Abstract
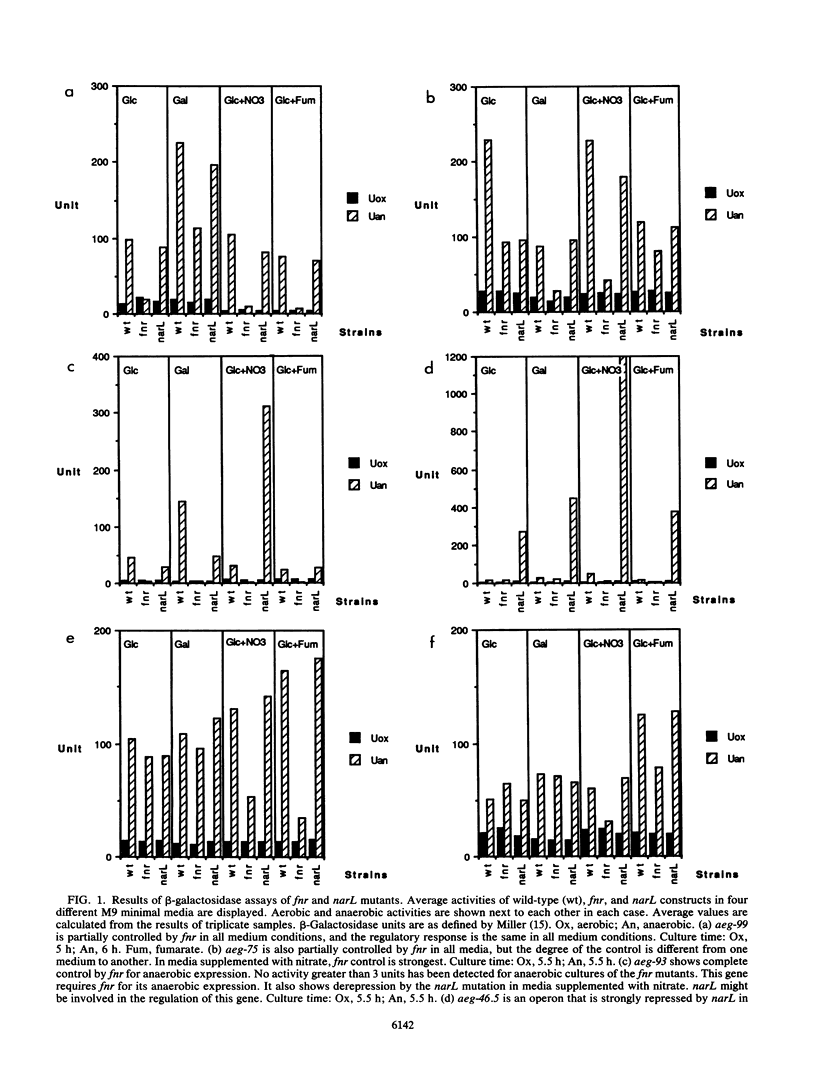
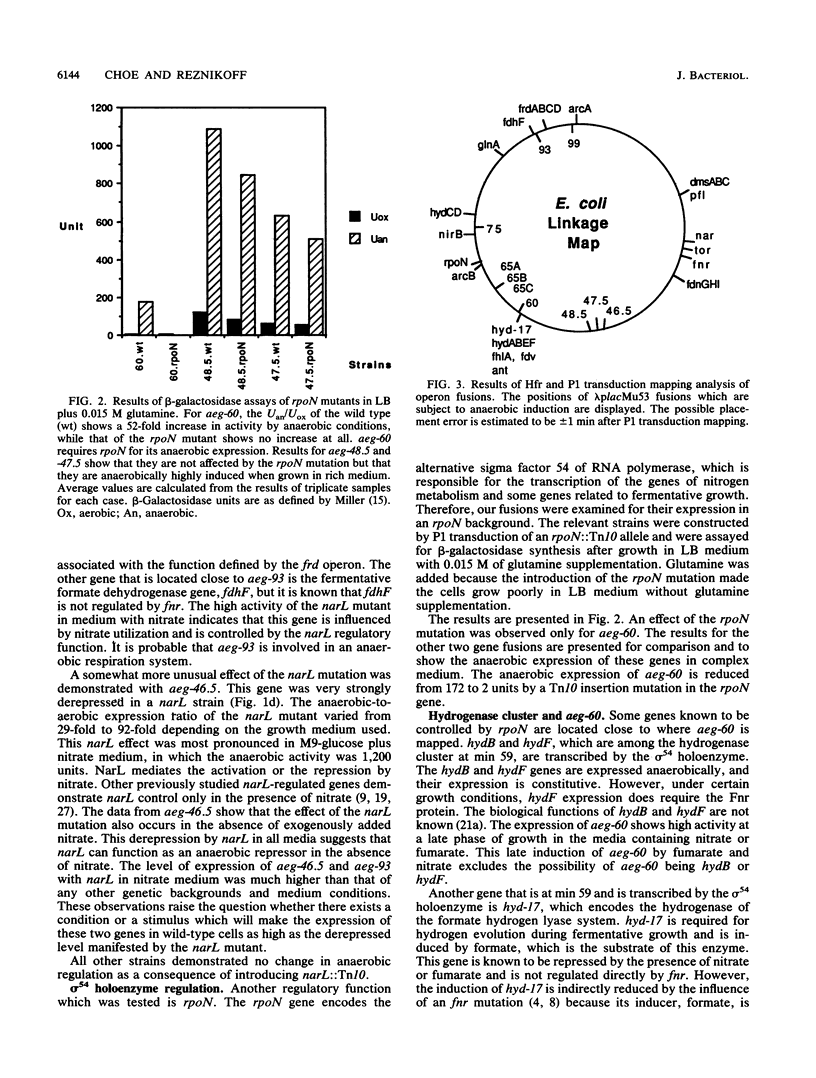
Genes that are expressed under anaerobic conditions were identified by operon fusion techniques with a hybrid bacteriophage of lambda and Mu, lambda placMu53, which creates transcriptional fusions to lacZY. Cells were screened for anaerobic expression on XG medium. Nine strains were selected, and the insertion point of the hybrid phage in each strain was mapped on the Escherichia coli chromosome linkage map. The anaerobic and aerobic expression levels of these genes were measured by beta-galactosidase assays in different medium conditions and in the presence of three regulatory mutations (fnr, narL, and rpoN). The anaerobically expressed genes (aeg) located at minute 99 (aeg-99) and 75 (aeg-75) appeared to be partially regulated by fnr, and aeg-93 is tightly regulated by fnr. aeg-60 requires a functional rpoN gene for its anaerobic expression. aeg-46.5 is repressed by narL. aeg-65A and aeg-65C are partially controlled by fnr but only in media containing nitrate or fumarate. aeg-47.5 and aeg-48.5 were found to be anaerobically induced only in rich media. The effects of a narL mutation on aeg-46.5 expression were observed in all medium conditions regardless of the presence or absence of nitrate. This suggests that narL has a regulatory function in the absence of exogenously added nitrate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aliabadi Z., Warren F., Mya S., Foster J. W. Oxygen-regulated stimulons of Salmonella typhimurium identified by Mu d(Ap lac) operon fusions. J Bacteriol. 1986 Mar;165(3):780–786. doi: 10.1128/jb.165.3.780-786.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkmann A., Sawers R. G., Böck A. Involvement of the ntrA gene product in the anaerobic metabolism of Escherichia coli. Mol Gen Genet. 1987 Dec;210(3):535–542. doi: 10.1007/BF00327209. [DOI] [PubMed] [Google Scholar]
- Birkmann A., Zinoni F., Sawers G., Böck A. Factors affecting transcriptional regulation of the formate-hydrogen-lyase pathway of Escherichia coli. Arch Microbiol. 1987 Jun;148(1):44–51. doi: 10.1007/BF00429646. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefoy V., Fons M., Ratouchniak J., Pascal M. C., Chippaux M. Aerobic expression of the nar operon of Escherichia coli in a fnr mutant. Mol Microbiol. 1988 May;2(3):419–425. doi: 10.1111/j.1365-2958.1988.