Abstract
Although social, physical, and cognitive activities have each been suggested to reduce the risk of Alzheimer’s Disease (AD), epidemiologic studies cannot determine which activity or combination of activities is most important. To address this question, mutant APP transgenic AD mice were reared long-term in one of four housing conditions (impoverished, social, social+physical, or complete enrichment) from 1½ through 9 months of age. Thus, a stepwise layering of social, physical, and enhanced cognitive activity was created. Behavioral evaluation in a full battery of sensorimotor, anxiety, and cognitive tasks was carried out during the final 5 weeks of housing. Only AD mice raised in complete enrichment (i.e., enhanced cognitive activity) showed: 1) protection against cognitive impairment, 2) decreased brain β-amyloid deposition, and 3) increased hippocampal synaptic immunoreactivity. The protection provided by enhanced cognitive activity spanned multiple cognitive domains (working memory, reference learning, and recognition/identification). Cognitive and neurohistologic benefits of complete enrichment occurred without any changes in blood cytokine or corticosterone levels, suggesting that enrichment-dependent mechanisms do not involve changes in the inflammatory response or stress levels, respectively. These results indicate that the enhanced cognitive activity of complete enrichment is required for cognitive and neurologic benefit to AD mice – physical and/or social activity are insufficient. Thus, our data suggest that humans who emphasize a high lifelong level of cognitive activity (over and above social and physical activities) will attain the maximal environmental protection against AD.
Keywords: Alzheimer’s disease, Transgenic mice, Cognitive activity, Physical activity, Social activity, β-amyloid, Synaptic immunoreactivity, Environmental enrichment
Introduction
Environmental factors have long been suspected of impacting the development of Alzheimer’s Disease (AD). In this context, retrospective studies have suggested that cognitive activity/occupational attainment during mid-life is associated with a reduced risk of AD (Friedland et al., 2001, Stern et al., 1994; Smyth et al., 2004; Valenzuela & Sachdev, 2006). Although prospective 5-year longitudinal studies in aged humans have similarly suggested that participation in cognitively stimulating activities decreases AD risk (Letenneur et al., 1999; Verghese et al., 2003; Wilson et al., 2002), controlled longitudinal studies over many years have not been done to assess the protective benefits of intellectual activity against AD. In addition, cross-sectional, prospective, and retrospective epidemiological studies have proposed that high levels of social (Scarmeas et al., 2001; Wang et al., 2002) or physical activity (Abbott et al., 2004; Larson et al., 2006; Podewils et al., 2005) reduce the risk of AD. The extent to which cognitive, social, and or physical activities protect against AD cannot be unequivocally determined in humans for several reasons: 1) retrospective studies cannot be controlled and rely on recall, which can be inaccurate, 2) well-controlled longitudinal studies over decades are impractical and usually do not establish that baseline performance levels are the same for each group, and 3) the impact of cognitive, social, and physical activities cannot be evaluated independently from one another, nor can these activities be unequivocally isolated from other life style factors (i.e., diet) that can impact AD risk. As well, such observational studies in humans cannot determine whether any of these three environmental activities is a promoter of intact cognition or simply a self-selected marker of same.
Fortunately, the availability of mutant amyloid precursor protein (APP) transgenic mice in recent years has made it possible to evaluate the potential of environmental activities (cognitive, social, and/or physical) to protect against or treat AD-like characteristics in well-controlled studies. These mouse models develop at least some AD neuropathology [e.g., β-amyloid (Aβ)-containing neuritic plaques] and become cognitively impaired during aging (Chen et al., 2000; Arendash et al., 2001a; 2004a; Westerman et al., 2002; Jensen et al., 2005; Nilsson et al., 2004). In the first study to evaluate the potential of environmental enrichment as a therapeutic in AD transgenic mice, we found that complete environmental enrichment (EE; continuous cognitive, social, and physical activity) improves cognitive performance in aged APPsw mice without affecting Aβ deposition (Arendash et al., 2004b). These results suggest that long-term intensive EE could stabilize or slow the cognitive decline of AD without necessitating a reduction in brain Aβ load. Indeed, “cognitive rehabilitation” (cognitive stimulation) in AD patients has resulted in improved performance in targeted tasks (Davis et al., 2001; Farina et al., 2002; Loewenstein et al., 2004), although these benefits have yet to translate into clearly improved overall cognition or quality of life. Nonetheless, a recent meta-analysis of 19 controlled cognitive stimulation (CS) studies in AD patients did reveal sizable effects of CS on executive functioning, verbal learning, and visual learning (Sitzer et al., 2006).
