Abstract
This study discovered that betulinic acid (BA) is a potent proteasome activator that preferentially activates the chymotrypsin-like activity of proteasomes. Chemical modifications can transform BA into proteasome inhibitors. Chemical modifications at the C-3 position of BA resulted in compounds, such as dimethylsuccinyl BA (DSB), with various inhibitory activities against human 20S proteasomes. Interestingly, the proteasomal activation by BA and the inhibitory activity of DSB could be abrogated by introducing a side chain at the C-28 position. In summary, this study discovered a class of small molecules that can either activate or inhibit human proteasome activity depending on side chain modifications.
Keywords: Betulinic acid, proteasome inhibitor, proteasome activator
1. Introduction
There are two major cellular proteolysis systems: lysosomes and proteasomes. Proteasomes are responsible for the majority of intracellular protein degradation. On the other hand, lysosomes are critical for breaking down proteins taken into cells through endocytosis [1, 2]. Proteasome is involved in many essential cellular functions, such as regulation of cell cycle, cell differentiation, signal transduction pathways, antigen processing for appropriate immune responses, stress signaling, inflammatory responses, and apoptosis. Proteasomes (20S) are cylindrical structures that contain four rings stacked on top of each other. Each ring is composed of seven subunits. The two outer rings contain α subunits and do not have enzymatic activity. The two inner rings are comprised of β subunits. These β subunits are where the proteolytic activities reside. There are three major proteolytic activities in the β subunits: a chymotrypsin-like (β5), a trypsin-like (β2), and a caspase-like (β1) activity. The 20S proteasome is normally associated with 19S components to form 26S proteasomes. The 19S components regulate the entry of proteins into the 20S proteasome [3]. The 19S complex, also termed PA700, activates proteasome degradation of ubiquitin-conjugated proteins. In addition to PA700, there are two other intracellular protein complexes, PA28 and PA200, that can activate 20S proteasomes using peptide substrate as models [4-8].
Many proteasome inhibitors have been identified and can be classified into two groups according to their source: Chemically synthesized small molecules and compounds derived from natural products [9]. The majority of chemically synthesized small molecule proteasome inhibitors are peptide derivatives. The peptide boronate PS341 (Bortezomib) was successfully developed into a useful anti-cancer drug for the treatment of multiple myeloma [10]. In addition to the chemically synthesized small molecules, natural products derived from plants, such as the triterpene celastrol and withaferin A from traditional herb medicine “Thunder-god vine” and Indian winter cherry, respectively, were also shown to inhibit proteasomes at low micromolar concentrations [11, 12].
In contrast to the development of proteasome inhibitors, drug-like small molecules that can activate or enhance proteasome activity are rare. Several types of small molecules, including denaturing reagents, lipids and peptide-based activators, were shown to activate 20S proteasome at relatively high concentrations [13]. For example, SDS at 0.05%, polylysine at 1 mg/ml, and peptide-based activators at 100 μM were shown to activate 20S proteasome [14]. In this study we discovered that BA is a proteasome activator and modifications at the C-3 position transformed BA into proteasome inhibitors.
2. MATERIALS AND METHODS
2.1. HIV-1 proteasome assay
HIV-1 proteasome assay kits were purchased from Calbiochem, San Diego, CA. The effect of BA and its analogs on the 20S proteasome activity was assayed following the protocol provided by the manufacturer. The major components of the assay mixture are human 20S proteasomes, fluorogenic peptide substrates and the proteasome activator PA28. The assay was designed to measured hydrolysis of the fluorogenic substrates Suc-Leu-Leu-Val-Tyr-AMC, (Z)-LLE-bNA, and Bz-VGR-AMC in the presence of the proteasome activator, PA28. Suc-Leu-Leu-Val-Tyr-AMC is frequently used to detect the chymotrypsin-like activity of 20S proteasomes. The trypsin-like and caspase-like activities of the 20S proteasome were determined using the fluorogenic substrates Bz-VGR-AMC and (Z)-LLE-bNA, respectively. Fluorescence generated from the proteolytic reaction in the presence of various concentrations of BA or its analogs was measured using a Bio-Tek fluorometer (Winooski, Vermont).
