle‐class status of participating families and the fact that the intervention was delivered by a single paediatrician (HH). In another randomised trial,11 a single, nurse‐led consultation emphasising ways to help very young infants settle to sleep independently resulted in intervention infants sleeping more than controls at age 12 weeks but in no change in maternal depression. All other sleep intervention trials have been limited by selection bias, small sample sizes, short follow‐up and/or lack of randomisation.12
The trial reported here was conducted within an existing universally available, state‐wide primary health care service, training the well‐child care providers themselves to manage infant sleep problems in families from a broad sociodemographic sample. We hypothesised that a brief behavioural intervention designed to reduce infant sleep problems would result in improved infant sleep and maternal well‐being. We also documented the costs of the intervention and costs to the healthcare system.
Methods
The trial was conducted in greater Melbourne (population 3.4 million), Victoria, Australia. Melbourne has 31 local government areas (LGAs), each with around 750 births per annum. LGAs were ranked by the census‐derived Socio‐Economic Indexes For Areas (SEIFA) Index of Relative Disadvantage (Australian Bureau of Statistics, 2001), and thence divided into tertiles representing low, middle and high socioeconomic status. From each tertile, two LGAs were selected; all Maternal and Child Health (MCH) nurses in these six LGAs participated.
Every infant born in the state of Victoria is assigned a MCH nurse, and 91% of parents attend the free health visit offered at 4 months of age.13 MCH nurses consecutively invited mothers of 4‐month‐old infants attending in October–November 2003 to take part in the Infant Sleep Study. Infants born before 32 weeks' gestation and mothers with insufficient English to complete questionnaires were excluded. The research team telephoned interested mothers and mailed the baseline questionnaire to be returned with written informed consent.
Randomisation
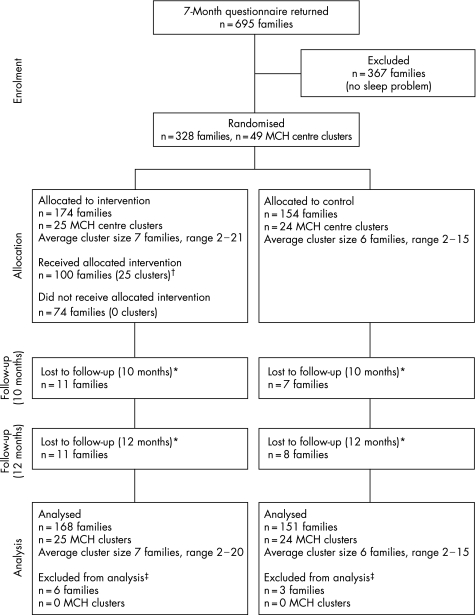
When infants turned 7 months, MCH centres (and therefore their mothers) were randomly allocated (stratifying by LGA) to intervention or control arms by an independent statistician (fig 1). Centres were ranked within each stratum according to the number of infants recruited at 4 months, randomising the largest centre and alternately allocating subsequent ones to avoid a marked imbalance in cluster sizes between trial arms. As all the centres were recruited prior to randomisation and ranked using a criterion that could not be influenced by the investigators, allocation concealment was achieved. Mothers reporting an infant sleep problem in the concurrent 7‐month questionnaire comprised the study sample.
Intervention
Intervention nurses attended two 2.5 h training sessions conducted by HH (paediatrician) and JB (child psychologist). Training incorporated didactic teaching, written information, role play and trouble shooting common problems (eg, partner conflict over sleep management).
Intervention mothers were advised by letter that their MCH nurse was trained to provide advice to help manage their infant's sleep. At the first consultation (8‐month well‐child visit) nurses elicited the nature of the sleep problem, identified solutions, and wrote an individualised sleep management plan with the mother. Two handouts discussed normal sleep patterns at 6–12 months and sleep associations and their causal role in sleep problems. Handouts on managing problem overnight feeding (ie, reducing volume/time spent feeding over a week) and dummies (ie, removal or teaching infant to replace own dummy) were also available.
Mothers were offered the choice of two behavioural interventions: i) “controlled crying” whereby parents respond to their infant's cry at increasing time intervals, to allow independent settling14,15 or ii) “camping out”, ie, sitting with their infant until they fell asleep and gradually removing parental presence over 3 weeks. Mothers maintained daily sleep diaries until the follow‐up appointment 2 weeks later, to facilitate recognition of sleep patterns and improvements and to help set further goals.