In a study to determine if complete EE can “protect” PDAPP+PS1 transgenic mice against cognitive impairment, we began EE in young adulthood and continued it through older age (Costa et al., 2006). Life-long EE was found to protect these AD transgenic mice against cognitive impairment across multiple cognitive domains (e.g., working memory, reference memory, and recognition/identification) and for both genders. Moreover, EE substantially decreased brain Aβ deposition in mice given both EE and behavioral testing (Costa et al., 2006). EE also was found to produce beneficial changes in hippocampal expression of genes related to Aβ sequestration, memory, and neuronal/synaptic plasticity. These results show that complete EE can protect against AD-like cognitive impairment and that both amyloid-dependent and amyloid-independent mechanisms are involved in that protection. Several other AD transgenic mouse studies have also reported EE-induced decreases in Aβ deposition (Lazarov et al., 2005) or EE-induced protection of cognitive function (Jankowsky et al., 2005).
The current study addresses the critical issue of which component of complete EE – social, physical, or cognitive activity – is most important for cognitive protection in AD transgenic mice. Some studies involving wild type (normal) rodents have reported that single housing can impair memory in some tasks (Schrijver et al., 2004; Voikar et al., 2005), while other studies failed to show social activity (i.e., social housing) improving cognitive performance compared to single housing (Williams et al., 2001). With a larger body of studies, physical (running wheel) activity in wild-type rodents has been reported to provide beneficial effects on gene expression (Cotman & Berchtold, 2002), growth factor levels (Johnson et al., 2003), and neurogenesis (van Praag et al., 1999, 2005; Kitamura et al., 2003). Voluntary wheel running has also been shown to improve spatial learning in “normal” young adult and aged rodents (van Praag et al., 1999, 2005; Anderson et al., 2000; Vaynman et al., 2004). In AD transgenic mice, however, divergent results have been reported for effects of physical activity, through voluntary wheel running activity, begun at 1½ - 2½ months of age. One study reported that wheel running decreased brain Aβ levels/deposition and enhanced the rate of Morris maze learning in the TgCRND8 model (Adlard et al., 2005). By contrast, another study reported wheel running to have no effect on brain Aβ deposition and no cognitive benefit in Morris maze learning for the APP-23 model (Wolf et al., 2004). Both studies found no memory retention benefit during the probe trial of Morris maze testing. These limited AD transgenic mouse findings, as well as the difficulties inherent to interpreting epidemiological human studies (Abbott et al., 2004 ; Podewils et al., 2005; Larson et al., 2006), suggest that the extent to which physical activity reduces the risk of AD has yet to be resolved.
In summary, no prior study involving AD transgenic mice has elucidated the relative contribution of social, physical, and cognitive activities to the cognitive protection provided by complete EE. The present study addressed this important issue in AD transgenic mice, as well as provided the initial analysis of housing effects on hippocampal synaptophysin staining and blood levels of corticosterone and cytokines in the same AD transgenic mice. It was found that enhanced cognitive activity, over and above that provided by social and/or physical activity, is required to protect against cognitive impairment in AD transgenic mice. Moreover, only the enhanced cognitive activity provided by complete EE reduced brain Aβ deposition and increased synaptic immunoreactivity in the hippocampus. Our results indicate that, regarding daily activities, a high level of cognitive activity is critical to protecting against AD-like cognitive impairment.
Materials and Methods
Animals and General Protocol
All mice contained a mixed background of C57B6, B6D2F1, SJL, and SW. Mice were generated from a cross between male mice, heterozygous for the mutant APPK670N, M671L gene (i.e., the APPsw, Swedish mutation derived from Tg2576 mice), and mutant PS1 (6.2 line) females bearing the M146V mutation. Mice were initially genotyped at the time of weaning and then had a confirmatory genotyping at 4 months of age. A total of 38 mice were used in three genotypic categories: non-transgenic mice, APP+PS1 double transgenic mice (e.g., mice bearing both APPsw and PS1 mutations), and APP single transgenic mice (e.g., mice bearing only the APPsw mutation). Mice were randomly divided into the various housing groups, where they lived through testing. Mice were housed and maintained in a specific pathogen-free facility under a 14 h /10 h light-dark cycle, with ad libitum access to rodent chow and water.
Beginning at 6 weeks of age, transgenic mice were moved from standard social cages to one of the following housing environments: an impoverished environment (n= 8), a social housed environment (n=8), a physical activity environment (n=5), or into a “complete” enrichment environment (n=6). An additional group of non-transgenic littermates (n=11) was socially housed as a control. At 6 months into their respective housing conditions (and at 7.5-8.5 months of age), all mice were tested in a 5-week behavioral battery, while still living in their designated housing. Following completion of the behavioral battery at 8.5-9.5 months of age, all animals were euthanized and brains of the APP+PS1 mice and non-transgenic controls were removed for histopathology and neurochemistry. All procedures used were reviewed and approved by the USF Institutional Animal Care and Use Committee (IACUC).
