Abstract
As a result of terrorism, accident, or war, populations potentially can be exposed to doses of ionizing radiation that could cause direct clinical effects within days or weeks. There is a critical need to determine the magnitude of the exposure to individuals so that those with significant risk have appropriate procedures initiated immediately, while those without a significant probability of acute effects can be reassured and removed from the need for further consideration in the medical/emergency system. In many of the plausible scenarios there is an urgent need to make the determination very soon after the event and while the subject is still present. In vivo EPR measurements of radiation-induced changes in the enamel of teeth is a method, perhaps the only such method, which can differentiate among doses sufficiently for classifying individuals into categories for treatment with sufficient accuracy to facilitate decisions on medical treatment. In its current state, the in vivo EPR dosimeter can provide estimates of absorbed dose with an error approximately ± 50 cGy over the range of interest for acute biological effects of radiation, assuming repeated measurements of the tooth in the mouth of the subject. The time required for acquisition, the lower limit, and the precision are expected to improve, with improvements in the resonator and the algorithm for acquiring and calculating the dose. The magnet system that is currently used, while potentially deployable, is somewhat large and heavy, requiring that it be mounted on a small truck or trailer. Several smaller magnets, including an intraoral magnet are under development, which would extend the ease of use of this technique.
Keywords: in vivo, retrospective dosimetry, EPR, teeth, radiation accidents, terrorism, after-the-fact dosimetry
1. Introduction and Overview of The Problem
There is a very signifciant possibility that terrorists in the near future will initiate an event in which large numbers of people will be potentially subject to significant doses of ionizing radiation. Such exposures also continue to be a very plausible scenario during the course of warfare. An accident could lead to similar concerns. In any such radiation event, the needs for rapid and accurate dose assessment are clear. Even if the exposures are unlikely to result in acute illness, experiences such as the reaction of the public to the Three Mile Island incident and the biological consequences of the disaster at Chernobyl have vividly illustrated the panic and uncertainty that can occur if populations potentially have been exposed to unknown doses of ionizing radiation. The panic and disruption are likely to be even greater in the event of the release of a “dirty bomb” or other acts of terrorism or in the course of nuclear warfare, which involve potential exposures to unknown amounts of radiation.
The appropriate management of such situations requires a capability to determine the magnitude of the exposure to individuals. While it is likely that many of the individuals will not have received clinically significant doses of radiation, the concern and probable panic from the uncertainty about the dose that has been received would be very damaging. And, of course, it is very desirable to be able to determine which individuals have received clinically significant exposures and the magnitude of the exposures. Individuals with significant risk could then have appropriate procedures initiated immediately, while those without a significant probability of acute effects could be reassured and removed from the need for further medical treatment. The determination of dose should be made while the subject is still present. As illustrated by the experience with hurricane Katrina as well as numerous other events, when major disruptions of infrastructure occur, it becomes extremely difficult to reconnect with individuals after they disperse from the scene.
Recent efforts in the development of radiation-mitigating agents (which is a prominent theme in the Centers for Medical Countermeasures for Radiation developed by NIH) further increase the needs for making a prompt and accurate assessment of risk of significant clinical effects from ionizing radiation. Many or most of these are likely to be most effective when they are delivered as soon as possible after the exposure occurs. Effective mitigating agents, like essentially all therapeutic measures, will have a significant risk of causing harm as well as having potential benefits. So they cannot be administered effectively in the absence of strong evidence of exposure, without having a high risk that they may do more harm than good. The mitigating agents also are likely to have a range of doses in which their use is appropriate, and therefore the accuracy of the dose assessment will be important for this use also.
There are several potential methodological approaches to determine the radiation dose to individuals after a radiation incident:
reconstruction of the dose patterns in the exposed environment
measurements based on objective biological changes such as radiation-induced changes in chromosomes
assessments based on clinical signs and symptoms
measurements based on a physical change associated with the individual
Reconstruction of the pattern of dose distribution would be useful but is unlikely to be able to be done in a timely and effective manner. In addition to the potential technical difficulties in making such an assessment promptly and accurately, there are a number of other factors that would make such assessments not likely to be useful for resolving the problem of dose estimation to the individuals. The accurate placement of the individual in the calculated radiation field will be difficult, especially under the chaotic conditions that are likely to accompany an incident in which radiation is released. In addition, there is likely to be a high degree of skepticism by the public as to the validity and accuracy of pronouncements of exposure and risk by the authorities, especially if the message is that no significant exposures occurred. The problems of credibility of official announcements have been demonstrated well in previous incidents in which radiation was released, such as the Chernobyl and Three Mile Island events.
The use of biological markers, such as chromosomal changes in blood cells to measure exposure, has some very attractive features. It has a long history of use to assess radiation exposures. The results would be based on measurements in the individual, indicating the total effective exposure to the individual, especially for the hematopoetic system. There are some additional well-characterized biological effects of ionizing radiation that occur in the dose range of interest. Unfortunately, these also have some very significant limitations for applications in an incident where large numbers of individuals are potentially involved and an immediate assessment is desired. The current methods such as dicentric assays and FISH require that samples be obtained from the individuals (which may be difficult under field conditions), sent to a laboratory where expert personnel can make the assessment (which severely limits the capacity to process large numbers of samples), the need to wait for relatively long periods of time for the changes to become manifest (which results in delays of up to several days before the results are available), and then a need to locate the individuals from whom the samples were taken. The quantitative relationship between some of the changes and radiation dose is not fully quantitative.