Measures
Mothers completed written questionnaires at 4 months (sociodemographic details), 7 months (prior to randomisation, to establish the presence of a sleep problem), and at 10 and 12 months (to measure outcomes). The primary outcome was maternal report of infant sleep (“Over the last 2 weeks, has your baby's sleep generally been a problem for you?” Yes/No), an established indicator of frequent and prolonged night wakings.6 Maternal well‐being was a secondary outcome, measured by the 10‐item Edinburgh Postnatal Depression Scale (EPDS)16 (community cut‐point for depression is a score >917) and the SF‐12,18 a 12‐item, validated, measure of physical and mental health with higher scores indicating better health. Other validated outcome measures were maternal sleep quality and quantity (using a 4‐point ordinal question,19 and dichotomised at the midpoint into good versus bad quality and enough versus not enough quantity), and maternal rating of infant temperament (5‐point Global Infant Temperament Scale20 higher scores indicate a more difficult baby). Mothers also rated their confidence in their nurse's health advice, on a 4‐point, ordinal, study‐designed scale. Intervention mothers' reported satisfaction with and usefulness of the sleep information in treating their infant's sleep problem, on 10‐cm visual analogue scales.6 They reported how often they used the strategies (from “none of the time” to “all of the time”) and degree of support they received from their partner when using the sleep strategies (4‐point scale ranging from “hardly ever supported me” to “supported me almost all the time”). All mothers were asked if they had received help other than that given in the study and, if so, from where (eg, stay at a parenting centre). In the 12‐month questionnaire, intervention mothers also rated specific advice/strategies given by their nurse as “helpful” or “unhelpful”.
Costs
At 10 and 12 months, mothers reported on the number of MCH visits for sleep advice and non‐MCH nurse professional health care (eg, parenting centres, family doctor) and non‐professional care (eg, books, relatives). Nurses provided information on number and duration of sleep visits for mothers in the intervention group. The cost of intervention materials (design and production) and nurse training programme (design and delivery) was calculated from research budgets, with total cost allocated equally over all intervention group mothers.
Nurse visits, outreach visits and telephone support costs were calculated using average MCH nurse salary data. Admissions to a parenting centre were valued using cost estimates from two of eight Melbourne centres. Other health service use costs were calculated using Medicare Benefits Schedule fees.21
Project approval was obtained from the Ethics in Human Research Committee of Melbourne's Royal Children's Hospital (23067B). The trial was conducted in accordance with the CONSORT statement for cluster randomised trials.22
Sample size
To detect a difference of 20% (70% in the control group and 50% in the intervention group) in the primary outcome, an individually randomised trial would require 103 infants in each arm to have 80% power at the 5% level of significance. This sample size was inflated by a design effect of 1.2 to 124 infants per trial arm to allow for correlation between responses within the same cluster (ie, MCH centre),23 with an expected average cluster size of 11 (ie, number of eligible mothers attending the centre) and intraclass correlation coefficient (ICC) of 0.02.
Analysis
Analyses were conducted on an “intention to treat” basis. Outcomes and costs were compared between the intervention and control groups at 10 and 12 months, adjusted for potential confounders identified in our previous research6,10 and determined a priori (see tables 1 and 2). Tests of interaction assessed whether the impact of the intervention on the sleep problem and the quantitative EPDS outcome was greater amongst mothers with high baseline depression scores (EPDS>9) than mothers with low scores.
Table 1 The 10‐ and 12‐month outcome results by randomisation group.