Enriched Environments
At 6 weeks of age, transgenic mice were placed into one of four test environments. In a step-wise ascension towards the “complete” enrichment paradigm, transgenic mice were put into an impoverished environment (IMP; 6 APP+PS1 and 2 APP mice), a social housed environment (SH; 4 APP+PS1 and 4 APP mice), a socially housed plus physical activity environment (PA; 5 APP+PS1), or into a “complete” enrichment environment (EE; 6 APP+PS1). Animals of the same gender, however of different genotypes, were housed together. Each housing group was gender-balanced at the beginning of the study. For the impoverished and social housing environments, APP+PS1 and APP mice performed identically, so their behavioral data were combined for statistical analysis. However, because APP+PS1 and APP mice were significantly different pathologically at euthanasia (e.g., lower Aβ plaques burdens in APP mice) the APP mice were eliminated from neuropathological and neurochemical analyses. A group of socially housed non-transgenic litermates were included as an additional control (NT; n=12).
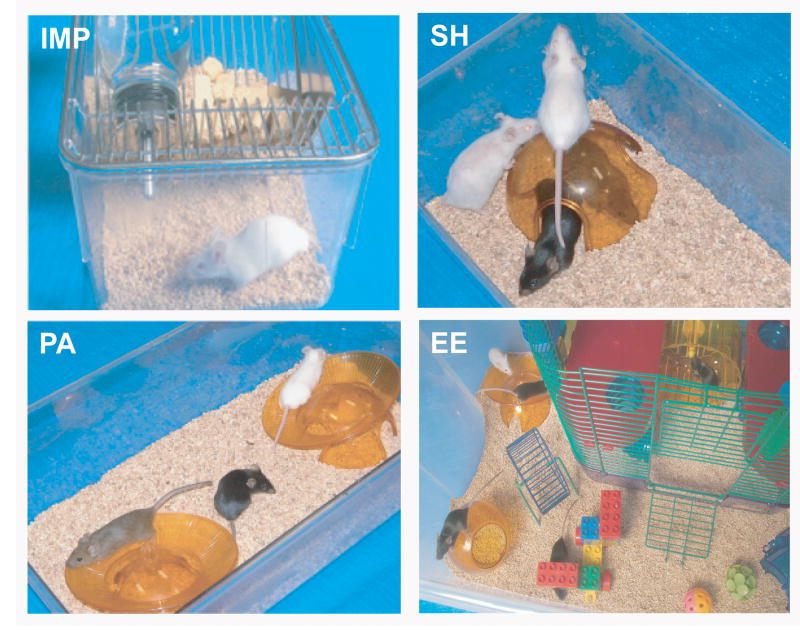
Animals living in an “impoverished environment” were housed individually (Fig. 1; IMP) and had access to food and water within their standard plexiglas mouse cage (6.5” wide, 10.5 ” long, 5.5” high). Socially housed mice lived in standard mouse cages with other mice (2-4 mice/cage) of the same gender, thus constituting a “social activity” group (Fig. 1; SH). Another group of socially housed mice had both a larger rat cage and access to running wheels. This “physical activity” group had cages that were 7” wide, 11” long, and 5” high, with each cage equipped with 2 running wheels (Fig. 1; PA). “Complete” enrichment mice had social, physical and cognitive stimulation. A 110 liter Sterilite container (19” wide, 32” long, 13.5” high), with an inner “CritterTrailTWO” rodent house, was used as housing for the “Complete” enrichment group (Fig. 1; EE). Housing for this group (5-7 mice/cage) not only involved various crawl-tubes and platforms within the rodent house, but also involved running wheels and toys in the courtyard surrounding the rodent house; these items were changed weekly for novelty. “Complete” enrichment mice were also placed in novel, complex environments for at least 1 hour 3 times a week over the course of the study. Thus, a stepwise ascension toward complete EE was created wherein the difference between impoverished and social housing was “social activity”; the difference between social and social+physical activity housing was “physical activity”; and the difference between social+physical and complete EE was enhanced “cognitive activity”. Parenthetically, we have found that mice in the later two housing environments show the same level of physical activity, particularly at night (Arendash et al., unpublished observations). Mice lived in their designated environment from 6 weeks of age until euthanasia at about 9 months of age. In order to provide equal social interaction, environments involving a social component (SH, PA, and EE) were supplemented with littermates of different genotypes at the time of weaning. These later mice were not included in any behavioral testing or pathologic/chemical analyses. All cages contained an igloo and neslets.
Fig. 1. Impoverished (IMP), social activity (SH), physical activity (PA), and complete environmental enrichment (EE) housing environments.
With this design, a step-wise ascension toward the complete EE paradigm was created. Transgenic mice were raised in one of these environments from 1.5 through 8.5 months of age. A group of socially housed non-transgenic mice were included as a standard housed control.