To determine the proteasome activation activity of the compounds, PA28 was omitted from the reaction mixtures. The rate of cleavage reaction is expressed as the slope of each line (ΔRFU(360/460)/minute) in the plots. The 50% effective concentration for activation (EC50) is defined as the activator concentration that reaches 50% of maximal reaction rate. The rate of reaction was plotted against the log-concentration of the activator to determine the EC50.
For proteasome inhibition, various concentrations of BA derivatives were tested in the presence of 16 μg/ml of PA28. The 50% inhibitory concentration (IC50) is defined as the inhibitor concentration that reduces the reaction rate by 50%. The velocity of reaction (ΔRFU(360/460)/minute) was plotted against the log-concentration of the inhibitor to determine the IC50.
2.2. Cell-based proteasome assay
To determine the effect of BA derivatives on proteasomes in culture cells, a Promega cell-based assay was used in this study. MT4 cells (4,000 cells) were treated with BA derivatives or the proteaome inhibitor LLM-f (Catalog #I-170, Boston Biochem, Cambridge, MA, USA) in serum free medium at 37°C for 3 hours. MT4 cells are human T cells isolated from a patient with adult T-cell leukemia. MT4 cells were obtained from the NIH AIDS Research and Reference Reagent Program. The drug-treated MT4 cells were incubated with the Promega Proteasome-Glo Cell-Based Assay Reagent (Promega Bioscience, Madison, WI) for 10 minutes. The chymotrypsin-like proteasome activity was detected as the relative light unit (RLU) generated from the cleaved substrate in the reagent. Luminescence generated from each reaction condition was detected with a PerkinElmer Victor-3 luminometer (Shelton, CT, USA).
3. Results
We and others have studied the anti-HIV-1 activity of BA and its derivatives [15-22]. In an effort to identify potential cellular targets of these compounds, BA was tested against human 20S proteasomes. The chemical structure of BA is shown in Figure S1 (supplemental data). BA did not inhibit the chymotrypsin-like activity of 20S proteasomes (Fig. 1). In fact, the chymotrypsin-like activity was enhanced in the presence of BA. This result raised the possibility that BA could act as a proteasome activator. To test this possibility, the proteasome activator, PA28, was left out of the reaction mixtures. In the absence of PA28, 20S proteasome is inactive. BA was able to activate the chymotrypsin-like activity of 20S proteasome in a dose dependent manner (Fig. 2a). The reaction rate reached a plateau with BA at 10 μg/ml. The concentration of BA required to achieve 50% activation (EC50) is approximately 2.5 μg/ml.
Figure 1. BA enhanced the chymotrypsin-like activity of 20S proteasomes.
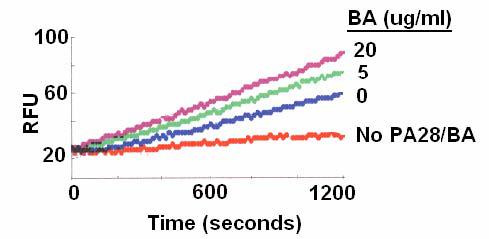
The cleavage of the proteasome substrate Suc-Leu-Leu-Val-Tyr-AMC in the presence of PA28 was quantified with the relative fluorescence units (RFU) measured at 360/460 nm over a 20 minute reaction time.
Figure 2. BA preferentially activated chymotrypsin-like proteasome activity.

Effects of BA on the chymotrypsin-like activity (a), trypsin-like activity (b), and caspase-like activity (c) of 20S proteasomes were determined in the absence of PA28.
Although BA can activate the proteasome activity, its mode of action is clearly different from that of PA28 or SDS (sodium dodecyl sulfate). PA28 and SDS are two proteasome activators that are commonly used in proteasome assays. Both PA28 and SDS can activate the three main proteasome activities: chymotrypsin-like, trypsin-like, and caspase-like activities. In contrast, BA did not activate the trypsin-like activity and exhibited minimal activation on the caspase-like activity of 20S proteasomes (Fig. 2b and 2c). PA28 activates proteasome by inducing conformational changes in 20S proteasome, which allows the proteasome substrates to access the proteolytic sites inside the 20S proteasome cylinder. On the other hand, SDS activates proteasomes presumably by partially denaturing the 20S proteasome, and that allows substrates to access the catalytic sites. Therefore, BA appears to activate the chymotrypsin-like activity in a mode distinct from that of PA28 or SDS.