| Outcome | Group | Adjusted comparative statistic (intervention relative to control) | |||
|---|---|---|---|---|---|
| Intervention | Control | Statistic (95% CI) | p Value | ICC | |
| 10 months | |||||
| Sleep problem, n (%) | 92 (56) | 100 (68) | 0.58 (0.36, 0.94) | 0.03 | 0 |
| Global Infant Temperament score, mean (SD) | 2.7 (0.9) | 2.9 (0.9) | −0.1 (−0.3, 0.1) | 0.31 | 0.028 |
| EPDS score >9, n (%) | 46 (28) | 51 (35) | 0.63 (0.35, 1.12) | 0.11 | 0 |
| Total EPDS score at 2 months, mean (SD) | 6.8 (5.1) | 7.8 (5.5) | −1.4 (−2.3, −0.4) | 0.007 | 0 |
| Poor/fairly poor sleep quality, n (%) | 102 (63) | 104 (72) | 0.69 (0.41, 1.15) | 0.16 | 0 |
| Not enough/not nearly enough sleep, n (%) | 64 (40) | 66 (46) | 0.76 (0.46, 1.24) | 0.27 | 0.009 |
| SF12 – physical health, mean (SD) | 51.6 (7.3) | 51.5 (7.6) | 0.2 (−1.6, 2.0) | 0.83 | 0.017 |
| SF12 – mental health, mean (SD) | 48.1 (10.3) | 45.0 (11.0) | 3.7 (1.5, 5.8) | 0.001 | 0 |
| 12 months | |||||
| Sleep problem, n (%) | 64 (39) | 80 (55) | 0.50 (0.31, 0.80) | 0.004 | 0 |
| Global Infant Temperament score, mean (SD) | 2.7 (0.9) | 2.9 (0.9) | −0.2 (−0.4, 0.0) | 0.07 | 0 |
| EPDS score >9, n (%) | 41 (25) | 41 (28) | 0.71 (0.39, 1.30) | 0.26 | 0 |
| Total EPDS score, mean (SD) | 5.9 (4.8) | 7.2 (5.2) | −1.7 (−2.6, −0.7) | 0.001 | 0 |
| Poor/fairly poor sleep quality, n (%) | 83 (52) | 92 (63) | 0.52 (0.32, 0.86) | 0.01 | 0 |
| Not enough/not nearly enough sleep, n (%) | 55 (34) | 65 (45) | 0.55 (0.33, 0.92) | 0.02 | 0 |
| SF12 – physical health, mean (SD) | 50.8 (7.2) | 52.7 (6.7) | −1.7 (−3.3, −0.1) | 0.04 | 0 |
| SF12 – mental health, mean (SD) | 49.7 (9.2) | 46.1 (10.7) | 3.9 (1.8, 6.1) | <0.001 | 0.001 |
The comparative statistic is mean difference for quantitative outcomes and odds ratio for dichotomous outcomes.
Sample size ranged from 148 to 159 in the intervention arm and 138 to 144 in the control arm at 10 months and ranged from 145 to 154 in the intervention arm and 134 to 139 in the control arm at 12 months. Analyses adjusted for whether the mother received paid professional help for the infant's sleep problem at the time of follow‐up and perceived nurse competency. Analysis of sleep problem was additionally adjusted for Socio‐Economic Indexes For Areas (SEIFA) Index of Relative Disadvantage code and mother's educational level. Analyses of maternal EPDS score, sleep quality, sleep quantity, and SF12 physical and health scores were additionally adjusted for baseline EPDS total score. Analyses of Global Infant Temperament score were additionally adjusted for baseline EPDS total score and Global Infant Temperament at baseline. EPDS, Edinburgh Postnatal Depression Scale.
Negative estimates of the intraclass correlation coefficient (ICC) are truncated at zero.
Figure 1 Flow chart of participants. *All lost to follow‐up due to failure to return questionnaires. Some families returned questionnaires at 10 but not 12 months and vice versa. †Take‐up of the intervention was voluntary. One hundred families reported receiving the intervention. ‡Did not return either the 10‐ or 12‐month follow‐up questionnaire. MCH, Maternal and Child Health.
To allow for clustering, quantitative outcomes and costs were analysed using random effects linear regression24 and dichotomous outcomes were analysed using logistic regression by fitting marginal models using generalised estimating equations (GEE) with “robust” estimates of standard error25 and a specified exchangeable correlation matrix. For GEE analyses where the ICC was negative, the results from standard logistic regression are reported. All quantitative outcomes and costs (except the Global Infant Temperament score) were skewed and, therefore, results were validated by non‐parametric bootstrapping.26 Bootstrap confidence intervals were similar to the confidence intervals from the main analyses, so the latter are reported. Analyses were conducted using Stata 8 (Stata, College Station, TX).
Results
Response rates were 739 of 1025 eligible mothers at 4 months (72%) and 695 (68%) at 7 months, of whom 328 reported a problem with their infant's sleep and were therefore participants in the trial. Intervention (n = 174) and control mothers (n = 154) had similar infant, sleep, maternal and sociodemographic characteristics (table 3). Most mothers (84%) reported insufficient sleep which was of poor quality (55%).