Behavioral Assessment
While still living in their selected housing environment, mice were tested in Y-maze, standard water maze, circular platform, platform recognition, and radial arm water maze tasks, in that order. All behavioral testing was conducted during the light cycle and according to our established procedures for each task (Jensen et al., 2005; Arendash et al., 2001a; 2004a; 2006).
Y-maze
In this single day task, each animal was placed in a walled Y-maze for a single 5-min trial to test both general activity (entries) and basic mnemonic processing (percent alternations). Each of the 3 Y-maze arms was 21 by 4 cm with 40 cm high walls. The total number of arm entries and the sequence of arm choices were recorded. Basic mnemonic function was measured as percentage of spontaneous alternation (ratio of arm choices differing from the previous two choices divided by the total number of entries). For example, the sequence of arm entries (2,3,1,3,2,1,2,3) has six alternation opportunities (total entries minus 2) and the percent alternation would be 67%.
Morris (Standard) Water Maze
A 100 cm circular pool was divided into 4 equal quadrants by drawing lines on the bottom of the pool. Quadrant 2 (goal quadrant) contained a clear, 9 cm diameter submerged platform, 1.5 cm below the water. Surrounding the pool were visual cues, which were placed proximal and distal to the pools edge. Visual/spatial cues consisted of large, brightly colored 2D and 3D objects, including a beach ball, poster, and inflatable pool toys. During testing, the pool water was maintained at 23-27° C. For each of the 10 days of acquisition, mice were given 4 trials. For each of the four successive 60-s trials per day, mice were started from a different quadrant; the same quadrant start pattern was used for all 10 days of acquisition. Latency to find the platform (maximum of 60 s) was recorded, and the average latency of the 4 trials was calculated for use in statistical evaluation. Once the mouse found the platform, it was allowed to stay for 30 s. If after 60 s the mouse did not find the platform, it was gently lead to the platform and given a 30 s stay. After 10 days of acquisition testing, memory retention was evaluated in a single 60 s probe trial the following day. For this trial, the submerged platform was removed and animals were released from the quadrant directly opposite the goal quadrant. This single trial was video recorded and the percent time spent in each quadrant, as well as the number of annulus crossings, was analyzed.
Circular Platform Task
The circular platform maze consisted of a 69 cm circular platform with 16 holes equally placed around the periphery of the walled maze. Surrounding the maze were two different colored shower curtains that had two-dimensional visual cues attached to them for use by the mice in navigating the maze. One of the holes contained an escape box that remained in the same location for all 8 days of testing. Animals were encouraged to find the escape hole by adding aversive stimuli of bright lights and fan wind to the maze. The aversive stimuli included two 150 W flood lamps hung 76 cm above the platform and one high-speed fan 15 cm above the platform. During a single 5-min daily trial, both the number of errors (head pokes into non-escape holes) and latency to find the escape hole (up to 300 s) were recorded. Although the escape hole remained the same for any given animal over 8 days of testing, the escape box was relocated after each animal’s trial to one of 3 other hole locations to control for olfactory cues. The maze surface was cleaned with dilute vinegar after each animal for added olfactory cue control.
Platform Recognition
The platform recognition task measures the ability to search for and identify/recognize a variably-placed elevated platform. It requires animals to switch to an identification/recognition strategy and to ignore the spatial cues present around a circular pool, which was the same pool used in earlier Morris water maze testing. A visible platform, 9 cm in diameter with an attached 10 × 40 cm ensign, was placed into the same 100 cm pool in which standard water maze testing was conducted. The visible platform was elevated 0.8 cm above the water’s surface. For 4 days of testing, animals were placed in the pool at the same start position for each of 4 trials, with the platform being moved to a different one of the 4 quadrants for each trial. The latency to ascend the platform was recorded (60 s maximum) and the daily 4 trials were averaged. A 30-s stay was given when the mice found the platform. Mice that did not find the platform within 60 s were gently guided to the platform by the experimenter and allowed to stay for 30 s.