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Bremer E., Silhavy T. J., Weinstock G. M. Transposable lambda placMu bacteriophages for creating lacZ operon fusions and kanamycin resistance insertions in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1092–1099. doi: 10.1128/jb.162.3.1092-1099.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter P. A., Gunsalus R. P. Oxygen, nitrate, and molybdenum regulation of dmsABC gene expression in Escherichia coli. J Bacteriol. 1989 Jul;171(7):3817–3823. doi: 10.1128/jb.171.7.3817-3823.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T. P., Magasanik B. Transcription of glnA by purified Escherichia coli components: core RNA polymerase and the products of glnF, glnG, and glnL. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8453–8457. doi: 10.1073/pnas.82.24.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S., Lin E. C. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1888–1892. doi: 10.1073/pnas.85.6.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. M., Gunsalus R. P. Regulation of Escherichia coli fumarate reductase (frdABCD) operon expression by respiratory electron acceptors and the fnr gene product. J Bacteriol. 1987 Jul;169(7):3340–3349. doi: 10.1128/jb.169.7.3340-3349.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman L. V., Gunsalus R. P. Nitrate- and molybdenum-independent signal transduction mutations in narX that alter regulation of anaerobic respiratory genes in Escherichia coli. J Bacteriol. 1990 Dec;172(12):7049–7056. doi: 10.1128/jb.172.12.7049-7056.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. F., DeMoss J. A. Location of sequences in the nar promoter of Escherichia coli required for regulation by Fnr and NarL. J Biol Chem. 1988 Sep 25;263(27):13700–13705. [PubMed] [Google Scholar]
- Morett E., Buck M. In vivo studies on the interaction of RNA polymerase-sigma 54 with the Klebsiella pneumoniae and Rhizobium meliloti nifH promoters. The role of NifA in the formation of an open promoter complex. J Mol Biol. 1989 Nov 5;210(1):65–77. doi: 10.1016/0022-2836(89)90291-x. [DOI] [PubMed] [Google Scholar]
- Sankar P., Shanmugam K. T. Biochemical and genetic analysis of hydrogen metabolism in Escherichia coli: the hydB gene. J Bacteriol. 1988 Dec;170(12):5433–5439. doi: 10.1128/jb.170.12.5433-5439.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar P., Shanmugam K. T. Hydrogen metabolism in Escherichia coli: biochemical and genetic evidence for a hydF gene. J Bacteriol. 