Summary
The Escherichia coli Rsd protein binds tightly and specifically to the RNA polymerase (RNAP) σ70 factor. Rsd plays a role in alternative σ factor-dependent transcription by biasing the competition between σ70 and alternative σ factors for the available core RNAP. Here, we determined the 2.6 Å-resolution X-ray crystal structure of Rsd bound to σ70 domain 4 (σ704), the primary determinant for Rsd binding within σ70. The structure reveals that Rsd binding interferes with the two primary functions of σ704, core RNAP binding and promoter –35 element binding. Interestingly, the most highly conserved Rsd residues form a network of interactions through the middle of the Rsd structure that connect the σ704-binding surface with three cavities exposed on distant surfaces of Rsd, suggesting functional coupling between σ704 binding and other binding surfaces of Rsd, either for other proteins or for as yet unknown small molecule effectors. These results provide a structural basis for understanding the role of Rsd, as well as its ortholog, AlgQ, a positive regulator of Pseudomonas aeruginosa virulence, in transcription regulation.
Keywords: RNA, polymerase/Rsd/Sigma, factor/Transcription, regulation/X-ray, crystallography
Introduction
In bacteria, the 450 kDa RNA polymerase (RNAP) holoenzyme, comprising the evolutionarily conserved catalytic core (subunit composition α2ββ′ω) combined with the initiation-specific σ subunit, directs transcription initiation 1. The principal control point of gene expression in bacteria is transcription initiation, and a major mechanism by which bacteria regulate transcription initiation is through regulation of σ activity 2. The activity of most σ factors is determined by their cellular level, their affinity for RNAP, and their interactions with regulatory proteins that bind and modulate σ factor function.
In Escherichia coli (Ec), the primary σ factor, σ70, has the highest affinity for core RNAP and is also the most abundant σ factor throughout the growth cycle 3; 4. Thus, it is not clear how alternative σ factors capture sufficient core RNAP to express genes under their control. The discovery of the σ70-binding protein Rsd provided a partial solution to this problem 5. Biochemical and genetic studies demonstrated that Rsd can indirectly stimulate transcription from alternative σ factor-dependent promoters by binding to σ70 with a stoichiometry of 1:1 5–10. Mapping of the primary Rsd-binding determinant on σ70 was localized to σ70 domain 4 (σ704; 10–12, a structural domain of σ70 that binds the RNAP β-subunit flap-tip, recognizes the –35 promoter element, and is a target for many transcriptional activators) 13–17.
The amino acid sequence of Rsd is 55% similar to the sequence of the Pseudomonas aeruginosa (Paer) AlgQ transcription regulator. Paer is an opportunistic pathogen and causative agent of microbial corrosion. AlgQ is important in regulating the production of alginate, neuraminidase, and pyoverdine; factors that are necessary for promoting Paer virulence 18–22. Ec Rsd can complement AlgQ in the production of pyoverdine 18. Thus, it has been proposed that Paer AlgQ binds to and modulates the activity of Paer σ70 in a manner similar to Ec Rsd 11; 18.
Here, we describe the 2.6 Å-resolution X-ray crystal structure of the Ec σ704/Rsd complex. The results provide a structural basis for future experiments addressing the role of both Rsd and AlgQ in regulating σ activity.
Results
Crystallization, structure determination, and overall structure of the σ704/Rsd complex
Rsd appears to interact with Ec σ70 primarily through determinants within σ704 10–12. Although Ec σ704 on its own is poorly behaved for structural studies 23, co-expression of Ec σ704 (Ec σ70 residues 541–613) with Rsd resulted in a soluble complex that was purified to homogeneity with high yield (Materials and Methods). Alongside the Ec σ704/Rsd complex, Paer AlgQ and Paer σ704 were co-expressed and purification of the complex attempted. Although there is evidence that the proteins interact (Ref. 11; L.F.W. and S.A.D., unpublished), the complex of Paer proteins was unstable and did not survive the purification procedure (data not shown).
Rod-like crystals with approximate dimensions of 180 × 20 × 20 μm, space group p64 (a = b = 84.111 Å, c = 84.219 Å), were grown using vapor diffusion (Materials and Methods). The crystals contained one 26.7 kDa σ704/Rsd complex per asymmetric unit, with a solvent content of 64%. The crystals diffracted isotropically to 2.6 Å-resolution. The structure of the complex was solved by Multiwavelength Anomalous Diffraction 24 using data from selenomethionyl-substituted protein crystals (Table 1; Figure S1). Cycles of iterative model building and crystallographic refinement converged to an R/Rfree of 0.239/0.267 at 2.6 Å-resolution (Table 1).
Table 1.
Crystallographic Analysis
| Diffraction Data | ||||||||
|---|---|---|---|---|---|---|---|---|
| Data Set | Beamline | Wavelength (Å) | Resolution (Å) | No. of Reflections (tot./unique) | Completeness (%) (tot./last shell) | I/σ(I) (tot./last shell) | Rsymc (%) (tot./last shell) | No. of Sites |
| Native | NSLSa
X29 |
1.1 | 50–2.6 (2.7–2.6) | 144,443/10,405 | 99.1 (98.8) | 17.2 (2.2) | 8 (48.6) | |
| SeMet1(λ1) | APS
SGXb 31-ID-D |
0.9793 (peak) | 20–2.9 (3.0-2.9) | 117,509/7,527 | 98.2 (87.1) | 8.5 (1.3) | 11.7 (44.7) | 4 |
| SeMet1(λ1) | APS
SGXb 31-ID-D |
0.9641 (remote) | 20–3.1 (3.21-3.1) | 132,551/6,187 | 99.5 (97.5) | 7.7 (1.4) | 13.5 (52.1) | 4 |
|
| ||||||||
| Crystal space group, P64; unit cell, a = b = 84.111 Å, c = 84.219 Å; Figure of Meritd (30 - 2.9 Å resolution), 0.35 | ||||||||
|
| ||||||||
| Refinement (against Native data set) | ||||||||
|
| ||||||||
| Resolution (Å) | 50 – 2.6 | |||||||
| No. of solvent molecules | 98 | |||||||
| Rcryst/Rfreee (%) | 24.0/27.3 | |||||||
| Rmsd bond lengths | 0.008 Å | |||||||
| Rmsd bond angles | 1.328° | |||||||
National Synchrotron Light Source, Brookhaven, NY;
Advanced Photon Source-Structural GenomiX, Argonne, IL;
Rsym =Σ|I − <I>|/ΣI, where I is observed intensity and <I> is average intensity obtained from multiple observations of symmetry related reflections;
Figure of merit calculated by SHARP (de la Fortelle et al. 1997);
Rcryst = Σ||Fobserved| − |Fcalculated||/Σ|Fobserved|, Rfree = Rcryst calculated using 5% random data omitted from the refinement.