While the continuing clinical treatment of exposed individuals ultimately will be strongly based on the signs and symptoms of the patient and not just on the measurements, the signs and symptoms will provide very limited guidance for early determination of radiation dose. That is because clinically manifested biological effects, such as the various radiation syndromes, require considerable time for the effects to become evident, except for extraordinarily high doses. While it has been suggested that the time to nausea or vomiting might be a useful guideline, this seems unsuitable in the context of the confusion and panic that is likely to occur in an event with the potential for mass exposure. Individuals vary in the degree that they experience nausea and vomiting, and the presence of other persons with these symptoms greatly affects the incidence of these symptoms. The general confusion and trauma may limit the ability of individuals to remember accurately when they experienced the symptoms. Also, it would be quite feasible for terrorists to include emetic agents within the radiation-releasing device.
Methods based on physical changes in the individual that would be measured immediately and would not require processing are an attractive alternative, in principle. These would not require the evolution or processing needed for the biologically based methods and would be more readily calibrated. While several different physical methods have been suggested, such as radiation-induced changes that can give rise to luminescence (Godfrey-Smith et. al, 1997), currently there is only one physical method that appears to be suitable at this time. This is electron paramagnetic resonance (EPR or, sometimes termed ESR). This is based on long-lived radiation-induced paramagnetic species that are stabilized in the matrix of teeth and bones or the keratin of fingernails, toenails, or hair. Ionizing radiation creates unpaired electron species as a direct result of its interactions with molecules. While most of these react immediately and disappear, some will be stabilized for long periods of time 108-1011 years if they are generated in an appropriate matrix. The hydroxyapatite component of bone and teeth is an especially effective such matrix. This phenomenon previously has been exploited to make measurements on isolated teeth, but not in vivo.
We report here such a method, based on in vivo EPR of teeth, which appears to meet most or all of the requirements for after-the-fact dosimetry applicable to individuals. (Several other papers in this issue report on the potential use of fingernails and toenails). The characteristics of in vivo EPR dosimetry based on teeth that are especially appropriate for the problem include:
Sufficient sensitivity to measure clinically relevant doses
Provides unambiguous data that is sufficient to make the differentiation into the designated dose subclasses
Applicable to individuals
Non-invasive
Can be measured at any time after the radiation exposure
Provides the data rapidly, while the subject is still present
Can operate in a variety of environments
Can be operated by minimally trained individuals
In assessing the potential value of in vivo EPR dosimetry, it is essential to focus on what are the real minimum requirements for this type of retrospective dosimetry. At the most basic level this would be to be able to determine reliably whether:
the individual has received a dose below that which has a significant probability of leading to near term acute symptomatology
the individual has received a dose that has a significant probability of leading to near term acute symptomatology
the individual has received a dose above that for which there is a significant probability that therapeutic intervention will not be effective.
It is important to keep in mind what information on exposure dose is needed for effective medical decision-making: A threshold of about 150 cGy and an accuracy of about ± 50 - 75 cGy would be more than adequate for this purpose. It appears that in vivo EPR dosimetry will readily meet these criteria. This would probably still be the case even with uncertainties as high as ± 100 cGy. This is because the state of knowledge of the quantitative relationship between dose and the clinical response of human subjects to ionizing radiation is limited. It probably also is true that even with more extensive data, the intrinsic variation in the response of individuals to ionizing radiation is sufficiently large that very precise information on the doses could not usefully improve the categorization of the subjects into action classes.
Of course, it would be desirable, for other purposes, to have the sensitivity and accuracy of the technique to be greater than that discussed above, for some uses such as estimating the probability of long term effects from the exposure such as carcinogenesis. Although not a subject of this paper, there are some potential means to achieve such capabilities with in vivo EPR dosimetry, especially by extending the time of measurement.
2. Use of In Vivo EPR Spectroscopy for Retrospective Dosimetry
Ionizing radiation generates free radicals proportionate to the dose. In aqueous systems these radicals react very rapidly and disappear, but in drier systems, especially those with organized matrices, they can persist indefinitely. This phenomenon has been recognized in bone and teeth for more than 50 years (Brady et al., 1968; Gordy et al., 1955; Swartz, 1965; Swartz et al., 1965) and has been shown to be a feasible method for retrospective dosimetry, based on the free radicals being stabilized in the hydroxyl apatite matrix.
Subsequently, this technique has been used productively on isolated teeth and bones to measure radiation doses from radiation accidents and heavy environmental contamination. Using this approach, it has been possible to measure doses in the range of a few cGy in isolated teeth. Teeth are especially attractive because the signal intensity is stronger in them because of the high stability of the unpaired electrons in enamel. This approach has been used extensively in analysis of exposures in the former Soviet Union (Chumak et al. 1998; Ishii et al. 1990a; Ivannikov et al., 1997; Ivannikov et al., 2002; Romanyukha et al., 1994; Romanyukha et al., 1999; Romanyukha et al., 2000), and in survivors from the atomic weapons in Japan (Hoshi et al., 1985; Ikeya et al., 1984; Ikeya et al., 1998). The results obtained with EPR dosimetry have been shown to correlate well with hematologically based assays and other types of dose estimates (Nakamura et al., 1996; Ilyinskikh et al., 1999; Ilyinskikh et al., 2000; Rossi et al., 2000; Serezhenkov et al., 1992; Sevan'kaev et al., 2005; Sevan'kaev et al., 2006). Those dosimetry studies were carried out in isolated teeth at conventional EPR frequencies (e.g. 9 GHz). This frequency has high sensitivity for estimation of dose with isolated teeth measured in vitro. The isolated teeth can be processed to enhance sensitivity, physically concentrating the components that have the EPR signal and removing aqueous components that lead to non-specific absorption of the microwave. Cooperative and comparative studies have confirmed the feasibility of obtaining highly accurate results with this technique (Chumak et al., 1999; IAEA, 2002; Kleinerman et al., 2006).