Table 3 Sample characteristics by randomisation group at baseline (7 months)*.
| Variable | Randomisation | |
|---|---|---|
| Control (n = 154) | Intervention (n = 174) | |
| Infant | ||
| Infant age, mean (SD) | 7.3 (0.6) | 7.4 (0.6) |
| Male, n (%) | 89 (58) | 89 (51) |
| Birth weight (g), mean (SD) | 3432 (488) | 3461 (507) |
| Family position, n (%) | ||
| First | 72 (47) | 83 (48) |
| Second | 55 (36) | 54 (31) |
| Third or more | 25 (16) | 37 (21) |
| Exclusively breast feeding (at | 93 (60) | 104 (60) |
| 4 months), n (%) | ||
| Difficult temperament, n (%) | 44 (29) | 40 (23) |
| Infant sleep | ||
| Sleep problem severity, n (%) | ||
| Mild | 37 (24) | 38 (22) |
| Moderate | 58 (38) | 66 (38) |
| Severe | 59 (38) | 70 (40) |
| Number of waking nights/week | ||
| Median (IQR) | 7 (5–7) | 7 (5–7) |
| 0–3, n (%) | 22 (14) | 28 (16) |
| 4–6, n (%) | 44 (29) | 46 (26) |
| 7, n (%) | 88 (57) | 100 (57) |
| Who responds to waking infant overnight? | ||
| Mother, n (%) | 135 (91) | 155 (90) |
| Father, n (%) | 6 (4) | 3 (2) |
| Both parents, n (%) | 8 (5) | 15 (9) |
| Sleep site, n (%) | ||
| Cot in own/sibling's room | 101 (66) | 122 (71) |
| Parent's room | 36 (24) | 30 (17) |
| Parent's bed | 16 (10) | 20 (12) |
| Family | ||
| Maternal age (years), mean (SD) | 33.2 (4.8) | 32.8 (4.3) |
| Relationship status, n (%) | ||
| Married/de facto | 145 (97) | 172 (99) |
| Parents' country of birth | ||
| (mother's/father's), n (%) | ||
| Australia or New Zealand | 124 (81)/ | 139 (80)/ |
| 124 (82) | 137 (79) | |
| Language other than English | 31 (20) | 32 (18) |
| at home, n (%) | ||
| Education status (mother's/ | ||
| father's), n (%) | ||
| Did not complete high school | 28 (18)/43 (28) | 29 (17)/49 (29) |
| Completed high school only | 51 (33)/44 (29) | 53 (30)/45 (26) |
| Completed tertiary/ | 75 (49)/66 (43) | 92 (53)/77 (45) |
| postgraduate degree | ||
| Socioeconomic status (SEIFA | ||
| ranking), n (%) | ||
| Low | 35 (23) | 24 (14) |
| Medium | 41 (27) | 63 (36) |
| High | 78 (51) | 87 (50) |
| Maternal health | ||
| Past history of depression, n (%) | 32 (21) | 36 (21) |
| EPDS | ||
| Mean (SD) | 8.4 (5.1) | 8.4 (5.3) |
| Median (IQR) | 8 (4–12) | 8 (4–12) |
| >9 (n (%)) | 64 (42) | 66 (38) |
| Maternal sleep, n (%) | ||
| Quality | ||
| Fairly/very good | 74 (48) | 77 (44) |
| Very/fairly bad | 80 (52) | 97 (56) |
| Quantity | ||
| Enough/more than enough | 19 (12) | 34 (20) |
| Not nearly/not quite enough | 135 (88) | 140 (80) |
| MCH centres, n | ||
| MCH centre clusters | 24 | 25 |
| Nurses | 31 | 38 |
| Families | 154 | 174 |
| Nurses per MCH centre cluster | ||
| Mean (SD) | 1.35 (0.49) | 1.52 (0.65) |
| Range | 1–2 | 1–3 |
*Sample size ranged from 299 to 328.
EPDS, Edinburgh Postnatal Depression Scale; IQR, interquartile range; MCH, Maternal and Child Health.
Infant sleep
At 10 months, 56% of intervention and 68% of control mothers reported infant sleep problems (OR 0.61, p = 0.04); at 12 months, this fell to 39% vs 55% (OR 0.53, p = 0.007). Thus to resolve one infant sleep problem, 8.3 infants would be need to be treated at 10 months and 6.3 infants at 12 months. After adjusting for potential confounders (table 1), the odds of reporting a sleep problem in the intervention group were 42% lower at 10 months and 50% lower at 12 months compared with controls.
Maternal well‐being
At 10 months (table 1), 28% of intervention mothers and 35% of control mothers scored >9 on the EPDS, indicating clinically significant depression (adjusted OR 0.63, p = 0.11); comparable figures at 12 months were 25% vs 28% (adjusted OR 0.71, p = 0.26). Intervention mothers had lower mean EPDS scores than controls at both 10 months (6.8 vs 7.8, adjusted mean difference −1.4, p = 0.007) and 12 months (5.9 vs 7.2, adjusted mean difference −1.7, p = 0.001) and higher mental health (SF‐12) scores at both 10 months (48.1 vs 45.0, p = 0.001) and 12 months (49.7 vs 46.1, p<0.001). Physical health was similar at 10 months but unexpectedly poorer in intervention mothers at 12 months (mean score 50.8 vs 52.7, adjusted mean difference −1.7, p = 0.04).