The structure reveals a σ704/Rsd complex with a stoichiometry of 1:1 (Figure 1). This is consistent with the gel filtration behavior of the σ704/Rsd complex during purification (data not shown), and with previous biochemical experiments indicating a 1:1 stoichiometry of the full-length σ70/Rsd complex in solution 10.
Figure 1. Structure of the σ704/Rsd complex.
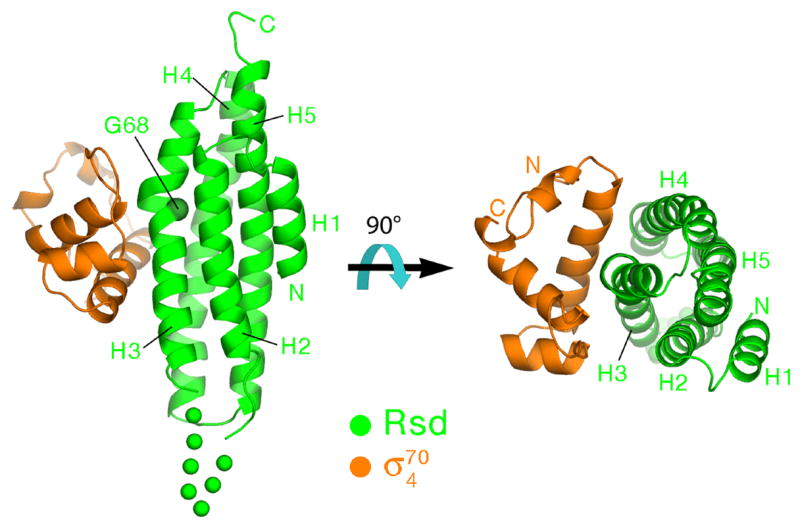
Ribbon diagrams showing two orthogonal views of the complex, color-coded as shown. The α-helices of Rsd, H1–H5, are labeled. The α-carbon of Gly68 at the position of the kink in H3, is shown as a CPK sphere and labeled. A 7-residue disordered segment connecting H2 to H3 is represented by green dots.
Rsd structure
The Rsd structure comprises four core α-helices (helices H2–H5; Figure 1) packed in an up-and-down bundle with a slight left-handed twist. An additional short N-terminal α-helix (H1) is tucked against the side of the bundle against H2 and H5. The Rsd fold is remarkably similar to the vinculin tail domain. Superposition of Rsd with the vinculin tail domain (chicken vinculin residues 901–1046; Ref. 25) over the entire length of Rsd (residues 1–153) but excluding exposed loops connecting the α-helices yields a root-mean-square deviation of 2.53 Å over 116 α-carbons (Figure S2). Rsd therefore belongs to the vinculin/α-catenin superfamily of proteins 26. Vinculin and α-catenin are structural components of the eukaryotic cytoskeleton, while Rsd is a bacterial transcriptional regulator. At this point we do not believe there is any functional significance to the observed structural similarity.
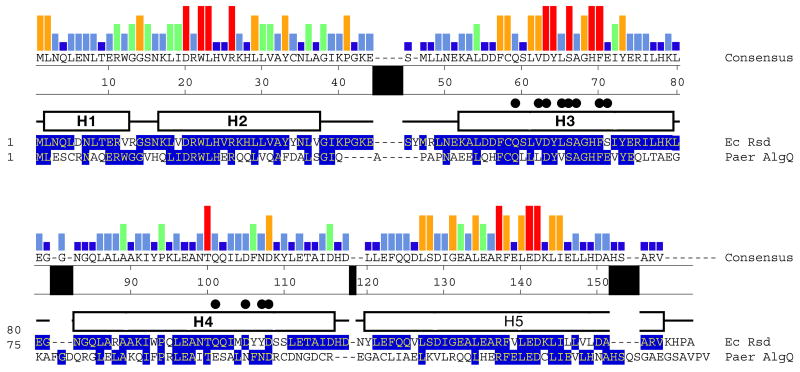
A distinctive structural feature of Rsd is a pronounced kink (~30°) in H3. The kink occurs around a highly conserved Gly residue at position 68 (Figure 1). In an alignment of 113 Rsd orthologs (Supplemental Information), Gly occurs at this position in 110 of the sequences (97% identity, Figure 2). Moreover, the primary Rsd binding interface with σ704 is centered at the kink (Figure 1). All of the absolutely conserved Rsd residues that contact σ704 lie near the H3 kink (Asp63, Ser66, Phe70; Figures 2 and 3). These observations suggest that the kink is a conserved feature of the Rsd structure that is important for σ704 binding.
Figure 2. Sequence conservation among Rsd orthologs.
The sequence on top shows the consensus sequence from an alignment of 113 Rsd orthologs (see supplemental information), while the histogram above it denotes the level of sequence conservation at each position (red bar, 100% conserved; dark blue bar, less than 20%). Shown are only two sequences from the full alignment, E. coli Rsd (top) and Paer AlgQ, represented in one-letter amino acid code and identified by the species at the right. The numbers at the beginning of each line indicate amino acid positions relative to the start of each protein sequence. The numbers at the top of the sequences indicate the amino acid position in E. coli Rsd. Amino acid identity with the consensus sequence is indicated by blue shading. The α-helices in the Rsd structure are indicated above the Rsd sequence as rectangles (labeled H1–H5), loops are indicated by a solid line. Rsd positions that contact σ704 are denoted by black dots above the helices.