With the recent development of lower frequency EPR (e.g. 1 GHz) for making measurements in vivo with good sensitivity, it becomes possible to assess the amount of irradiated dose in vivo, eliminating the need to have isolated teeth. This is feasible because the lower frequency has greater tolerance for the presence of water, and also can measure relatively large volumes, such as several teeth. The sensitivity of in vivo EPR is less than that obtained with isolated teeth because of the lower frequency and the need to make the measurements in unprocessed samples within the confines of the mouth. These obstacles now have been overcome and the feasibility of this approach has been shown in animals, and its potential for use in human subjects was demonstrated by making measurements in isolated human teeth and comparing these measurements with isolated rat teeth and with rat teeth measured in situ in vivo (Miyake et al., 2000). Building on these results, measurements were made in human subjects using the placement of irradiated teeth in the mouths of volunteers (Iwasaki et al., 2005). We describe here the most recent results, including the first measurements in subjects whose teeth were irradiated during the course of radiation therapy. This paper focuses on general aspects; more specific aspects including methods of dose reconstruction and technical details of EPR measurements are covered in two related papers in this same issue (Demidenko et al., Williams, et al.)
3. Description of the In Vivo EPR Spectrometer for Dosimetry
3.1. Overview
EPR is a magnetic technique with considerable analogy to NMR, requiring an exciting field and a magnetic field with an appropriate relationship between them to achieve resonance absorption by the unpaired electron. The in vivo EPR dosimeter uses a lower frequency EPR spectrometer (1.2 GHz) with specially designed resonators that can make measurements in teeth in situ in the mouth; a magnet system that can comfortably and effectively encompass the human head; data processing to maximize sensitivity and provide an output useable by modestly trained personnel; and instrumentation that is suitable for use at the site of the potential exposures.
3.2. The magnet
The properties of the magnet are a crucial aspect for the ability to have the potential for widespread field applications. There is a need for having satisfactory homogeneity over the volume that will be studied (typically this requires uniformity on the order of ± 0.1 G), while minimizing the size and complexity of the magnet. The usual size and weight of a conventional electromagnet makes it difficult to meet the logistical requirements for field deployment, and such magnets also require considerable amounts of power. Therefore, we have pursued several alternative approaches that can meet the requirements. These involve the use of permanent magnets and/or specially configured magnets that are small but provide the necessary homogeneity at the site of the measurement.
The first approach, which already has been implemented for the initial studies, is to use a large conventional permanent magnet with a gap that can accommodate the subject. At the present time this requires that subjects be brought to the magnet, which adds significant time and logistical problems for dealing rapidly with potential exposures, but there certainly would be many circumstances where this would be entirely acceptable. This configuration also would be quite suitable for field deployment, using a small pick-up truck or trailer to transport it and to serve as the operating platform. This development is quite straightforward and we anticipate having such a unit available within a few months (and if necessary, the existing system probably could be deployed within hours to days to meet urgent needs until the transportable versions are regularly available). Figure 1 shows this magnet with a volunteer within it.
Figure 1.
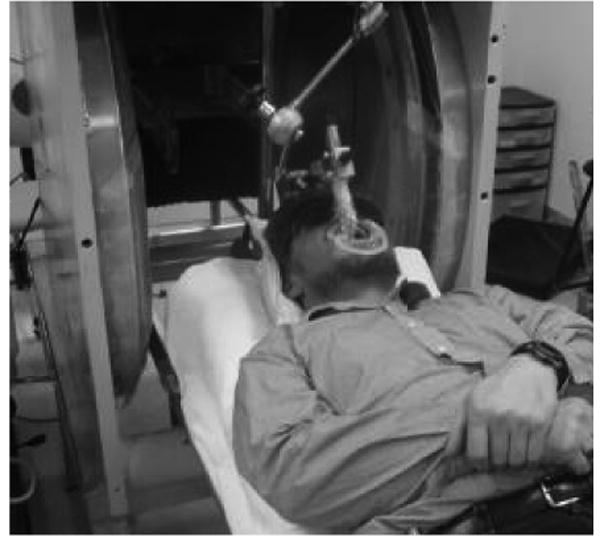
Clinical magnet with a volunteer in it.
The second approach that is being actively pursued is the development of a smaller magnet that will accommodate the head. This is planned to be an integral unit to which the resonator and modulation coils also are mounted. Several designs for the magnet have been successfully developed, which indicates that it will be quite feasible to achieve the required homogeneity of the magnetic field using a permanent magnet. Alternatively these configurations could be based on electromagnets. We are pursuing two types of magnets for this purpose: a flat magnet and a conventional two pole magnet. Figure 2 shows the calculated design for a flat permanent magnet with the desired characteristics. The open access aspects of this device are very attractive. The potential limiting aspect is the size and position of the homogeneous volume. The design indicates that this will be satisfactory, and construction is proceeding on a prototype based on a permanent magnet. Figure 3 shows the design capabilities for a type of magnet that could be used in a helmet-like configuration. While a bit more confining in terms of positioning, this type of magnet can more readily have a higher volume of sufficient homogeneity. This type of magnet also seems quite feasible and prototypes based on permanent magnets are in the process of being constructed.
Figure 2.