Fewer intervention than control mothers reported poor sleep quality at 10 months (63% vs 72%, adjusted p = 0.16) and 12 months (52% vs 63%, adjusted p = 0.01), and insufficient sleep at 10 months (40% vs 46%, adjusted p = 0.27) and 12 months (34% vs 45%, adjusted p = 0.02).
In subgroup analyses (table 2), the effect of the intervention on depression symptoms at 10 months was greater for mothers whose initial EPDS score was >9.
Table 2 Test of EPDS score at baseline as a modifier of the intervention effect on percentage with sleep problems and mean EPDS scores by randomisation group.
| Outcome | EPDS baseline score | Group | Adjusted comparative statistic (intervention relative to control) | p Value for interaction | |||
|---|---|---|---|---|---|---|---|
| Intervention | n | Control | n | ||||
| Sleep | |||||||
| Sleep problem at 10 months, n (%) | >9 | 34 (54) | 63 | 43 (72) | 60 | 0.34 (0.14, 0.81) | 0.24 |
| ⩽9 | 58 (58) | 100 | 57 (66) | 87 | 0.71 (0.38, 1.32) | ||
| Sleep problem at 12 months, n (%) | >9 | 30 (47) | 64 | 36 (62) | 58 | 0.49 (0.22, 1.07) | 0.88 |
| ⩽9 | 34 (34) | 99 | 44 (50) | 88 | 0.48 (0.26, 0.90) | ||
| EPDS score | |||||||
| Total EPDS score at 10 months, mean (SD) | >9 | 9.5 (5.6) | 63 | 11.8 (5.0) | 60 | −2.1 (−3.9, −0.2) | 0.05 |
| ⩽9 | 5.1 (4.0) | 100 | 5.1 (4.1) | 87 | −0.2 (−1.4, 0.9) | ||
| Total EPDS score at 12 months, mean (SD) | >9 | 8.9 (4.8) | 64 | 10.5 (4.6) | 58 | −1.6 (−3.2, 0.1) | 0.56 |
| ⩽9 | 4.0 (3.8) | 99 | 5.0 (4.3) | 88 | −1.3 (−2.6, 0.1) | ||
The comparative statistic is odds ratio for sleep problem and mean difference for EPDS score. EPDS, Edinburgh Postnatal Depression Scale.
All analyses adjusted for whether a mother received paid professional help for the infant's sleep problem and level of nurse competency. The test of interaction for the sleep problem outcome was additionally adjusted for Socio‐Economic Indexes For Areas (SEIFA) Index of Relative Disadvantage code and mother's education.
Usefulness of sleep strategies
Table 4 shows the strategies intervention mothers found helpful. Mothers were generally satisfied with the intervention strategies (median 7.7, interquartile range (IQR) 5.5–9.2) and found them useful (median 7.3, IQR 3.0–9.1). The majority (56%) used the sleep strategies “most” to “almost all of the time”; only 7% reported no use. Most mothers (80%) reported partner support with sleep strategies.
Table 4 Intervention mothers' ratings of specific intervention components (n (%))*.
| Intervention | Helpful | Unhelpful | Did not receive/do this† |
|---|---|---|---|
| Information about normal sleep patterns in 6–12‐month‐old infants | 43 (70) | 6 (10) | 12 (20) |
| Having someone to talk to | 56 (93) | 3 (5) | 1 (2) |
| Filling out a sleep diary for the child | 36 (59) | 9 (15) | 16 (26) |
| Learning what thing(s) made the child's sleep problem better/worse | 46 (79) | 4 (7) | 8 (14) |
| Having sleep cycles (ie, “heavy” and “light” sleep) explained | 36 (60) | 13 (22) | 11 (18) |
| Information about managing dummies | 17 (30) | 10 (17) | 30 (53) |
| Information about managing night feeds | 24 (42) | 8 (14) | 25 (44) |
| Using “controlled crying” | 47 (78) | 9 (15) | 4 (7) |
| Using “camping out” in a chair or bed | 10 (17) | 11 (18) | 39 (65) |
| Putting child to bed awake | 47 (78) | 9 (15) | 4 (7) |
| Advice on how to look after own well‐being (eg, rest once a day) | 24 (40) | 6 (10) | 30 (50) |
*Sample size of intervention mothers who reported that they had received the information/strategies ranged from 57 to 61.