1988 Dec;170(12):5446–5451. doi: 10.1128/jb.170.12.5446-5451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers G., Böck A. Anaerobic regulation of pyruvate formate-lyase from Escherichia coli K-12. J Bacteriol. 1988 Nov;170(11):5330–5336. doi: 10.1128/jb.170.11.5330-5336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers G., Böck A. Novel transcriptional control of the pyruvate formate-lyase gene: upstream regulatory sequences and multiple promoters regulate anaerobic expression. J Bacteriol. 1989 May;171(5):2485–2498. doi: 10.1128/jb.171.5.2485-2498.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M., Baker T. A., Schnitzler G., Deischel S. M., Goel M., Dove W., Jaacks K. J., Grossman A. D., Erickson J. W., Gross C. A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989 Mar;53(1):1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. W., Neidhardt F. C. Proteins induced by anaerobiosis in Escherichia coli. J Bacteriol. 1983 Apr;154(1):336–343. doi: 10.1128/jb.154.1.336-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro S., Guest J. R. Regulation and over-expression of the fnr gene of Escherichia coli. J Gen Microbiol. 1987 Dec;133(12):3279–3288. doi: 10.1099/00221287-133-12-3279. [DOI] [PubMed] [Google Scholar]
- Spiro S., Roberts R. E., Guest J. R. FNR-dependent repression of the ndh gene of Escherichia coli and metal ion requirement for FNR-regulated gene expression. Mol Microbiol. 1989 May;3(5):601–608. doi: 10.1111/j.1365-2958.1989.tb00207.x. [DOI] [PubMed] [Google Scholar]
- Stewart V., Berg B. L. Influence of nar (nitrate reductase) genes on nitrate inhibition of formate-hydrogen lyase and fumarate reductase gene expression in Escherichia coli K-12. J Bacteriol. 1988 Oct;170(10):4437–4444. doi: 10.1128/jb.170.10.4437-4444.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V., Parales J., Jr Identification and expression of genes narL and narX of the nar (nitrate reductase) locus in Escherichia coli K-12. J Bacteriol. 1988 Apr;170(4):1589–1597. doi: 10.1128/jb.170.4.1589-1597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V., Parales J., Jr, Merkel S. M. Structure of genes narL and narX of the nar (nitrate reductase) locus in Escherichia coli K-12. J Bacteriol. 1989 Apr;171(4):2229–2234. doi: 10.1128/jb.171.4.2229-2234.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman J. W., Clark D. P. Anaerobically induced genes of Escherichia coli. J Bacteriol. 1986 Jul;167(1):362–367. doi: 10.1128/jb.167.1.362-367.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]