Activation of proteasome by BA raised the possibility that BA derivatives might also activate 20S proteasomes. There are three sites on the BA structure that can be used for chemical modifications: C-3, C-20, and C-28. We have previously shown that the anti-HIV-1 activity of BA derivatives is highly dependent on where their side chain modification resides [21, 22]. A series of BA derivatives with modification at C-3 and C-28 positions were tested for their effect on 20S proteasomes. Instead of activation of proteasomes, some of the tested BA derivatives inhibited the chymotrypsin-like activity at low μg/ml concentrations (Table 1).
Table 1.
Effect of BA derivatives on the chymotrypsin-like activity of 20S proteasome.
 | |||
|---|---|---|---|
| Compounds | Structures | IC50# (μg/ml) | |
| R1 | R2 | ||
| DSB*1 (2) | 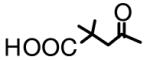 |
OH | 4.0 |
| A15*1 (3) |  |
OH | 8.2 |
| A16*1 (4) | OH | >20 | |
| A18*1 (5) | OH | >20 | |
| LH68*1 (6) | 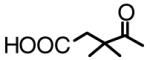 |
OH | 6.5 |
| LH141*2 (7) |
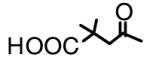 |
>20 | |
| IC9564*2 (8) |
H | >20 | |
A typical example of BA derivatives that inhibited 20S proteasomes is shown in Figure 3. The BA derivative 3',3'-dimethyl-succinyl BA (DSB, 2)) inhibited the chymotrypsin-like activity of 20S proteasome by 50% at a concentration of approximately 4 μg/ml (Fig. 3a). However, DSB with an additional side chain modification at position C-28 (compound LH141, 7) was inactive against proteasomes (Table 1). The BA derivative IC9564 (8) with the same C-28 side chain as LH141 was also inactive (Table 1). This C-28 side chain also abrogated the ability of BA to activate 20S proteasomes. IC9564 did not activate 20S proteasome in the absence of PA28 (data not shown). These results suggested that the C-3 modifications could transform BA from a proteasome activator into an inhibitor and addition of a side chain at C-28 could nullify the activation activity of BA and inhibitory activity of DSB.
Figure 3. The BA derivative, DSB, preferentially inhibited chymotrypsin-like proteasome activity.

The chymotrypsin-like activity (a), trypsin-like activity (b), and caspase-like activity (c) of 20S proteasomes were determined in the presence of various concentrations of DSB as indicated.
The trypsin-like activity of 20S proteasomes was less sensitive to DSB. It took approximately 12 μg/ml of DSB to inhibit the trypsin-like activity by 50% (Fig. 3b). On the other hand, DSB did not inhibit the caspase-like activity of proteasomes (Fig. 3c). These results suggested that BA derivatives can become a new class of proteasome inhibitors that preferentially inhibit chymotrypsin-like activity of proteasomes.
Inhibition of proteasomes by DSB could be a result of competition with the cellular proteasome activator, PA28, in the reaction mixture. To test this possibility, PA28 was replaced with 0.3% SDS as proteasome activator in the assay mixture. Under this assay condition, DSB inhibited the proteasome activity by 50% at approximately 3 μg/ml (Fig. 4). This inhibitory activity is comparable to that when PA28 was used as the proteasome activator (Figure 3a). Therefore, the data suggest that the inhibitory activity of DSB is not due to the inhibition of PA28 binding to proteasomes.
Figure 4. DSB inhibited SDS-activated chymotrypsin-like proteasome activity.

The SDS-activated chymotrypsin-like activity of 20S proteasomes was determined in the presence of various concentrations of DSB as indicated.