†Not all mothers received or used each strategy as sleep management was tailored to specific needs.
Costs
Mean costs for the intervention versus control group were £96.93 (SD 249.37) versus £116.79 (SD 330.31), respectively (mean difference −£19.44 (95% CI −83.70 to 44.81), p = 0.55). In the intervention group 100 mothers attended their nurse to discuss infant sleep for an average of 1.52 visits, while in the control group 30 mothers attended for an average of 1.32 visits. Across all mothers, this gives an average reported use of nurse sleep‐related visits of 0.9 and 0.3 for intervention versus control mothers, respectively. The mean duration of the first sleep visit was 25 min and 19 min for subsequent visits.
Control mothers were more likely than intervention mothers to have sought help from professional services for infant's sleep (33% vs 18%, p = 0.02; see table 5).
Table 5 Number and percentage of mothers who accessed sources of help and advice (other than MCH nurse service).
| 8–10 months | 10–12 months | |||
|---|---|---|---|---|
| Intervention, n = 163 | Control, n = 147 | Intervention, n = 163 | Control, n = 146 | |
| Professional, n (%) | ||||
| Parenting centre: day | 4 (2.5) | 7 (4.8) | 3 (1.8) | 4 (2.7) |
| Parenting centre: overnight | 4 (2.5) | 11 (7.5) | 3 (1.8) | 4 (2.7) |
| Telephone support | 2 (1.2) | 3 (2.0) | 5 (3.1) | 5 (3.4) |
| Nurse visit | 0 (0) | 3 (2.0) | 1 (0.6) | 5 (3.4) |
| Family doctor | 6 (3.7) | 9 (6.1) | 5 (3.1) | 7 (4.8) |
| Paediatrician | 2 (1.2) | 3 (2.0) | 1 (0.6) | 3 (2.1) |
| Chiropractor/osteopath | 3 (1.8) | 5 (3.4) | 2 (1.2) | 3 (2.1) |
| All professional sources, n (%)*† | 14 (8.6) | 31 (21.1) | 18 (11.0) | 26 (17.8) |
| Non‐professional, n (%) | ||||
| Mother (in‐law) | 25 (15.3) | 15 (10.2) | 17 (10.4) | 13 (8.9) |
| Friends/parent group | 39 (23.9) | 35 (23.8) | 31 (19.0) | 22 (15.1) |
| Written information/video | 20 (12.3) | 20 (13.6) | 12 (7.4) | 11 (7.5) |
| All non‐professional sources‡,§ | 48 (29.4) | 43 (29.3) | 35 (21.5) | 26 (17.8) |
| All non‐professional/professional sources¶** | 54 (33.1) | 64 (43.5) | 46 (28.2) | 39 (26.7) |
*Odds ratio (95% CI) 0.37 (0.17 to 0.80), p = 0.01 at 8–10 months; †odds ratio (95% CI) 0.55 (0.30 to 0.98), p = 0.04 at 10–12 months; ‡odds ratio (95% CI) 1.01 (0.62 to 1.63), p = 0.98 at 8–10 months; §odds ratio (95% CI) 1.18 (0.75 to 1.86), p = 0.47 at 10–12 months; ¶odds ratio (95% CI) 0.65 (0.40 to 1.05), p = 0.08 at 8–10 months; **odds ratio (95% CI) 1.04 (0.66 to 1.64), p = 0.88 at 10–12 months.
Discussion
A brief behavioural intervention delivered by primary healthcare professionals reduced infant sleep problems, improved maternal mental health, and reduced the amount of paid professional help sought for infant sleep problems 2 and 4 months after intervention. Benefits occurred across a broad sociodemographic range and at lower cost to the healthcare system.
This is the first trial examining the impact of a primary care infant sleep intervention on infant sleep and maternal well‐being. Over 95% of mothers and all clusters remained in the trial. Although only 72% of eligible mothers entered the study at 4 months, their mean EPDS score (mean 6.8, standard deviation (SD) 4.9) was similar to that reported in sample of women in Victoria 8–9 months postpartum (mean 7.6, SD 4.8).27 The prevalence of reported sleep problems (47%) was similar to that in our earlier study6 but higher than the 36% reported by mothers in another community study of Australian infants aged 7–9 months.5 This might suggest that mothers experiencing early sleep problems were more likely to enter the study but should not limit generalisability to English‐speaking mothers with infant sleep problems in the general population.