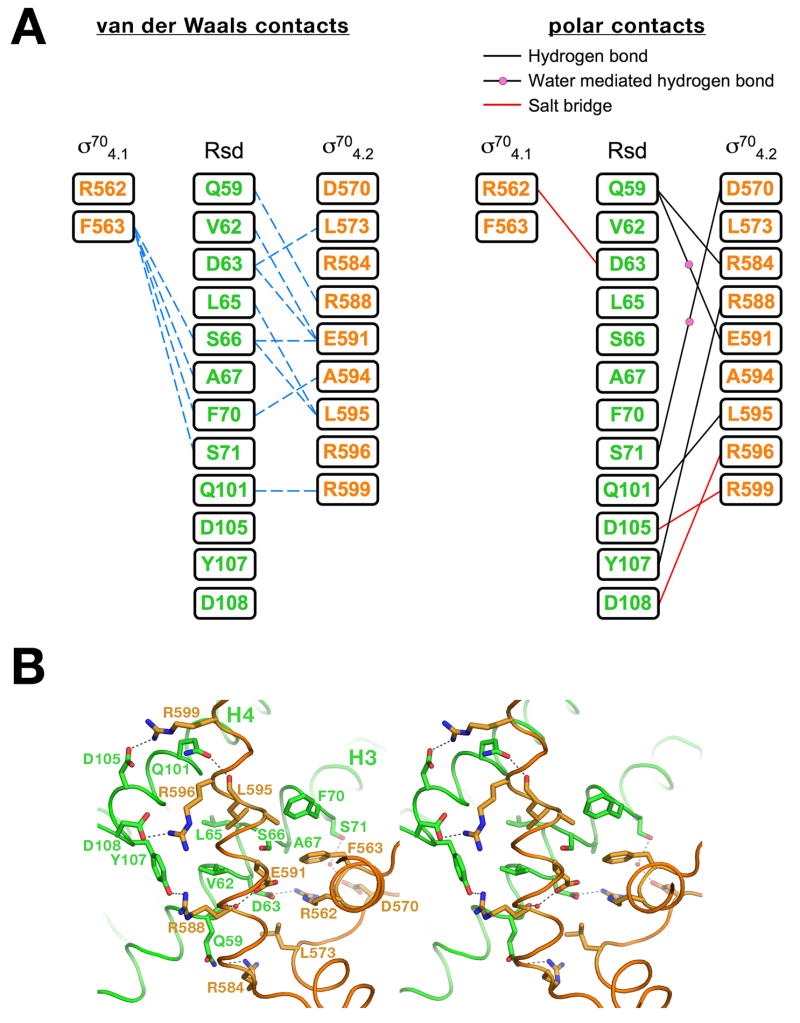
Figure 3. The σ704/Rsd interactions.
A) Schematic diagram denoting molecular interactions between Rsd and σ704. Van der Waals interactions (<4 Å) are listed on the left, polar interactions (hydrogen bonds, salt bridges) are listed on the right.
B) Stereo view of the σ704/Rsd interface. Protein α-carbon backbones are shown as worms, color-coded as in Figure 1. The two α-helices of Rsd harboring amino acid residues that interact with σ704 (H3 and H4) are labeled. Amino acid side chains that participate in interprotein interactions are shown, with carbon atoms color-coded as the backbone worm, nitrogen atoms colored blue, and oxygen atoms colored red. Interprotein polar interactions (hydrogen bonds or salt bridges) are indicated by dashed lines.
Interactions between σ704 and Rsd
Binding of σ704 to Rsd buries a modest 779 Å2 of protein surface area. Nevertheless, the protein/protein complex is very stable, as it survives several purification steps without dissociating. All of the interactions between σ704 and Rsd are schematically diagrammed in Figure 3A, and a stereo view of the interface with the involved amino acids is shown in Figure 3B.
The σ704/Rsd interface, centered about the kink in Rsd-H3, involves hydrophobic interactions with a patch of highly conserved residues from H3 exposed on the surface of Rsd (Figure 3). Rsd residues Val62 and Leu65 are conserved as hydrophobic residues, while Ser66 (which makes van der Waals contacts through its β-carbon), Ala67, and Phe70 are absolutely conserved (Figure 2). The interface includes polar interactions involving mostly residues from Rsd-H4. The protein/protein interaction is also stabilized by two salt bridges between pairs of highly conserved, oppositely-charged residues (σ704-Arg562/Rsd-Asp63; σ704-Arg596/Rsd-Asp108).
The structural observations are completely consistent with biochemical and genetic studies on the σ704/Rsd interaction. Previous studies implicated σ704 region 4.2 as the primary determinant in the σ70/Rsd interaction 10–12, which is borne out by the structure (Figure 3A). Detailed genetic studies have implicated residues in σ704 that the structure shows are involved in, or are near, the σ704/Rsd interface (σ704-590/591/593/595/596/598; 10–12. A more recent study showed that mutation of Rsd-Asp63 (involved in a conserved salt bridge with σ704-Arg562) to Ala caused a severe defect in Rsd binding to σ70 9.
Conserved residues in Rsd form an interacting network
The alignment of Rsd orthologs (Supplementary information) reveals 13 absolutely conserved residues distributed throughout helices H2–H5 (4 conserved residues in H2, 5 in H3, 1 in H4, 3 in H5; red bars in histogram of Figure 2). Only three of the absolutely conserved residues, all on H3, contact σ704 (Rsd Asp63, Ser66, and Phe70; Figure 2). Remarkably, all 13 absolutely conserved residues form an interacting network that extends through the middle of the Rsd structure (Figure 4). The interacting network includes both polar (green lines in Figure 4B) and van der Waals (orange lines, Figure 4B) interactions. The network links the σ704 binding surface with three exposed cavities on the Rsd surface (labelled I, II, and III in Figure 4).
Figure 4. Interacting network of conserved residues in Rsd.