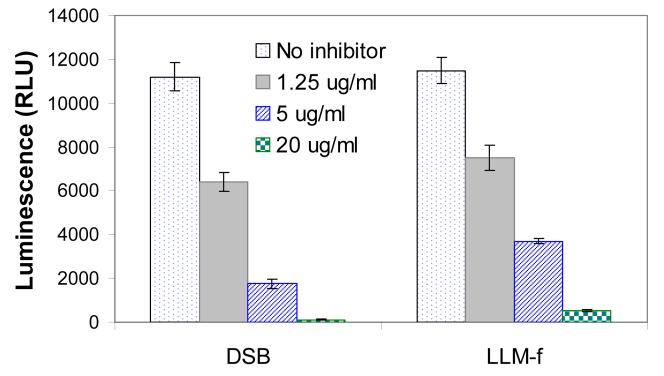
Although DSB could inhibit purified 20S proteasomes, it is not clear whether the compound can effectively inhibit proteasomes in the cells. To determine the effect of DSB on proteasomes in living cells, MT4 cells were treated with DSB or a known proteasome inhibitor Ac-Leu-Leu-Met-CHO (LLM-f). The chymotrypsin-like activity of proteasomes in the cells was analyzed using a Promega cell-based proteasome assay. DSB effectively inhibited the proteasome activity with an IC50 approximately 2 ug/ml (Figure 5). Thus, DSB is approximately 2-fold more potent in the cell-based assay than in the assays using purified proteasomes. It is not clear why DSB is slightly more potent in the cell-based assay than in the purified proteasome assay. One possible explanation is that DSB might have accumulated to a higher concentration in the cells.
Figure 5. DSB inhibited the chymotrypsin-like proteasome activity in MT4 cells.
MT4 cells were treated with various concentrations of DSB or LLM-F as indicated. Each data point represents the mean +/− SD of three duplicated experiments.
4. Discussion
The results of this study demonstrated that BA could directly interact with purified proteasomes and preferentially activate the chymotrypsin-like activity of proteasomes with minimal effect on the trypsin-like and caspase-like activities of proteasomes. This is consistent with the finding that BA-induced degradation of transcription factors in LNCaP cells could be abrogated by proteasome inhibitors, such as MG132 [23]. Other than proteasome activation, BA was shown to induce apoptosis by direct effects on mitochondria leading to cytochrome-c release [24-27]. Cytochrome-c release from mitochondria regulates the downstream caspase activation and ultimately leads to cell death. BA was shown to inhibit the growth of melanoma cell lines at low micromolar concentrations. Due to its potential anti-cancer activity, BA is currently undergoing phase II clinical trials for dysplastic melanocytic nevus (web site:ClinicalTrials.gov).
While BA is promising for its anti-cancer activity, BA derivatives have been extensively studied for their potential as anti-HIV agents. The mechanism of anti-HIV action is dependent on side chain locations. BA derivatives with C-3-hydroxyl esterified compounds inhibited HIV-1 replication by interfering with HIV-1 maturation [28, 29]. DSB (also designated as Bevirimat) is a typical C-3 substituted BA derivative exhibiting potent anti-HIV-1 activity [15, 16]. DSB is currently under phase II clinical trial as an orally active anti-HIV-1 drug. DSB at a daily dose of 100 mg resulted in a peak plasma concentration over 30 μg/ml (Martin, D. et al. Abstract 551, 12th Conference on Retroviruses and Opportunistic Infection, 2005). The inhibitory activity of DSB on proteasomes might have the potential to cause side effects if the compound is used for AIDS therapy, especially if the plasma concentration of DSB is higher than the concentration that inhibits proteasomes.
In contrast to the anti-HIV-1 maturation activity of the C-3 derivatives, BA derivatives with a side chain modification at C-28 inhibited HIV-1 entry. For example, IC9564 is a C-28 substituted BA derivative that blocks HIV-1 entry [17]. Unlike DSB, IC9564 did not affect 20S proteasome.
Although targeting proteasomes might be a concern if the C-3 substituted BA derivatives are used as anti-viral agents, the most important attribute of this study is the potential clinical and biomedical applications of the proteasome activators and inhibitors due to the importance of proteasomes in multiple cellular processes. For example, the proteasome inhibitor Bortezomib was successfully developed into a useful anti-cancer drug for the treatment of multiple myeloma [10]. In addition, over-expression of the proteasome activator PA28 was shown to enhance survival of Huntington's disease (HD) neuronal model cells [30].
In summary, the results of this study indicate that BA preferentially activates the chymotrypsin-like activity of proteasomes. BA can be transformed into proteasome inhibitors by simple chemical modifications at the C-3 position of the molecules. It would be interesting to study whether BA could have beneficial effects on neurodegenerative diseases, such as Huntington's disease. In addition, BA derivatives that inhibit proteasomes might have the potential to be developed into therapeutics for cancers or inflammatory diseases.