Radial Arm Water Maze
Spatial working memory was assessed in a “win-stay” version of the radial arm water maze (RAWM) task. In the same 100 cm pool utilized for Morris Water Maze and Platform Recognition testing, an aluminum insert was introduced in order to divide the pool into 6 equally spaced swim arms (30.5 cm length × 19 cm width) radiating from a central circular swim area (40 cm diameter). The insert extended 5 cm above the surface of the water, allowing the mice to easily view surrounding visual cues, which were generously placed outside of the pool. Visual/spatial cues consisted of large brightly colored 2D and 3D objects, including a beach ball, poster, and inflatable pool toys. During testing, the pool water was maintained at 23-27° C. In one of the arms, a transparent 9 cm submerged escape platform was placed 1.5 cm below the water near the wall end. Each mouse was given five 1-min trials per day for nine days. The last of the four consecutive acquisition trials (Trial 4, T4) and a 30-min delayed retention trial (Trial 5, T5) are indices of working memory. On any given day, the escape platform location was placed at the end of one of the 6 arms, with the platform moved to a different arm in a semi-random fashion for each day of testing. In contrast to the stationary platform of Morris water maze, moving the escape platform forced the animal to learn a new platform location daily, therefore evaluating working memory. On each day, different start arms for each of the five daily trials were selected from the remaining five swim arms in a semi-random sequence that involved all five arms. For any given trial, the mouse was placed into that trial’s start arm, facing the center swim area, and given 60 s to find the platform. When the mouse made an incorrect choice, it was gently pulled back to that trial’s start arm and an error was recorded. An error was also recorded if the mouse failed to make a choice in 20 s (in which case it was returned to that trails start arm), or if the animal entered the platform-containing arm, but failed to locate the platform. A 30-s stay was given once the mouse had found the platform. If the mouse did not find the platform within a 60-s trial, it was guided by the experimenter to the platform, allowed to stay for 30 s, and was assigned a latency of 60 s. Both errors (incorrect arm choices) and escape latency were recorded for each daily trial.
Tissue and Blood Collection
Following behavioral testing on the final test day, a blood sample (0.5 ml) was taken from the submandibular vein, plasma was separated, and stored at -80°C for later analysis of corticosterone levels. Two days after plasma collection, animals were deeply anesthetized with pentobarbital and a second blood sample was collected intra-cardially, and stored at -80°C for later analysis of plasma cytokines. Animals were then perfused transcardially with 100 ml of 0.9% saline. Post mortem brains were immediately removed and bisected sagitally. The left hemisphere was placed in 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4) over night and then transferred to a graded series of sucrose solutions (10%, 20%, and finally 30%), wherein tissues remained until sectioning.
Corticosterone Quantification
Corticosterone (CS) levels were measured using a radioimmunoassay (RIA), utilizing the methodology of Newman et al. (2001). Serum levels of CS were determined using a double-antibody RIA kit purchased from ICN Biomedicals (Costa Mesa, CA). Samples from all mice were assayed in duplicate. Approximately 5 ng/ml was the minimum detectable concentration.
Blood Cytokine Levels
Relative cytokine levels were determined through the use of a custom Mouse Cytokine Antibody Array purchased from RayBiotech Incorporated, according to the manufacturer’s protocol. Ten separate anti-mouse-cytokine antibodies were provided on membranes. Cytokines analyzed included; IL1-α, IL1β, IL-2, IL-4, IL-6, IL-10, IL-12, IL-12 (p70), TFN-α, IFNγ, and GMCSF. Membranes were first treated with blocking buffer and then incubated with 1 ml of diluted (1:10) plasma for 1 h. Samples were decanted and 1x secondary biotinylated antibodies were added and incubated for one h. A second 2 h incubation was conducted with diluted (1:1000) labeled-streptavidin, completing the conjugated secondary antibody complex. The final detection step involved incubation for 1 min with provided detection buffers. Using Fuji film AR x-ray film, signals from membranes were detected and developed using back-lit photography. For duplicated samples, analysis of signal mean intensities minus background signal intensity were determined using Kodak 1D Image Analysis Software. In order to account for the large variability in signal intensities among the various cytokines, signals were standardized on a zero to one scale based on minimum and maximum mean intensity readings for each cytokine. Standardized values were then used to compare cytokine levels between animals.
Immunohistochemistry
Half brains that had been stored in 30% sucrose solution were removed, rinsed in dH2O, and frozen on the stage of a sliding microtome. At the level of the hippocampus, 25 μm thick coronal sections were collected and placed into wells containing PBS solution for later Aβ and synaptophysin immunochemical staining/analysis. Five sections, spaced 175 μm apart, were taken for each immunostain. Immunohistochemical staining was done based on the manufacturer’s protocol using a Vectastain ABC Elite kit (Vector Laboratories, Burlingame, CA) coupled with the diaminobenzidine reaction. Rabbit polyclonal anti-pan Aβ antibody (diluted 1:1,000; incubated at 4°C overnight, Biosource, Camarillo, CA) or rabbit polyclonal anti-human synaptophysin antibody (undiluted; incubated at 4°C overnight, DAKO, Carpinteria, CA) were used as primary antibodies. Phosphate-buffered saline (0.1 mM, pH 7.4) or normal rabbit serum (isotype control) was used instead of primary antibody or ABC reagent as a negative control. Although brain sections were not treated with formic acid prior to immunostaining, we have found concordance between brain Aβ immunostaining, thioflavin S staining, and ELISA-based Aβ levels within the same animals in a prior study (Costa et al., 2006).