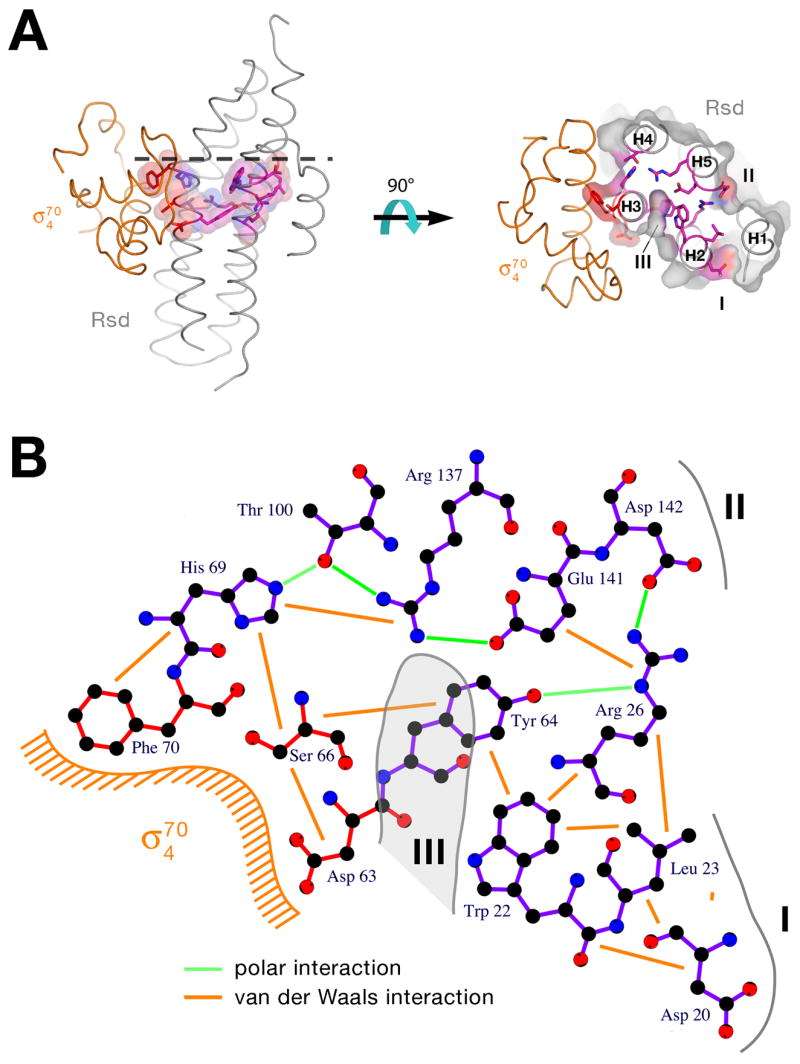
A) Two orthogonal views of the σ704/Rsd complex (similar views as Figure 1). The α-carbon backbones are shown as worms, with σ704 colored orange, and Rsd colored grey. The side chains of the 13 absolutely conserved Rsd residues are shown (nitrogen, blue; oxygen, red), with carbon atoms colored magenta, except the three residues that interact directly with σ704 (Asp63, Ser66, Phe70) are red. In the left view, transparent van der Waals surfaces for the conserved residues (color-coded as the atoms) is also shown, showing the continuous network through the middle of the Rsd structure. In the right view, the Rsd structure is shown as a cross-section, sliced at the level of the dashed line in the left view. On the right, the transparent molecular surface of Rsd is shown, colored grey except where the conserved residues are surface exposed. The Rsd helices (H1–H5), viewed mostly end-on, are labeled, as are the three cavities containing surface-exposed conserved residues (I, II, and III).
B) Schematic diagram showing the 13 conserved Rsd residues (bonds color-coded as in A, carbon atoms colored black) and illustrating the polar (hydrogen bonds or salt bridges, green lines) and van der Waals (< 4 Å, orange lines) interactions. The relative locations of σ704, and cavities I, II, and II are indicated.
Taking the σ704 binding surface of Rsd as the front, Cavity I is located on the side of the molecule and is lined with conserved residues Asp20 and Leu23 (Figure 4). Cavity II is located on the back of Rsd and is lined with conserved residues Arg26 and Asp142. Most interesting is Cavity III, a deep (approximately 8 Å), narrow (approximately 3 Å in diameter) ‘hole’ penetrating into the structure between H2 and H3. Cavity III is large enough to accommodate a water molecule. Cavity III is lined by conserved residues Trp22, Tyr64, Arg137, and Glu141. Most of the surface of Cavity III is lined with polar atoms (Trp22 NE1; Tyr64 O; Leu65 O; Gly68 N; Tyr73 OH; Arg137 NH1; Glu141 OE2), but one surface comprises the hydrophobic face of the aromatic ring of Tyr64. Note that conserved residues Arg137 and Glu141, which form a salt bridge (Arg137 NH1–Glu141 OE2 distance = 3.62 Å), are completely buried in the interior of the Rsd structure except for the exposure of Arg137 NH1 and Glu141 OE2 on the solvent-accessible surface of Cavity III.
Ecσ704 structure and the relationship of Rsd with T4 AsiA
Structures of σ4 from σ70-family members have been observed individually (Thermus aquaticus [Taq] σA4; Ref. 13), in complex with –35 element promoter DNA (Taq σA4; Ref. 13), in complex with anti-σ factors (Ec σE4; Ref. 27; Aquifex aeolicus σ284; Ref. 28), and in complex with core RNAP (Taq σA4; Ref. 16; Thermus thermophilus σA4; Ref. 17). In all of these cases mentioned above, the σ4 domain comprises a compact structural core of three α-helices (corresponding to residues 551–599 of Ec σ704) that is essentially identical in all of the structures. The last two α-helices of the structural core make up the helix-turn-helix motif that is responsible for recognition of the promoter –35 element 13. In one case, however, the interaction of Ec σ704 with the anti-σ and appropriator AsiA from bacteriophage T4 induces a dramatic structural rearrangement of σ704 29.
In the σ704/Rsd complex, the structure of Ec σ704 is nearly identical to the undistorted σ4 structures that have been observed previously. For instance, superimposing the 49-residue structural core of Ec σ704 (residues 551–599) from the σ704/Rsd complex with Taq σA4 (PDB ID 1KU3, residues 376–424; Ref. 13) results in an rmsd in α-carbon positions of 1.6 Å (Figure S3). Regions to the N- and C-terminus of the structural core deviate in structure, but these regions are known to be structurally variable depending on the functional context 13.