Supplementary Material
5. Acknowledgement
This study is supported by NIH grants: AI52022 and AI65310.
Abbreviations
- BA
betulinic acid
- DSB
dimethylsuccinyl betulinic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- 1.Goldberg AL, Dice JF. Intracellular protein degradation in mammalian and bacterial cells. Annu. Rev. Biochem. 1974;43:835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg AL, John AC. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu. Rev. Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- 3.Rechsteiner M, Hill CP. Mobilizing the proteolytic machine: cell biological roles of proteasome activators and inhibitors. Trends Cell Biol. 2005;15:27–33. doi: 10.1016/j.tcb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Hill CP, Masters EI, Whitby FG. The 11S regulators of 20S proteasome activity. Curr. Top. Microbiol. Immunol. 2002;268:73–89. doi: 10.1007/978-3-642-59414-4_4. [DOI] [PubMed] [Google Scholar]
- 5.Ustrell V, Hoffman L, Pratt G, Rechsteiner M. PA200, a nuclear proteasome activator involved in DNA repair. EMBO J. 2002;21:3516–3525. doi: 10.1093/emboj/cdf333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rechsteiner M, Realini C, Ustrell V. The proteasome activator 11 S REG (PA28) and class I antigen presentation. Biochem. J. 2000;345:1–15. [PMC free article] [PubMed] [Google Scholar]
- 7.DeMartino GN, Slaughter CA. The proteasome, a novel protease regulated by multiple mechanisms. J. Biol. Chem. 1999;274:22123–22126. doi: 10.1074/jbc.274.32.22123. [DOI] [PubMed] [Google Scholar]
- 8.Voges D, Zwickl P, Baumeister W. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu. Rev. Biochem. 1999;68:1015–1068. doi: 10.1146/annurev.biochem.68.1.1015. [DOI] [PubMed] [Google Scholar]
- 9.Borissenko L, Groll M. 20S proteasome and its inhibitors: crystallographic knowledge for drug development. Chem. Rev. 2007;107:687–717. doi: 10.1021/cr0502504. [DOI] [PubMed] [Google Scholar]
- 10.Kane RC, Bross PF, Farrell AT, Pazdur R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8:508–513. doi: 10.1634/theoncologist.8-6-508. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Chen D, Cui QC, Yuan X, Dou QP. Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006;66:4758–4765. doi: 10.1158/0008-5472.CAN-05-4529. [DOI] [PubMed] [Google Scholar]
- 12.Yang H, Shi G, Dou QP. The tumor proteasome is a primary target for the natural anticancer compound Withaferin A isolated from “Indian winter cherry”. Mol Pharmacol. 2007;71:426–437. doi: 10.1124/mol.106.030015. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka K, Ii K, Ichihara A, Waxman L, Goldberg AL. A high molecular weight protease in the cytosol of rat liver. I. Purification, enzymological properties, and tissue distribution. J. Biol. Chem. 1986;261:15197–15203. [PubMed] [Google Scholar]
- 14.Wilk S, Chen WE. Synthetic peptide-based activators of the proteasome. Mol. Biol. Rep. 1997;24:119–24. doi: 10.1023/a:1006851428691. [DOI] [PubMed] [Google Scholar]
- 15.Kashiwada Y, Hashimoto F, Cosentino LM, Chen CH, Garrett PE, Lee KH. BA and dihydroBA derivatives as potent anti-HIV agents. J. Med. Chem. 1996;39:1016–1017. doi: 10.1021/jm950922q. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto F, Kashiwada Y, Cosentino LM, Chen CH, Garrett PE, Lee KH. Anti-AIDS agents-XXVII. Synthesis and anti-HIV activity of BA and dihydroBA derivatives. Bioorg. Med. Chem. 1997;5:2133–2143. doi: 10.1016/s0968-0896(97)00158-2. [DOI] [PubMed] [Google Scholar]