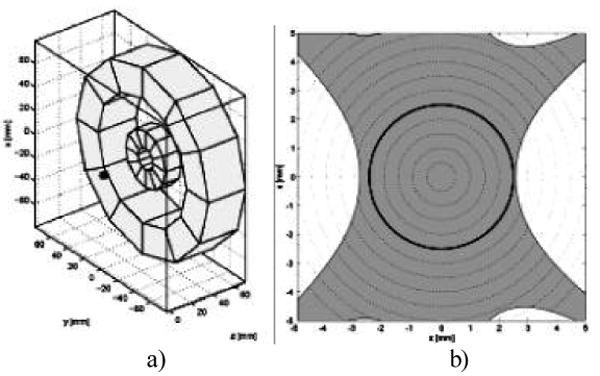
Flat permanent magnet design. (a) Topical Application (b) Field calculation. Field inhomogeneity is calculated on the x-z plane, y=0, containing the 5mm DSV, black circumference. Field inhomogeneity within 500 ppm is in light grey.
Figure 3.
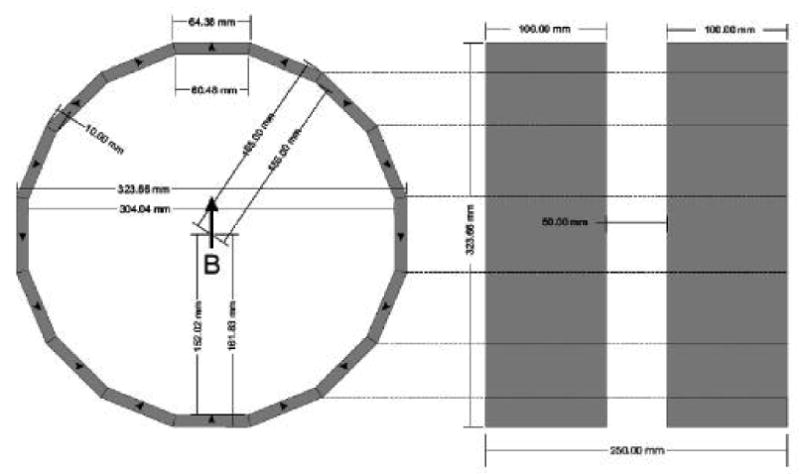
Design of a helmet type magnet, with 42 mT, 60ppm homogeneity.
The third approach is to develop a magnet that could fit within the mouth, probably being integrated with the resonator. This is a challenging task but if achieved, would be a very desirable development. Such a small device could be widely distributed and be brought rapidly to the site where deployment is needed. If fully accomplished the relationship between the resonator and the magnet would be fixed, completely removing one potential source of uncertainty. Figure 4 shows the design of such a magnet. This is being actively refined and we hope to be able to initiate construction in the near future.
Figure 4.
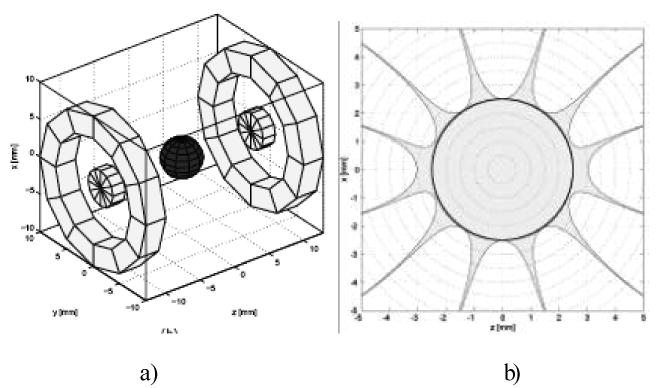
Permanent intra-oral magnet design. a) Grey rings and disks denote permanent material with uniform magnetization in the +z direction. The grey sphere gives the 5mm DSV, b) Field calculation of symmetric intra-oral permanent magnet dipole design. Field inhomogeneity is calculated on the x-z plane, y=0, containing the 5mm DSV, black circumference. Field inhomogeneity within 500 ppm is in light grey.
In summary the magnet should not be a limiting factor for field-deployable EPR dosimetry. A design that is completely feasible for field use is being used currently and variants that would be even more convenient and more readily deployed are in active development.
3.3. Resonators
The resonators are a key element of the dosimeter, being the most important component for achieving high sensitivity. The geometric constraints for obtaining data from the mouth and the irregular shape of the teeth require the development of special resonators. Some of the basic aspects are covered in another article within this issue (Williams, et al.) and therefore will not be repeated here. Ikeya and colleagues have described the general conceptual approach, anticipating that the measurements would be made at high frequencies (Ikeya, 1993; Ishii et al., 1990b; Yamanaka et al., 1993). While this turned out to be difficult because of limitations due to poor penetration and stability with the higher frequencies, subsequent developments by others (Zdravkova et al., 2002; Zdravkova et al., 2003a, 2003b) at lower frequencies now have made it practical to implement this approach with the requisite sensitivity. The EPR signal is located principally in the enamel of the teeth, so the optimization of the sensitive volume of the resonator focuses on probing the maximum amount of enamel. The highest amount of enamel is on the biting surfaces of the molars. Unlike the incisors, the molars have minimum probability of having potentially confusing UV induced EPR signals. While initially we focused on measurements of single teeth, recently we recognized that all of the teeth will be exposed in subjects who have been exposed to ionizing radiation. This insight, coupled with the understanding that the distribution of the sensitive volume is symmetrical above and below the loop of the resonator, has led to a recent focus on positioning the loop of the resonator to probe pairs of upper and lower teeth. The result is a dramatic increase in the amplitude of the radiation-induced EPR signal (see figure 5).
Figure 5.