Pineda et al.30 proposed that the T4-type phage AsiA orthologs and Rsd orthologs are members of a related family that interact similarly with their cognate σ factors. Although Jishage & Ishihama 5 concluded in their analysis that there was no significant sequence similarity between Rsd and T4 AsiA, Pineda et al. 30 present an alignment between T4-type phage AsiA orthologs and the N-terminal 82 residues of Rsd and suggest that it indicates significant similiarity. However, the Pinda et al. 30 alignment between T4-phage AsiA and Rsd[1–82] contains many gaps for such a short segment, and despite this the sequence identity between T4-phage AsiA and Rsd is only about 7% (6 out of 82 Rsd residues), which is similar to the sequence identity expected for the alignment of completely random sequences (6%). Indeed, structural comparison of T4 AsiA 31 and Rsd does not support the idea that these proteins belong to a related family of proteins (Figure S4). Moreover, T4 AsiA dramatically distorts the σ704 structure 29, while Rsd does not.
Rsd binding to σ704 occludes both RNAP and promoter DNA binding by σ704
In the RNAP holoenzyme, σ4 interacts with the core RNAP by binding to the RNAP β-subunit flap-tip-helix 14–17. Analysis of the σ704/Rsd complex predicts that Rsd would sterically interfere with the binding of σ704 to the β-flap-tip-helix (Figure 5). Rsd also directly interacts with several σ704 residues that interact with the β-flap-tip in the RNAP holoenzyme (σ704-Phe563, Leu595, and Arg599). Indeed, a core RNAP binding defect results from mutation of σ704-Phe563 32; 33.
Figure 5. Rsd sterically occludes core RNAP and –35 element promoter DNA binding by σ704.

The σ704/Rsd complex is shown with the α-carbon backbones as worms (Rsd, green; σ704, orange). Superimposed is the position of the β-flap-tip (T. aquaticus RNAP β-subunit residues 759–788, corresponding to E. coli RNAP β-subunit residues 887–916), shown as a cyan backbone worm as it would be interacting with σ4 in the context of the RNAP holoenzyme 17. Also superimposed is the –35 element DNA as it would be interacting with σ4 13. Both the β-flap-tip and the –35 element sterically clash with Rsd. Below is a diagram schematically illustrating amino acid residues of σ4 that are involved in interactions with Rsd, the RNAP σ-subunit flap (Murakami et al., 2002; Vassylyev et al., 2002), and –35 element promoter DNA (Campbell et al., 2002). Shown in single-letter amino acid code is the sequence of Ecσ704. Conserved regions 4.1 and 4.2 are indicated above the sequence. Dots below the sequence mark σ4 residues involved in interactions with Rsd (green dots), the RNAP β-flap (cyan dots), or –35 element promoter DNA (grey dots, DNA phosphate backbone; magenta dots, sequence-specific interactions). Residues that interact with Rsd and are also involved in interactions with the β-flap or –35 element DNA are boxed.
In the RNAP holoenzyme open promoter complex, in addition to the β-flap-tip helix, σ704 also binds the promoter DNA –35 element. Analysis of the σ704/Rsd complex predicts that Rsd would sterically interfere with the binding of σ704 to the –35 element DNA (Figure 5). Rsd also directly interacts with several σ704 residues that interact with the promoter DNA (σ704-Arg562, Leu573, Arg584, and Arg588; Ref. 13). Both σ704-Arg584 and Arg588 have been shown to be important for sequence-specific recognition of the –35 element 34; 35; 36. We conclude that σ704 bound to Rsd would not be able to interact with the RNAP β-flap-tip nor would it be able to interact with the promoter –35 element.
Conclusions
Rsd was identified on the basis of its tight binding to Ec σ70 5. The binding is specific for σ70, as Rsd does not associate with alternative σ factors 5. Data are consistent with Rsd playing a role in assisting alternative σ factor-dependent transcription by biasing the competition between σ70 and alternative σ factors for the available core RNAP through its interaction with σ70 6–9.
The structure of Rsd bound to σ704, the primary determinant for Rsd binding within σ70 10–12, reveals that Rsd binding interferes with the two primary functions of σ4, core RNAP binding and promoter –35 element binding (Figure 5). Interestingly, the most highly conserved residues of Rsd form a network of interactions through the middle of the Rsd structure that connect the σ704-binding surface with three cavities exposed on distant surfaces of Rsd. This suggests functional coupling between σ704 binding and other binding surfaces of Rsd, either for other proteins or for as yet unkown small molecule effectors. Rsd is known to participate in protein/protein interactions in addition to the one with σ704, including with the core RNAP 37; 38, and with other σ70 domains (L.F.W. and S.A.D., unpublished). The possibility that Rsd may interact with small molecule effectors, particularly through Cavity III (Figure 4), which does not seem appropriate for a protein/protein interaction, has not, to our knowledge, been considered previously. The identification of a putative small molecule ligand for Rsd could greatly facilitate the understanding of Rsd’s role in the regulation of transcription. Finally, due to the high sequence similarity between σ704 from Ec and Paer, and between Ec Rsd and Paer AlgQ, the structure of the Ec σ704/Rsd complex provides a structural basis for understanding the mechanism of action of AlgQ, a global regulator of virulence in the pathogen Paer.
Materials and Methods
Construction of the Ecσ704/Rsd co-expression cassette
The Ec σ704/Rsd co-expression plasmid was constructed in three steps based upon the procedure of 39. First, DNA encoding full-length Rsd (residues 1–158) was amplified by the polymerase chain reaction (PCR), digested with NdeI and BamHI restriction endonucleases, and cloned between the NdeI and BamHI sites of pET21a (Novagen) generating pET21aRsd. Second, DNA encoding Ec σ704 (Ec σ70 residues 541–613) was amplified by PCR, cleaved with NdeI and HindIII, and cloned between the NdeI and HindIII sites of a pET28a-based plasmid, creating pSKB2Ecσ704. Finally, using pSKB2Ecσ704 as a template, the DNA encoding the pET28a-based translation initiation regions and the coding sequence of Ec σ704 was amplified by PCR, cleaved with BamHI and HindIII, and cloned between the BamHI and HindIII sites of pET21aRsd, creating pET21aRsd/Ecσ704.
Expression and Purification of the Ecσ704/Rsd complex
The expression plasmid pET21aRsd/Ecσ704 was transformed into BL21 (DE3) Ec cells and transformants were selected in the presence of the appropriate antibiotic. Cultures were grown at 37 °C to an A600 nm ~0.6 and induced with 1 mM IPTG for 3 hours at 30°C. Cells containing overexpressed proteins were harvested by centrifugation and stored at minus 80°C.