Image Analysis
Quantitative image analysis was done based on previous methods with modifications (Tan et al., 2002). Images were acquired using an Olympus BX60 microscope with an attached digital camera system (DP-70, Olympus, Tokyo, Japan), and the digital image was routed into a Windows PC for quantitative analysis using SimplePCI software (Compix, Inc. Imaging Systems, Cranberry Township, PA). Images of five 25 μm sections (150 μm apart) through both anatomic regions of interest (hippocampus and entorhinal cortex) were captured from each animal, and a threshold optical density was obtained that discriminated staining from background. Each region of interest was manually edited to eliminate artifacts. For Aβ burden analysis, data are reported as percentage of immuno-labeled area captured (positive pixels) relative to the full area captured (total pixels). To evaluate synaptophysin immunoreactivity, after the mode of all images was converted to gray scale, the average intensity of positive signals from each image was quantified in the CA3 region of hippocampus as a relative number from zero (white) to 255 (black). Each analysis was done by a single examiner blinded to sample identities.
Statistical Analysis
Behavioral Analysis
Behavioral performance was statistically evaluated to determine any group difference based on housing condition or transgenicity. For both of the single day tasks (Y-maze and Morris water maze retention) one-way ANOVAs were used. For multi-day tasks (Morris water maze acquisition, circular platform, platform recognition and RAWM) both one-way ANOVAs and two-way repeated measure ANOVAs were performed. Prior to analysis, Morris water maze (MWM) data was broken down into five-2 day blocks and the RAWM data was divided into three-3 day blocks, to aid in data presentation and analysis. After ANOVA analysis, post hoc pair-by-pair differences between groups (planned comparisons) were resolved using the Fisher LSD test. All group comparisons were considered significant at p<0.05. While very few in number, any outliers or non-performers (e.g. repeated circulars, consistent floaters) in any given task were eliminated from behavioral statistical analysis of that task.
Neurohistologic Analysis
Neuroimmunologic data analyses involving Aβ burden and synaptophysin immunoreactivity were performed using ANOVA. In order to test if relationships were present between neurohistologic, plasma, and behavioral measures, correlation analysis was performed using the Systat analytical software package.
FA and DFAs