There is no accepted definition of an infant sleep problem. However, mothers who report an infant sleep problem are known to have babies with longer and more frequent night wakings and greater settling difficulties, and to suffer poorer mental health.6 Parents reporting a child sleep problem are also more accurate reporters of their child's sleep patterns than parents not reporting a child sleep problem when compared with over‐night infra‐red camera footage.28
Neither nurses nor mothers could be blind to group membership during the intervention and mothers' self‐reported outcomes. This may have biased reports of the intervention mothers towards an apparent benefit. However, intervention mothers reported a worse physical health outcome which argues against such a bias.
The trial demonstrated a sustained reduction in infant sleep problems, similar in size to that reported in other trials of behavioural interventions with shorter follow‐up periods.12 At 12 months, mean SF‐12 mental health scores for intervention and control mothers were 50 and 46, respectively. In a large, representative Australian population survey, adults scoring 40–49 vs ⩾50 on the SF‐12 were almost four times as likely to have a current mental disorder, and more than twice as likely to have impaired daily functioning.29 Thus this trial's SF‐12 mental health scores suggest an important impact on mental health disability. Similarly, the improvement in maternal sleep quality and quantity at 12 months might reduce other problems associated with maternal sleep deprivation, that is, maternal overload and dysfunction30 and later child behaviour problems.31
The chosen perspective of the economic evaluation meant we excluded resource use and costs that fell solely on mothers, for example out‐of‐pocket costs in accessing services. If the intervention reduces use of professional health services (apart from MCH nurses), it may well also reduce personal costs to mothers as mothers usually live within walking distance of their nurse's centre. Although the costs for mothers in the intervention arm were reduced relative to the control arm, the confidence intervals for the difference suggest that the cost could be greater in the intervention arm, in which case one would need to consider incremental costs. Equally, intervention costs could be even lower, as we included costs of material development which would not be re‐incurred if the programme were taken up more widely.
In conclusion, this brief intervention was effective, feasible, and acceptable to parents and primary healthcare providers alike. Effects were consistent across measures and over time, and occurred at a lower cost to the healthcare system. The challenge now is to translate this intervention to the wider population in a sustainable and feasible way.
What is already known on this topic
Infant sleep problems and postnatal depression are common, impact adversely on infants and mothers and often co‐exist.
Behavioural sleep interventions, when delivered by a paediatric trainee, reduce both infant sleep problems and postnatal depression symptoms.
What this study adds
Behavioural sleep interventions, delivered by primary health care professionals, can also reduce infant sleep problems and improve maternal well‐being.
Benefits occur in families from a variety of socioeconomic backgrounds.
Behavioural sleep interventions are acceptable and feasible to deliver in a busy primary care setting, reduce the need for mothers to access other sources of professional help and are cost effective.
Acknowledgements
We would like to thank the MCH nurses and families of the cities of Bayside, Darebin, Hobson's Bay, Manningham, Monash and the shire of Yarra Ranges who took part in this study.
Contributions
The trial design was formulated by HH and MW. JB, HH and AH implemented the trial. Data management and analysis was implemented by OU, LG and AH with HH, MW and JB contributing to the analysis plan. HH produced the first draft of the paper with all other authors contributing to subsequent drafts. HH will act as guarantor for the paper.
Abbreviations
EPDS - Edinburgh Postnatal Depression Scale
GEE - generalised estimating equations
ICC - intraclass correlation coefficient
IQR - interquartile range
LGA - local government area
MCH - Maternal and Child Health
SEIFA - Socio‐Economic Indexes For Areas
Footnotes
Funding: This project was funded by the National Health and Medical Research Council Project, grant number 237120 and The Pratt Foundation. Dr Hiscock was supported by the Murdoch Childrens Research Institute. None of the funders played a role in the study design, collection analysis or interpretation of the data or in the decision to submit.
Competing interests: None.