The Ec σ704/Rsd complex (Ec σ704 contains an N-terminal His6-tag and PreScission cleavage site derived from the vector) was purified by HiTrap Ni2+-charged affinity chromatography (GE Healthcare), and the N-terminal His6-tag was removed using PreScission protease (GE Healthcare). The sample was further purified by a second, subtractive HiTrap Ni2+-charged affinity chromatography step to remove uncleaved His6-σ704 protein and the His6-tag, ion exchange chromatography (HiTrap Q Sepharose; GE Healthcare) and gel filtration chromatography (Superdex 75; GE Healthcare). Purified σ704/Rsd complex was concentrated to ~16 mg/ml using centrifugal filtration units (Vivascience) and exchanged into buffer containing 10 mM Tris-HCl (pH 8.0), 0.2 M NaCl, 5 mM DTT. Selenomethionyl-substituted complex was prepared for MAD analysis by suppression of methionine biosynthesis 40, and purified using similar procedures.
Crystallization and Structure Determination
Rod-like crystals were initially obtained by vapour diffusion using a sitting drop setup in 96-well plates using a sparse matrix screen (Hampton Research) at 4°C by mixing 1 μL of protein solution with 1 μL of well solution (0.2 M KCl, 0.05 M MgCl2, 0.05 M Tris-HCl, pH 7.5, 10% PEG4000) and equilibrating against 100 μL of well solution. Attempts to reproduce the original crystallization condition in a larger 24-well plate using a hanging drop vapour diffusion setup and a grid screen around the original condition yielded needle-like crystals. Crystal growth was optimized using a combination of grid screening and microseeding using Seed Beads (Hampton Research).
Crystals were prepared for cryocrystallography by incubating in cryosolution (0.2 M KCl, 0.05 M MgCl2, 0.05 M Tris-HCl, pH 7.5, 10% PEG4000, 25% glycerol) for 1 minute. The crystals were then flash frozen in a vial of liquid nitrogen and stored at liquid nitrogen temperature. MAD data were collected from the crystals of selnomethioyl-substituted complex at two wavelengths corresponding to the peak and one remote value of the X-ray absorption spectrum (λ1 and λ2, respectively, Table 1).
Four Se sites (out of a possible six Se sites) were located using SnB 41 with the anomalous signal from the SeMet(λ1) dataset. Phases were calculated using SHARP 42. The experimental electron density map, after density modification using SOLOMON 43, was excellent (Figure S1). Model building was performed manually using O 44. Iterative model building and refinement against the native amplitudes were performed using CNS 45. The final model contains Rsd residues 1–42 and 50–158 (the loop region 43–49 is disordered), Ec σ704 residues 546–613, one Mg2+-ion, and 98 water molecules. Analysis of the structure using PROCHECK 46 showed 91% of the residues in the most favored regions of the Ramachandran plot, and 9% in additional allowed regions (no residues in generously allowed or disallowed regions), and an overall G factor of 0.3.
Sequence alignment
The sequence alignment of 113 Rsd orthologs (Supplemental information) was generated by using the Ec K-12 Rsd sequence to BLAST the NCBI non-redundant database, followed by sequence retrieval. The downloaded sequences, which included both Rsd and Paer AlgQ, were then aligned using PCMA (ftp://iole.swmed.edu/pub/PCMA/) (PMID: 12584134).
Accession codes
The coordinates for the refined σ704/Rsd structure have been deposited in the Protein Data Bank (accession code 2P7V).
Supplementary Material
Acknowledgments
We are indebted to Stephen K. Burley, Stephen Busby, Ann Hochschild, Annie Kolb, Ivo Lorenz, Joseph Marcotrigiano, Bryce Nickels, and Andy Yuan for helpful discussions. We thank Stephen R. Wasserman (SGX-CAT) and Howard Robinson (NSLS X29) for support at the synchrotron facilities. Financial support for beamline X29 at the National Synchrotron Light Source comes principally from the Offices of Biological and Environmental Research and of Basic Energy Sciences of the US Department of Energy, and from the National Center for Research Resources of the National Institutes of Health. The SGX Collaborative Access Team (SGX-CAT) beamline facilities at Sector 31 of the Advanced Photon Source were provided by SGX Pharmaceuticals, Inc., who constructed and operates the facility. Use of the Advanced Photon Source was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. Figures 1, 3B, 4A, and 5 were made using MacPyMOL 47. Figure 4B was made using LIGPLOT 48. G.A.P. was supported by a National Research Service Award (NIH F32 GM072326-01). W.J.L. was supported by National Institutes of Health MSTP grant GM07739 and by The W.M. Keck Foundation Medical Scientist Fellowship. This work was supported by National Institutes of Health grant GM53759 to S.A.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murakami K, Darst SA. Bacterial RNA polymerases: the wholo story. Curr Opinion Struct Biol. 2003;13:31–39. doi: 10.1016/s0959-440x(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 2.Gross CA, Chan C, Dombroski A, Gruber T, Sharp M, Tupy J, Young B. The functional and regulatory roles of sigma factors in transcription. Cold Spring Harbor Symp Quant Biol. 1998;63:141–155. doi: 10.1101/sqb.1998.63.141. [DOI] [PubMed] [Google Scholar]