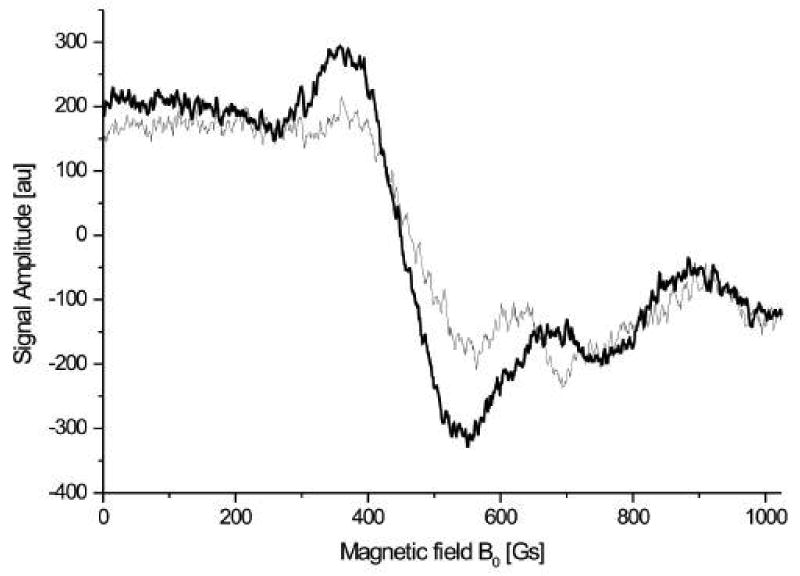
Spectra from measuring two teeth (upper and lower) simultaneously.
3.4. Data acquisition and data processing (analysis)
There are several challenges for data acquisition and processing for in vivo EPR dosimetry. With a goal to make the measurements quickly while achieving sufficient sensitivity, it is essential that all aspects be optimized. The EPR signals observed in irradiated teeth include at least three components:
The radiation-induced signal due to radiation-induced unpaired electrons that are stabilized in the hydroxyapatite
The partially overlapping background signal (Iwasaki et al., 2004) that is present in all teeth
Noise, which may arise from the instruments and/or the environment around the instrument
With isolated teeth, the dose-response for the tooth can be determined with the use of added well-known doses, with extrapolation back to the original dose. This enables one to deal explicitly with the contribution due to background signals plus any coherent noise that might be present. This option, of course, cannot be used with teeth in vivo. Instead, we either need to make an assumption about the nature and intensity of the background signal or to establish the means to measure it in the presence of the radiation-induced signals. While the latter potentially is feasible because we know the characteristics of the background signal from extensive measurements of unirradiated teeth, those measurements indicate that the background signal is relatively constant and therefore can be removed by use of an empirical calibration curve based on the measurements in vivo of teeth whose radiation dose is well characterized. In the future if further refinements are needed, we could obtain the background signal of the particular teeth being measured by careful measurements under conditions that differentiate between the radiation and background signals. The parameters that are likely to be used for this purpose are the modestly different g-factor and power saturation characteristics of the background signal.
Because the signal/noise of the spectra are likely to be limited because the signal is relatively weak and we wish to minimize the acquisition time in order to maximize throughput, we have devoted considerable effort to post-processing methods for isolating the radiation-induced signal. Some of the approaches that we have used and the initial results of those methods are summarized in a companion paper. Briefly, we can utilize our knowledge of the spectral properties of the radiation induced signals (which can be determined by the use of data from in vitro or in vivo measurements of teeth with known radiation doses measured under spectroscopically rigorous conditions—especially the use of long acquisition times) and the measurements of standards that are included in the measurement procedures. While significant progress has been made, the methods are not yet fully optimized. Currently the limiting factor for obtaining highly reproducible results among measurements is the ability to place the resonator in exactly the same optimal position relative to the tooth or teeth.
3.5. Results from measurements in vivo
While in principle the sensitivity and accuracy of measurements made with the apparatus using isolated teeth should be similar to those made within the mouth, there are several considerations that require that this assumption be tested rigorously. In particular, the presence of the oral structures could degrade the quality of the measurement because of effects on the microwaves that are used in the measurements. In order to make EPR measurements under conditions that are virtually identical to the way in which measurements would be made with subjects that had potentially been exposed to radiation, we have carried out a series of studies in which one or two molar teeth that previously have been irradiated to a known dose are inserted into the mouth of volunteers, usually at sites where the normal dentition is missing. Figure 6 shows typical spectra obtained from a volunteer in which teeth with various doses were measured in a series of studies on the same day. The conditions for the acquisition are shown in the figure legend. Of particular note is that these spectra were obtained with a measurement time of less than 5 minutes. When this experiment was repeated five times on five days, we obtained the dose response relationship indicated in Figure 7. This type of dose resolution is what is required for EPR dosimetry to fully meet the requirements for effective triage of clinically significant exposures. It should be noted, however, that the procedure used to obtain these results included five repetitions, each of which involved about five minutes acquisition per tooth. While such a procedure would be impractical for triage in the field for large numbers of subjects, it shows the potential to achieve this capability. On the basis of our analysis of the procedures, we believe that the current limiting step is the ability to place the resonator at the same position completely reproducibly and accordingly; this is now a very high priority for our developmental efforts. It also should be noted that these measurements were made on a single tooth, while in the event of a real exposure, all of the teeth will have been exposed and therefore the measurement will be based on acquisition of spectra simultaneously from two or more teeth.
Figure 6.