To group behavioral, plasma, and histologic measures by common factors, Factor Analyses (FA) were performed using Systat software. Regardless of genotype or housing, FA used all collected data to relate measures into individual factors. In an initial FA, all 15 behavioral measures were included. This enabled the determination of how different behavioral tasks related to one another, as well as how performance of one task might predict performance in other tasks. Follow-up FA’s included not only the 15 behavioral measures, but also the histologic and plasma measures as well. These FA’s extracted inter-relations within behavioral, histologic and plasma data. To determine if the 5 experimental groups (NT, IMP, SH, PA, and EE) were distinguishable from one another based on the behavioral measures set, DFA was performed using Systat software. DFA was performed using all 15 measures as well as with only the behavioral measures that loaded in factor 1 of factor analysis. Both a direct entry and stepwise-forward DFA were conducted in each case. The direct entry method uses all measures available, while the step-wise forward method selects measures based on their variance contribution and adds them in a step-wise fashion to best discriminate between groups. A more complete description of FA and DFA multi-metric statistical analyses can be found in Leighty et al. (2004).
Results
Behavioral Analysis
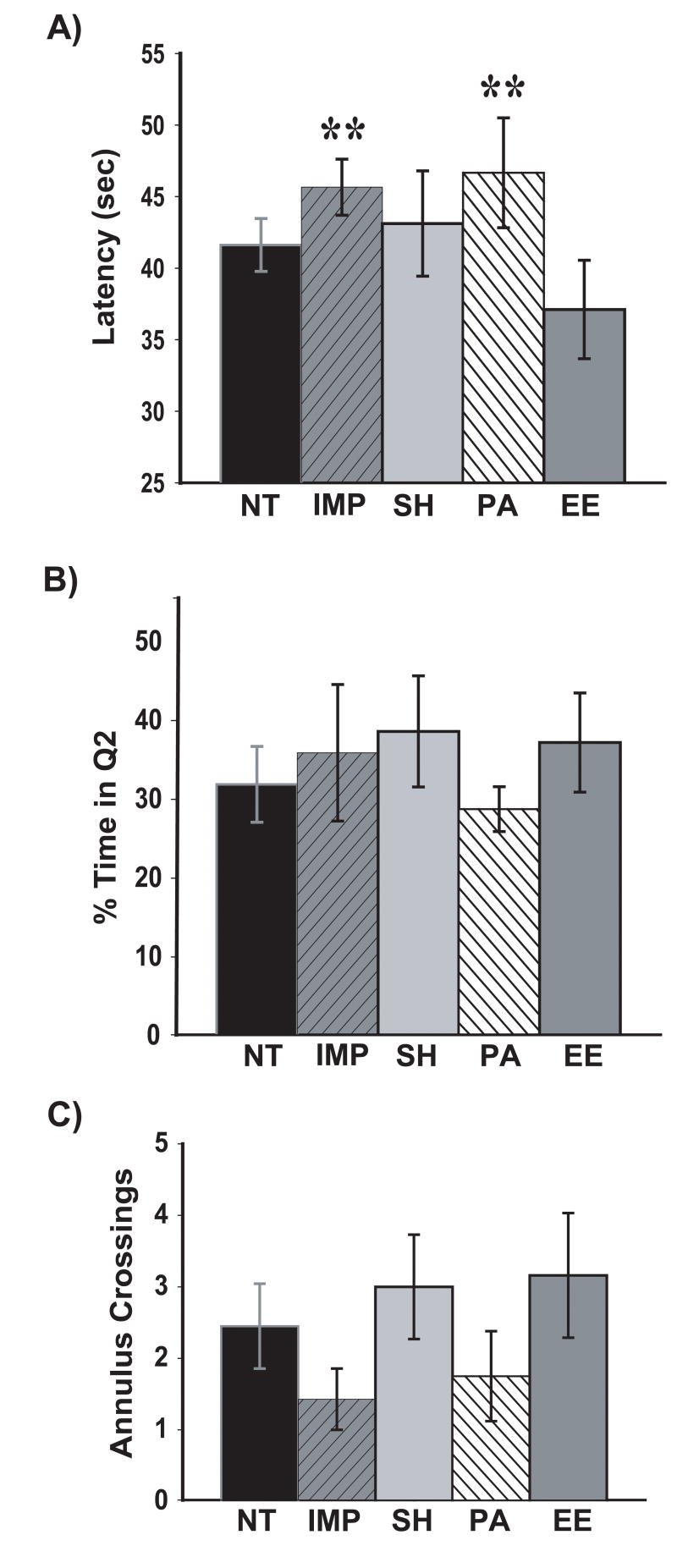
In Morris water maze (MWM) acquisition, escape latency data was analyzed over five 2 day blocks. Over all 5 blocks of testing, mice raised in complete environmental enrichment (EE) exhibited significantly lower escape latencies in comparison to both impoverished (IMP) and physical activity (PA) mice (Fig. 2A), although no effect of transgenicity was evident between non-transgenic (NT) and control socially housed (SH) mice. Evaluating acquisition across individual blocks revealed that EE mice had significantly lower escape latencies on Block 2 compared to all other groups (data not shown), indicating an ability of EE mice to improve their learning of this task faster than other transgenic groups. Also underscoring this faster acquisition rate of EE mice was a significant block by housing interaction [F(4,128)=1.92; p<0.025]. During the MWM probe trial, no housing group spent significantly more time searching in the quadrant formerly containing the submerged platform (Q2) than any other group (Fig 2B; F(2,29)=0.31; p= 0.87]. As well, there were no group differences in annulus crossings (Fig 2C; [F(4,29)=1.13; p= 0.36). Thus, for both indices of reference memory in Morris maze testing, EE mice did not perform significantly better than other transgenic groups.
Fig. 2. Morris water maze overall acquisition and probe-trial memory retention.
(A) EE mice showed better spatial learning than several other transgenic groups overall. (B and C) No group differences were observed for percent time spent in the former platform-containing quadrant (Q2) or in annulus crossing. * = EE group significantly lower latencies than IMP and PA (p<0.05). Abbreviations: NT, non-transgenic socially-housed mice; IMP, transgenic singly-house (impoverished) mice; SH, transgenic socially-housed mice; PA, transgenic socially-housed mice with physical activity; EE, transgenic “complete” environmentally-enriched mice.