- 3.Maeda H, Fujita N, Ishihama A. Competition among seven Escherichia coli sigma subunits: Relative binding affinities to the core RNA polymerase. Nucleic Acids Res. 2000;28:3497–3503. doi: 10.1093/nar/28.18.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jishage M, Ishihama A. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: Intracellular levels of sigma 70 and sigma 38. J Bacteriol. 1995;177:6832–6835. doi: 10.1128/jb.177.23.6832-6835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jishage M, Ishihama A. A stationary phase protein in Escherichia coli with binding activity to the major sigma subunit of RNA polymerase. Proc Natl Acad Sci USA. 1998;95:4953–4958. doi: 10.1073/pnas.95.9.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jishage M, Ishihama A. Transcriptional organization and in vivo role of the Escherichia coli rsd gene, encoding the regulator of RNA polymerase sigma D. J Bacteriol. 1999;181:3768–3776. doi: 10.1128/jb.181.12.3768-3776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jishage M, Kvint K, Shingler V, Nystrom T. Regulation of sigma factor competition by the alarmone ppGpp. Genes & Development. 2002;16:1260–1270. doi: 10.1101/gad.227902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurie AD, Bernardo LM, Sze CC, Skarfstad E, Szalewska-Palasz A, Nystrom T, Shingler V. The role of the alarmone (p)ppGpp in sigma N competition for core RNA polymerase. J Biol Chem. 2003;278:1494–1503. doi: 10.1074/jbc.M209268200. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell JE, Oshima T, Piper SE, Webster CL, Westblade LF, Karimova G, Ladant D, Kolb A, Hobman JL, Busby SJW, Lee DJ. The Escherichia coli regulator of sigma 70 protein, Rsd, can up-regulate some stress-dependent promoters by sequestering sigma 70. J Bacteriol. 2007;189:3489–3495. doi: 10.1128/JB.00019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westblade LF, Ilag LL, Powell AK, Kolb A, Robinson CV, Busby SJW. Studies of the Escherichia coli Rsd-sigma 70 complex. J Mol Biol. 2004;335:685–692. doi: 10.1016/j.jmb.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Dove SL, Hochschild A. Bacterial two-hybrid analysis of interactions between region 4 of the sigma 70 subunit of RNA polymerase and transcriptional regulators Rsd from Escherichia coli and AlgQ from Pseudomonas aeruginosa. J Bacteriol. 2001;183:6413–6421. doi: 10.1128/JB.183.21.6413-6421.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jishage M, Dasgupta D, Ishihama A. Mapping of the Rsd contact site on the sigma 70 subunit of Escherichia coli RNA polymerase. J Bacteriol. 2001;183:2952–2956. doi: 10.1128/JB.183.9.2952-2956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell EA, Muzzin O, Chlenov M, Sun JL, Olson CA, Weinman O, Trester-Zedlitz ML, Darst SA. Structure of the bacterial RNA polymerase promoter specificity sigma factor. Mol Cell. 2002;9:527–539. doi: 10.1016/s1097-2765(02)00470-7. [DOI] [PubMed] [Google Scholar]
- 14.Geszvain K, Gruber TM, Mooney RA, Gross CA, Landick R. A hydrophobic patch on the flap-tip helix of E. coli RNA polymerase mediates sigma 70 region 4 function. J Mol Biol. 2004;343:569–587. doi: 10.1016/j.jmb.2004.08.063. [DOI] [PubMed] [Google Scholar]
- 15.Kuznedelov K, Minakhin L, Niedziela-Majka A, Dove SL, Rogulja D, Nickels BE, Hochschild A, Heyduk T, Severinov K. A role for interaction of the RNA polymerase flap domain with the sigma subunit in promoter recognition. Science. 2002;295:855–857. doi: 10.1126/science.1066303. [DOI] [PubMed] [Google Scholar]
- 16.Murakami K, Masuda S, Darst SA. Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 Å resolution. Science. 2002;296:1280–1284. doi: 10.1126/science.1069594. [DOI] [PubMed] [Google Scholar]
- 17.Vassylyev DG, Sekine S, Laptenko O, Lee J, Vassylyeva MN, Borukhov S, Yokoyama S. Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 Å resolution. Nature. 2002;417:712–719. doi: 10.1038/nature752. [DOI] [PubMed] [Google Scholar]
- 18.Ambrosi C, Tiburzi F, Imperi F, Putignani L, Visca P. Involvement of AlgQ in transcriptional regulation of pyoverdine genes in Pseudomonas aeruginosa PAO1. J Bacteriol. 2005;187:5097–5107. doi: 10.1128/JB.187.15.5097-5107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deretic K, Konyecsni WM. Control of mucoidy in Pseudomonas aeruginosa: Transcriptional regulation of algR and identification of the second regulatory gene, algQ. J Bacteriol. 1989;171:3680–3688. doi: 10.1128/jb.171.7.3680-3688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato JL, Chiu L, Kitano K, DeVault JD, Kimbara K, Chakrabarty AM, Misra TK. Nucleotide sequence of a regulatory region controlling alginate synthesis in Pseudomonas aeruginosa: Characterization of the algR2 gene. Gene. 1989;84:31–38. doi: 10.1016/0378-1119(89)90136-4. [DOI] [PubMed] [Google Scholar]
- 21.Cacalano G, Kays M, Saiman L, Prince A. Production of the Pseudomonas aeruginosa neuraminidase is increased under hyperosmolar conditions and is regulated by genes involved in alginate expression. J Clin Invest. 1992;89:1866–1874. doi: 10.1172/JCI115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlictman D, Kubo M, Shankar S, Chakrabarty AM. Regulation of nucleoside diphosphate kinase and secretable virulence factors in Pseudomonas aeruginosa: Roles of algR2 and algH. J Bacteriol. 1995;177:2469–2474. doi: 10.1128/jb.177.9.2469-2474.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Severinova E, Severinov K, Fenyö D, Marr M, Brody EN, Roberts JW, Chait BT, Darst SA. Domain organization of the Escherichia coli RNA polymerase sigma 70 subunit. J Mol Biol. 1996;263:637–647. doi: 10.1006/jmbi.1996.0604. [DOI] [PubMed] [Google Scholar]
- 24.Hendrickson WA. Determination of macromolecular structures from anomalous diffraction of synchrotron radiation. Science. 1991;254:51–58. doi: 10.1126/science.1925561. [DOI] [PubMed] [Google Scholar]
- 25.Bakolitsa C, Cohen DM, Bankston LA, Bobkov AA, Cadwell GW, Jennings L, Critchley DR, Craig SW, Liddington RC. Structural basis for vinculin activation at sites of cell adhesion. Nature. 2004;430:583–586. doi: 10.1038/nature02610. [DOI] [PubMed] [Google Scholar]