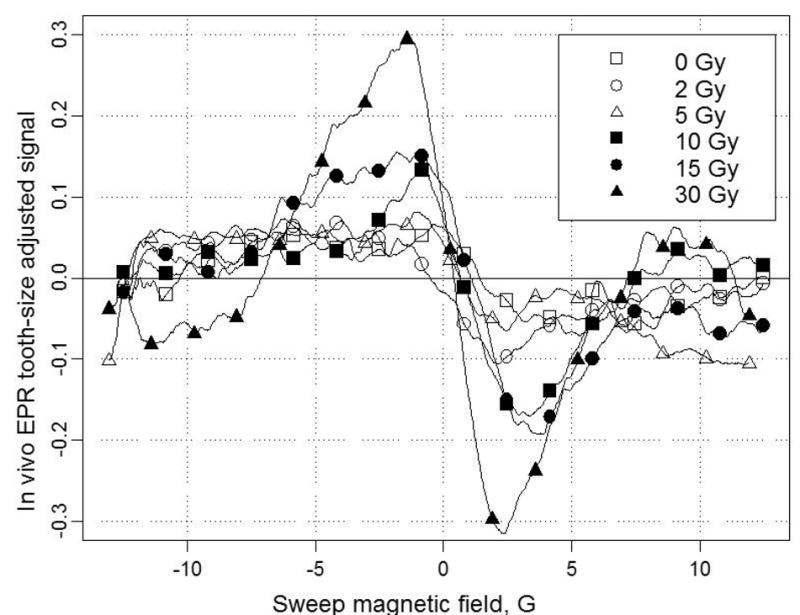
Typical EPR spectra in the mouth of a volunteer with various doses measured on the same day.
Figure 7.
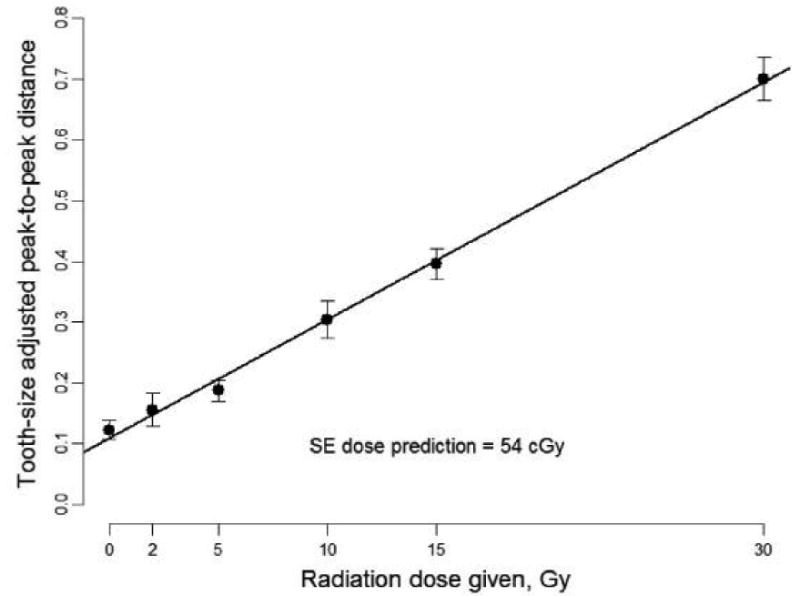
Dose response relationship for 6 irradiated teeth with the peak-to-peak EPR signal amplitude averaged over 5 days.
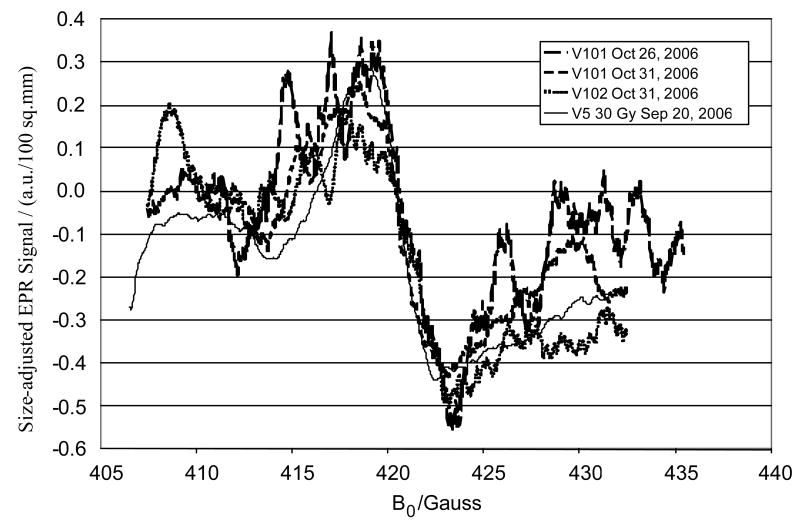
We have begun to make measurements in volunteers whose teeth have been irradiated in the course of radiation therapy. The dose to the teeth is known quite accurately from the treatment plan. This provides us with an opportunity to develop the method further under more realistic conditions. Figure 8 shows the first results in a volunteer. Because of the status of his dentition, measurements were made with the loop positioned over a canine tooth and a premolar tooth and therefore we did not probe the maximum amount of enamel that would be available with intact molars. The results, however, were very encouraging. As noted in Figure 8 the calculated dose was 25.5 Gy while the measured dose on the two repetitions (done on different days) was 32 and 29 Gy.
Figure 8.
In vivo L-band EPR spectra from two volunteers with radiation therapy. V5 line represents in vivo L-band EPR spectra from a 30Gy irradiated teeth that served as comparison.
4. Conclusions
In its current state, the in vivo EPR dosimeter can provide estimates of absorbed dose with an error approximately ± 50 cGy over the range of interest for acute biological effects of radiation, assuming repeated measurements of the tooth in the mouth of the subject. This is expected to improve, with improvements in the resonator, the algorithm for calculating dose, and the uniformity of the magnetic field. In its current state of development, it probably is sufficient for most applications related to terrorism or nuclear warfare, and for decision-making for individuals concerning acute effects from exposure to ionizing radiation.