References
- 1.Murray L, Cooper P J.Postpartum depression and child development. New York: Guilford Press, 1997 [DOI] [PubMed]
- 2.Beck C T. The effects of postpartum depression on child development: a meta‐analysis. Arch Psychiatr Nurs 19981212–20. [DOI] [PubMed] [Google Scholar]
- 3.Boyce P, Stubbs J M. The importance of postnatal depression. Med J Aust 1994161471–472. [DOI] [PubMed] [Google Scholar]
- 4.Appleby L, Warner R, Whitton A.et al A controlled study of fluoxetine and cognitive‐behavioural counselling in the treatment of postnatal depression. BMJ 1997314932–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong K L, Quin R A, Dadds M R. The sleep patterns of normal children. Med J Aust 19941202–206. [DOI] [PubMed] [Google Scholar]
- 6.Hiscock H, Wake M. Infant sleep problems and postnatal depression: a community based study. Pediatrics 20011071317–1322. [DOI] [PubMed] [Google Scholar]
- 7.Leeson R, Barbour J, Ray K L.et al Management of infant sleep problems in a residential unit. Child Care Health Dev 19942089–100. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong K L, Van Haeringen A R, Dadds M R.et al Sleep deprivation or postnatal depression in later infancy: separating the chicken from the egg. J Paediatr Child Health 199834260–262. [DOI] [PubMed] [Google Scholar]
- 9.Stoleru S, Nottleman E, Belmont B.et al Sleep problems in children of affectively ill mothers. J Child Psychol Psychiatry 199738831–841. [DOI] [PubMed] [Google Scholar]
- 10.Hiscock H, Wake M. Does a behavioural infant sleep intervention improve infant sleep and maternal mood? A randomised controlled trial. BMJ 20023241062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Symon B, Marley J E, Martin A J.et al Effect of a consultation teaching behaviour modification on sleep performance in infants: a randomised controlled trial. Med J Aust 2005182215–218. [DOI] [PubMed] [Google Scholar]
- 12.Ramchandani P, Wiggs L, Webb V.et al A systematic review of treatments for settling problems and night waking in young children. BMJ 2000320209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Office for Children Annual data reports for maternal and child health ‐ years prior to 2005. http://www.office‐for‐children.vic.gov.au/early‐years‐services/library/data/annual_mch_pre2005 (accessed 6 August 2007)
- 14.France K G, Henderson J M T, Hudson S. Fact, act and tact. A three‐stage approach to treating the sleep problems of infants and young children. Child Adolesc Psychiatr Clin N Am 19965581–599. [Google Scholar]
- 15.Ferber R.Solve your child's sleep problems. New York: Simon & Schuster, 1986
- 16.Cox J L, Holden J M, Sagovsky R. Detection of postnatal depression: development of a 10‐item Edinburgh Postnatal Depression Scale. Br J Psychiatry 1987150782–786. [DOI] [PubMed] [Google Scholar]
- 17.Murray L, Carothers A D. The validation of the Edinburgh Post‐natal Depression Scale on a community sample. Br J Psychiatry 1990157288–290. [DOI] [PubMed] [Google Scholar]
- 18.Sanderson K, Andrews G. The SF‐12 in the Australian population: cross‐validation of item selection. Aust N Z J Public Health 200226343–345. [DOI] [PubMed] [Google Scholar]
- 19.Buysse D, Reynolds C, Monk T.et al The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 199828193–213. [DOI] [PubMed] [Google Scholar]
- 20.Sanson A, Prior M, Garino E.et al The structure of infant temperament: factor analysis of the Revised Infant Temperament Questionnaire. Infant Behav Dev 19871097–104. [Google Scholar]
- 21.Department of Health and Aging Medicare benefits schedule. http://www.health.gov.au/mbsonline (accessed 6 August 2007)
- 22.Campbell M K, Elbourne D R, Altman D G. CONSORT statement: extension to cluster randomised trials. BMJ 2004328702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donner A K N. Design and analysis of cluster randomization trials. In: Health Research. London: Arnold, 200062–64.
- 24.Goldstein H.Multilevel statistical models. London: Arnold, 1995
- 25.Zeger S L, Liang K ‐ Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 198642121–130. [PubMed] [Google Scholar]
- 26.Davison A C, Hinkley D V.Bootstrap methods and their application. Cambridge: Cambridge University Press, 1997
- 27.Brown S, Lumley J. Maternal health after childbirth: results of an Australian population based survey. Br J Obstet Gynaecol 1998105156–161. [DOI] [PubMed] [Google Scholar]
- 28.Minde K, Popiel K, Leos N.et al The evaluation and treatment of sleep disturbances in young children. J Child Psychol Psychiatry 199334521–533. [DOI] [PubMed] [Google Scholar]
- 29.Sanderson K, Andrews G. Prevalence and severity of mental health ‐ related disability and relationship to diagnosis. Psychiatr Serv 20025380–86. [DOI] [PubMed] [Google Scholar]
- 30.McVeigh C A. An Australian study of functional status after childbirth. Midwifery 199713172–178. [DOI] [PubMed] [Google Scholar]
- 31.Kahn R S, Zuckerman B, Bauchner H.et al Women's health after pregnancy and child outcomes at 3 years: a prospective cohort study. Am J Pub Health 2002921312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]