- 26.Murzin AG, Brenner SE, Hubbard T, Chothia C. SCOP: A structural classification of proteins database for the investigation of sequences and structures. J Mol Biol. 1995;247:536–540. doi: 10.1006/jmbi.1995.0159. [DOI] [PubMed] [Google Scholar]
- 27.Campbell EA, Tupy JL, Gruber TM, Wang S, Sharp MM, Gross CA, Darst SA. Crystal structure of Escherichia coli sigma E with the cytoplasmic domain of its anti-sigma RseA. Mol Cell. 2003;11:1067–1078. doi: 10.1016/s1097-2765(03)00148-5. [DOI] [PubMed] [Google Scholar]
- 28.Sorenson MK, Ray SS, Darst SA. Crystal structure of the flagellar sigma/anti-sigma complex sigma 28/FlgM reveals an intact sigma factor in an inactive conformation. Mol Cell. 2004;14:127–138. doi: 10.1016/s1097-2765(04)00150-9. [DOI] [PubMed] [Google Scholar]
- 29.Lambert LJ, Wei Y, Schirf V, Demeler B, Werner MH. T4 AsiA blocks DNA recognition by remodeling sigma 70 region 4. EMBO J. 2004;23:2952–292. doi: 10.1038/sj.emboj.7600312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pineda M, Gregory BD, Szczypinski B, Baxter KR, Hochschild A, Miller ES, Hinton DM. A family of anti-sigma70 proteins in T4-type phages and bacteria that are similar to AsiA, a transcription inhibitor and co-activator of bacteriophage T4. J Mol Biol. 2004;344:1183–1197. doi: 10.1016/j.jmb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Urbauer JL, Simeonov MF, Urbauer RJ, Adelman K, Gilmore JM, Brody EN. Solution structure and stability of the anti-sigma factor AsiA: implications for novel functions. Proc Natl Acad Sci USA. 2002;99:1831–1835. doi: 10.1073/pnas.032464699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharp MM, Chan CL, Lu CZ, Marr MT, Nechaev S, Merritt EW, Severinov K, Roberts JW, Gross CA. The interface of sigma with core RNA polymerase is extensive, conserved, and functionally specialized. Genes Dev. 1999;13:3015–3026. doi: 10.1101/gad.13.22.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregory BD, Nickels BE, Garrity SJ, Severinova E, Minakhin L, Urbauer RJ, Urbauer JL, Heyduk T, Severinov K, Hochschild A. A regulator that inhibits transcription by targeting a intersubunit interaction of the RNA polymerase holoenzyme. Proc Natl Acad Sci USA. 2004;101:4554–4559. doi: 10.1073/pnas.0400923101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siegele DA, Hu JC, Walter WA, Gross CA. Altered promoter recognition by mutant forms of the sigma 70 subunit of Escherichia coli RNA polymerase. J Mol Biol. 1989;206:591–603. doi: 10.1016/0022-2836(89)90568-8. [DOI] [PubMed] [Google Scholar]
- 35.Gardella T, Moyle T, Susskind MM. A mutant Escherichia coli sigma 70 subunit of RNA polymerase with altered promoter specificity. J Mol Biol. 1989;206:579–590. doi: 10.1016/0022-2836(89)90567-6. [DOI] [PubMed] [Google Scholar]
- 36.Gregory BD, Nickels BE, Darst SA, Hochschild A. An altered-specificity DNA-binding mutant of Escherichia coli sigma 70 facilitates the analysis of sigma 70 function in vivo. J Mol Biol. 2005;56:1208–1219. doi: 10.1111/j.1365-2958.2005.04624.x. [DOI] [PubMed] [Google Scholar]
- 37.Arifuzzaman M, Maeda M, Itoh A, Nishikata K, Takit C, Saito R, Ara T, Nakahigashi K, Huang HC, Hirai A, Tsuzuki K, Nakamura S, Altaf-Ul-Amin M, Oshima T, Baba T, Yamamoto N, Kawamura T, Ioka-Nakamichi T, Kitagawa M, Tomita M, Kanaya S, Wada C, Mori H. Large-scale identification of protein-protein interactions of Escherichia coli K-12. Genome Res. 2006;16:686–691. doi: 10.1101/gr.4527806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ilag LL, Westblade LF, Deshayes C, Kolb A, Busby SJW, Robinson CV. Mass spectrometry of Escherichia coli RNA polymerase: Interactions of the core enzyme with sigma 70 and Rsd protein. Structure. 2004;12:269–275. doi: 10.1016/j.str.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Campbell EA, Darst SA. The anti-sigma factor SpoIIAB forms a 2:1 complex with sigma F, contacting multiple conserved regions of the sigma factor. J Mol Biol. 2000;300:17–28. doi: 10.1006/jmbi.2000.3838. [DOI] [PubMed] [Google Scholar]
- 40.Doublie S. Preparation of selenomethionyl proteins for phase determination. Methods Enzymol. 1997;276:523–530. [PubMed] [Google Scholar]
- 41.Weeks CM, Miller R. The design and implementation of SnB v2.0. J appl Crystallogr. 1999;32:120–124. [Google Scholar]
- 42.de La Fortelle E, Irwin JJ, Bricogne G. SHARP: A maximum-likelihood heavy-atom parameter refinement and phasing program for the MIR and MAD methods. In: Bourne P, Watenpaugh K, editors. Crystallographic Computing. Vol. 7. 1997. pp. 1–9. [Google Scholar]
- 43.Abrahams JP, Leslie AGW. Methods used in the structure determination of bovine mitochondrial F1 ATPase. Acta crystallographica. 1996;D52:30–42. doi: 10.1107/S0907444995008754. [DOI] [PubMed] [Google Scholar]
- 44.Jones TA, Zou J-Y, Cowan S, Kjeldgaard M. Improved methods for building protein models in electron denstiy maps and the location of errors in these models. Acta crystallographica. 1991;A47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 45.Adams PD, Pannu NS, Read RJ, Brunger AT. Cross-validated maximum likelihood enhances crystallographic simulated annealing refinement. Proc Natl Acad Sci USA. 1997;94:5018–5023. doi: 10.1073/pnas.94.10.5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK - A program to check the stereochemical quality of protein structures. J appl Crystallogr. 1993;26:283–291. [Google Scholar]
- 47.DeLano WL. The PyMOL molecular graphics system. 2002 http://www.pymol.org.
- 48.Wallace AC, Laskowski RA, Thornton JM. LIGPLOT: A program to generate schematic diagrams of protein-ligand interactions. Protein Eng. 1995;8:127–134. doi: 10.1093/protein/8.2.127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.