Acknowledgments
This work was supported by “In Vivo EPR Dosimetry System for Retrospective Measurement of Clinically Significant Acute Radiation Exposures,” Dept. of Defense # MD A905-02-C-0011 (DTRA) and used the facilities of the “EPR Center for the Study of Viable Systems”, NIH (NIBIB) grant P41 EB002032. We wish to thank the National Diseases Research Interchange (NDRI) for procurement of the teeth used in our experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brady JM, Aarestad NO, Swartz HM. In vivo dosimetry by electron spin resonance spectroscopy. Health Phys. 1968;15:43–47. doi: 10.1097/00004032-196807000-00007. [DOI] [PubMed] [Google Scholar]
- Chumak V, Sholom S, Pasalskaya L. Application of high precision EPR dosimetry with teeth for reconstruction of doses to Chernobyl populations. Radiation Protection Dosimetry. 1999;84(1–4):515–520. [Google Scholar]
- Chumak V, Likhtarev I, Sholom S, Meckback R, Krjuchkov V. Chernobyl experience in field of retrospective dosimetry: reconstruction of doses to the population and liquidators involved in the accident. Radiat Prot Dos. 1998;77:91–95. [Google Scholar]
- Demidenko E, Williams B, Sucheta A, Dong R, Swartz H. Radiation dose reconstruction from L-band in vivo EPR spectroscopy of intact teeth: Comparison of methods. Radiation Measurements. doi: 10.1016/j.radmeas.2007.05.025. This issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey-Smith DI, Pass B. A new method of retrospective radiation dosimetry: optically stimulated luminescence in dental enamel. Health Physics. 1997;72:744–749. doi: 10.1097/00004032-199705000-00010. [DOI] [PubMed] [Google Scholar]
- Gordy W, Ard W, Shields H. Microwave spectroscopy of biological substances. Paramagnetic resonance in X-irradiated amino acids and proteins. Proc Nat Acad Sci U S A. 1955;41:983–996. doi: 10.1073/pnas.41.11.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi M, Sawada S, Ikeya M, Miki T. ESR dating and dosimetry. Ionic; Tokyo: 1985. ESR dosimetry for A-bomb survivors; pp. 407–414. [Google Scholar]
- IAEA Report. Report of a coordinated research project. Vienna: 2002. Use of electron paramagnetic resonance dosimetry with tooth enamel for retrospective dose assessment. IAEA-TECDOC-1331. [Google Scholar]
- Ikeya M. New application of electron spin resonance – dating, dosimetry and microscopy. Singapore: Word Scientific; 1993. [Google Scholar]
- Ikeya M, Miyajima J, Okajima S. ESR dosimetry for atomic bomb survivors using shell buttons and tooth enamel. Jpn J Appl Phys. 1984;23:697–699. [Google Scholar]
- Ilyinskikh NN, Ilyinskikh IN, Porovskiy VA, Natarajan AT, Suskov II, Smirenniy LN, Ilyinskikh EN. Biodosimetry results obtained by various cytogenetic methods and electron spin resonance spectrometry among inhabitants of a radionuclide contaminated area around the Siberian chemical plant (Tomsk-7) Mutagenesis. 1999;14:473–478. doi: 10.1093/mutage/14.5.473. [DOI] [PubMed] [Google Scholar]
- Ilyinskikh NN, Ilyinskikh IN, Shakirov NN, Smirnov BV, Ilyinskikh EN. Monitoring of radiation-exposed people close to Mayak nuclear facility in the Chelyabinsk region (Russia) using different biodosimetric methods. Environ Monit Assess. 2000;61:345–359. [Google Scholar]
- Ishii H, Ikeya M. An electron spin-resonance system for in vivo human tooth dosimetry. Japanese Journal of Applied Physics. Part 1. 1990a;29:871–875. [Google Scholar]
- Ishii H, Ikeya M, Okano S. ESR dosimetry of teeth of residents close to the Chernobyl reactor accident. J Nucl Sci Tech. 1990b;27:1153–1155. [Google Scholar]
- Ivannikov AI, Skvortzov VG, Stepanenko VF, Tikunov DD, Fedosov IM, Romanyukha AA, Wieser A. Wide-scale EPR retrospective dosimetry: results and problems. Radiat Prot Dosim. 1997;71:175–180. [Google Scholar]
- Ivannikov AI, Zhumadilov Zh, Gusev BI, Miyazawa Ch, Liao L, Skvortsov VG, Stepanenko VF, Takada J, Hoshi M. Individual dose reconstruction among residents living in the vicinity of the Semipalatinsk Nuclear Test Site using EPR spectroscopy of tooth enamel. Health Phys. 2002;83:183–196. doi: 10.1097/00004032-200208000-00004. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Grinberg O, Walczak T, Swartz HM. In vivo measurements of EPR signals in whole human teeth. Appl Radiat Isot. 2005;62:87–90. doi: 10.1016/j.apradiso.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Walczak T, Grinberg O, Swartz HM. Differentiation of the observed low frequency (1200 MHz) EPR signals in whole human teeth. Appl Radiat and Isotopes. 2005;62:133–139. doi: 10.1016/j.apradiso.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Kleinerman RA, Romanyukha AA, Schauer DA, Tuckerd JD. Retrospective Assessment of Radiation Exposure Using Biological Dosimetry: Chromosome Painting, Electron Paramagnand the Glycophorin A Mutation Assay. Radiation Research. 2006;166:287–302. doi: 10.1667/RR3273.1. [DOI] [PubMed] [Google Scholar]