- 17.Holz-Smith SL, Sun IC, Jin L, Matthews TJ, Lee KH, Chen CH. Role of human immunodeficiency virus (HIV) type 1 envelope in the anti-HIV activity of the BA derivative IC9564. Antimicrob. Agents Chemother. 2001;45:60–66. doi: 10.1128/AAC.45.1.60-66.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun IC, Chen CH, Kashiwada Y, Wu JH, Wang HK, Lee KH. Anti-AIDS agents 49. Synthesis, anti-HIV, and anti-fusion activities of IC9564 analogues based on BA. J. Med. Chem. 2002;45:4271–4275. doi: 10.1021/jm020069c. [DOI] [PubMed] [Google Scholar]
- 19.Mayaux JF, Bousseau A, Pauwels R, Huet T, Henin Y, Dereu N, Evers M, Soler F, Poujade C, De Clercq E, Le Pecq JB. Triterpene derivatives that block entry of human immunodeficiency virus type 1 into cells. Proc. Natl. Acad. Sci. 1994;91:3564–3568. doi: 10.1073/pnas.91.9.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soler F, Poujade C, Evers M, Carry JC, Henin Y, Bousseau A, Huet T, Pauwels R, De Clercq E, Mayaux JF, Le Pecq JB, Dereu N. BA derivatives: a new class of specific inhibitors of human immunodeficiency virus type 1 entry. J. Med. Chem. 1996;39:1069–1083. doi: 10.1021/jm950669u. [DOI] [PubMed] [Google Scholar]
- 21.Huang L, Xiong Y, Aiken C, Chen CH. Bifunctional anti-HIV-1 small molecules with two novel mechanisms of action. Antimicrob. Agents Chemother. 2004;48:663–665. doi: 10.1128/AAC.48.2.663-665.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang L, Ho P, Lee KH, Chen CH. Synthesis and anti-HIV activity of bi-functional BA derivatives. Bioorg. Med. Chem. 2006;14:2279–2289. doi: 10.1016/j.bmc.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Chintharlapalli S, Papineni S, Ramaiah SK, Safe S. Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Res. 2007;67:2816–2823. doi: 10.1158/0008-5472.CAN-06-3735. [DOI] [PubMed] [Google Scholar]
- 24.Pisha E, Chai H, Lee IS, Chagwedera TE, Farnsworth NR, Cordell GA, Beecher CW, Fong HH, Kinghorn AD, Brown DM, Wani MC, Wall ME, Hieken TJ, Das Gupta TK, Pezzuto JM. Discovery of BA as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995;1:1046–1051. doi: 10.1038/nm1095-1046. [DOI] [PubMed] [Google Scholar]
- 25.Fulda S, Jeremias I, Pietsch T, Debatin KM. BA: a new chemotherapeutic agent in the treatment of neuroectodermal tumors. Klin. Padiatr. 1999;211:319–322. doi: 10.1055/s-2008-1043808. [DOI] [PubMed] [Google Scholar]
- 26.Fulda S, Friesen C, Los M, Scaffidi C, Mier W, Benedict M, Nunez G, Krammer PH, Peter ME, Debatin KM. BA triggers CD95 (APO-1/Fas)- and p53-independent apoptosis via activation of caspases in neuroectodermal tumors. Cancer Res. 1997;57:4956–4964. [PubMed] [Google Scholar]
- 27.Schmidt ML, Kuzmanoff KL, Ling-Indeck L, Pezzuto JM. BA induces apoptosis in human neuroblastoma cell lines. Eur J Cancer. 1997;33:2007–2010. doi: 10.1016/s0959-8049(97)00294-3. [DOI] [PubMed] [Google Scholar]
- 28.Li F, Goila-Gaur R, Salzwedel K, Kilgore NR, Reddick M, Matallana C, Castillo A, Zoumplis D, Martin DE, Orenstein JM, Allaway GP, Freed EO, Wild CT. PA-457: a potent HIV inhibitor that disrupts core condensation by targeting a late step in Gag processing. Proc. Natl. Acad. Sci. U S A. 2003;100:13555–13560. doi: 10.1073/pnas.2234683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J, Xiong Y, Dismuke D, Forshey BM, Lundquist C, Lee KH, Aiken C, Chen CH. Pharmacologic Inhibition of HIV-1 Replication by a Novel Mechanism: Specific Interference with the Final Step of Virion Maturation. J. Virol. 2004;78:922–929. doi: 10.1128/JVI.78.2.922-929.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seo H, Sonntag KC, Kim W, Cattaneo E, Isacson O. Proteasome Activator Enhances Survival of Huntington's Disease Neuronal Model Cells. PLoS ONE. 2007;2:e238. doi: 10.1371/journal.pone.0000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.