- Miyake M, Liu KJ, Walczak T, Swartz HM. In vivo dosimetry of accidental exposures to radiation: experimental results indicating the feasibility of practical use in human subjects. Appl Rad Isotop. 2000;52:1031–1038. doi: 10.1016/s0969-8043(00)00053-1. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Miyazawa C, Akiyama M, Sawada S, Awa AA. A close correlation between electron spin resonance (ESR) dosimetry from tooth enamel and cytogenetic dosimetry from lymphocytes of Hiroshima atomic-bomb survivors. Int J Radiat Biol. 1998;73:619–727. doi: 10.1080/095530098141870. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Miyazawa C, Akiyama M, Sawada S, Awa AA. Biodosimetry: chromosome aberration in lymphocytes and electron paramagnetic resonance in tooth enamel from atomic bomb survivors. World Health Statistics Quarterly. 1996;49(1):67–71. [PubMed] [Google Scholar]
- Romanyukha AA, Ignatiev EA, Ivanov DV, Vasilyev AG. The distance effect on the individual exposures evaluated from the Soviet nuclear bomb test at Totskoye test site in 1954. Radiat Prot Dosim. 1999;86:53–58. [Google Scholar]
- Romanyukha AA, Ignatiev EA, Vasilenko EK, Drozhko EG, Wieser A, Jacob P, Keriim-Markus IB, Kleschenko ED, Nakamura N, Miyazawa C. EPR dose reconstruction for Russian nuclear workers. Health Phys. 2000;78:15–20. doi: 10.1097/00004032-200001000-00004. [DOI] [PubMed] [Google Scholar]
- Romanyukha AA, Regulla D, Vasilenko E, Wieser A. South Ural nuclear workers: comparison of individual doses from retrospective EPR dosimetry personal monitoring. Appl Radiat Isot. 1994;45:1195–1199. doi: 10.1016/0969-8043(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Rossi AM, Wafcheck CC, de Jesus EF, Pelegrini F. Electron spin resonance dosimetry of teeth of Goiania radiation accident victims. Appl Radiat Isot. 2000;52(5):1297–303. doi: 10.1016/s0969-8043(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Serezhenkov VA, Domracheva EV, Klevezal GA, Kulikov SM, Kuznetsov SA, Mordvintcev PI, Sukhovskaya LI, Schklovsky-Kordi NE, Vanin AF, Voevodskaya NV, Vorobiev AI. Radiation dosimetry for residents of the Chernobyl region: a comparison of cytogenetic and electron spin resonance methods. Radiat Prot Dosim. 1992;42:33–36. [Google Scholar]
- Sevan'kaev A, Khvostunov I, Lloyd D, Voisin P, Golub E, Nadejina N, Nugis V, Sidorov O, Skvortsov V. The suitability of FISH chromosome painting and ESR-spectroscopy of tooth enamel assays for retrospective dose reconstruction. J Radiat Res (Tokyo) 2006;47(A) A:75–80. doi: 10.1269/jrr.47.a75. [DOI] [PubMed] [Google Scholar]
- Sevan'kaev AV, Lloyd DC, Edwards AA, Khvostunov IK, Mikhailova GF, Golub EV, Shepel NN, Nadejina NM, Galstian IA, Nugis VYu, Barrios L, Caballin MR, Barquinero JF. A cytogenetic follow-up of some highly irradiated victims of the Chernobyl accident. Radiat Prot Dosim. 2005;113:152–161. doi: 10.1093/rpd/nch435. [DOI] [PubMed] [Google Scholar]
- Swartz HM. Long-lived electron spin resonances in rats irradiated at room temperature. Radiat Res. 1965;24:579–586. [PubMed] [Google Scholar]
- Swartz HM, Molenda RP, Lofberg RT. Long-lived radiation-induced electron spin resonances in an aqueous biological system. Biochem Biophys Res Comm. 1965;21:61–65. doi: 10.1016/0006-291x(65)90426-2. [DOI] [PubMed] [Google Scholar]
- Williams BB, Sucheta A, Dong R, Sakata Y, Iwasaki A, Burke G, Grinberg O, Lesniewski P, Kmiec M, Swartz HM. Experimental procedures for sensitive and reproducible in situ EPR tooth dosimetry. Radiation Measurements. doi: 10.1016/j.radmeas.2007.05.001. This issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka C, Ikeya M, Hara H. ESR cavities for in vivo dosimetry of tooth enamel. Appl Radiat Isotop. 1993;44:77–80. doi: 10.1016/0969-8043(93)90199-k. [DOI] [PubMed] [Google Scholar]
- Zdravkova M, Crokart N, Trompier F, Asselineau B, Gallez B, Gaillard-Lecanu E, Debuyst R. Retrospective dosimetry after criticalicality accidents using low-frequency EPR: A study of whole human teeth irradiated in a mixed neutron and gamma-radiation field. Radiation Research. 2003a;160:168–173. doi: 10.1667/rr3026. [DOI] [PubMed] [Google Scholar]
- Zdravkova M, Wieser A, El-Faramawy N, Gallez B, Debuyst R. An in vitro L-band electron paramagnetic resonance study of highly irradiated whole teeth. Radiat Prot Dosimetry. 2003b;101(14):497–502. doi: 10.1093/oxfordjournals.rpd.a006036. [DOI] [PubMed] [Google Scholar]
- Zdravkova M, Denis JM, Gallez B, Debuyst R. Sensitivity of whole human teeth to fast neutrons and gamma-rays estimated by L-band EPR spectroscopy. Radiat Meas. 2002;35:603–608. doi: 10.1016/s1350-4487(02)00125-7. [DOI] [PubMed] [Google Scholar]