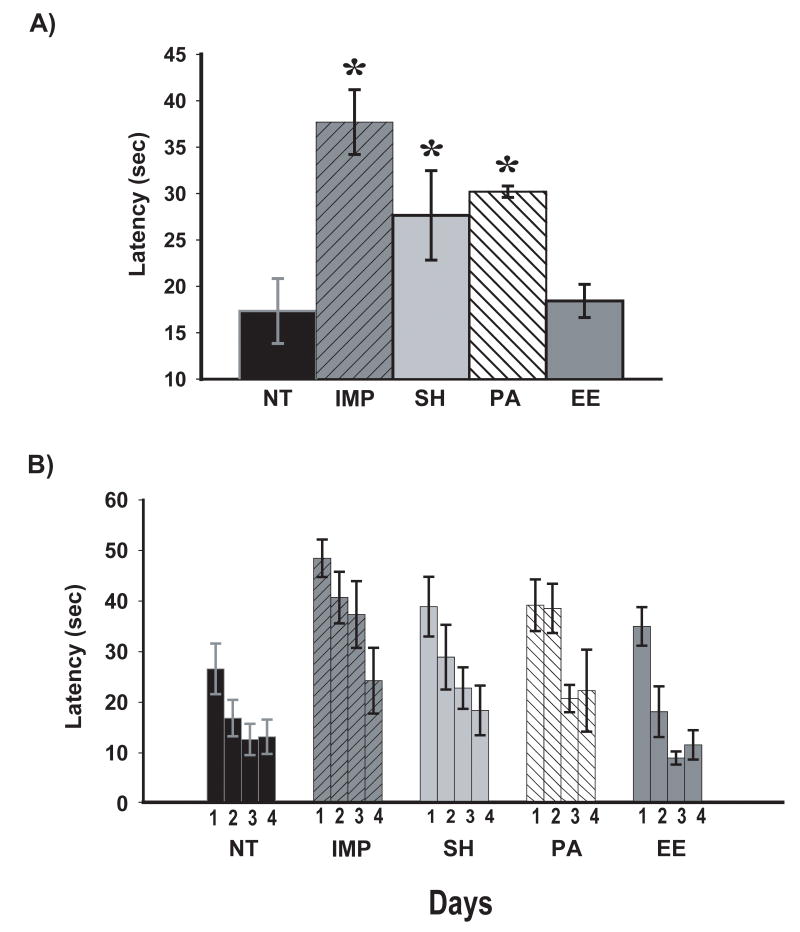
In platform recognition testing, an overall groups effects was present across all 4 days of testing [F(4,28)=5.04; p<0.005]. Post hoc planned comparisons of overall escape latencies revealed that IMP, SH, and PA groups were impaired overall vs. NT controls, whereas EE mice performed identical to NT controls (Fig. 3A). Inspection of performance over individual days shows that both NT and EE groups quickly reduced their escape latencies across the 4 days of testing (Fig. 3B). This rapid reduction in escape latency was not observed for IMP, SH and PA mice, and is consistent with the overall performance of EE mice being faster at changing from the spatial (cued) strategy of the MWM to the recognition/identification strategy of platform recognition. By Day 4, however, there were no group differences in escape latency, indicating that: 1) all housing groups were able to eventually reduce their escape latencies to levels comparable to NT controls, and 2) no defects in vision or ability to understand the purpose of the platform were present. This is primarily why the group by day interaction was not significant [P(3,84)=1.02; p=n.s.]. Moreover, all animals collectively improved their performance across the 4 days of testing, as evidenced by a strong overall effect of training [P(3,84)=25.91; p<0.00001]. Thus, “complete” enrichment is needed to protect AD transgenic mice against impaired strategy-switching, with physical activity and/or social interaction being insufficient.
Fig. 3. Recognition/Identification performance in platform recognition testing overall (A) and for each of the 4 days of testing (B).
Over all 4 days of testing (A), all transgenic groups except EE mice were significantly poorer in performance compared to NT controls. Across individual test days (B), NT mice and EE mice rapidly reduced their escape latency, while IMP, SH, and PA mice had a slower reduction in latency. * = significantly higher escape latencies than NT group at p<0.05 or higher levels of significance. Abbreviations: as in Figure 1.
In radial arm water maze (RAWM) testing of working memory, escape latencies were evaluated across three 3-day blocks for T1 (randomized initial trial), T4 (final acquisition trial) and T5 (delayed retention trial); T4 and T5 are indices of working memory. An overall groups effect was present for both T4 [F(4,28)=4.91; p<0.005] and T5 [F(4,28)=4.89; p<0.005]. Post hoc analysis of overall T4 and T5 latencies revealed that IMP, SH, and PA groups were significantly impaired vs. NT controls, whereas performance of EE mice was not different from NT controls and significantly better than the SH transgenic control group (Fig. 4A; p<0.05 for both T4 and T5). By the final block of testing, complete separation of EE and NT mice from all other groups was observed (Fig. 4B and C) For this important final block of testing, overall group effects were evident for both T4 [F(4,28)=2.77; p<0.05, Fig. 3B] and T5 [F(4,28)=4.90; p<0.005, Fig. 4C]. On T5 of the final block, NT control mice had significantly lower escape latencies than IMP, SH, and PA mice (p< 0.01). By contrast, EE mice were identical to NT mice and significantly better than all other Tg+ groups (p<0.025). There were no group differences in swim speed in this task [F(94,28)=1.08; p=0.38], as indexed by the number of seconds taken per arm choice during T4 and T5 overall [IMP, 12.8±1.2; SH, 14.0±1.1; PA, 11.6±1.5; EE, 10.6±1.2; NT, 11.6±1.3]. These RAWM data underscore findings from platform recognition in showing that “complete” EE protects AD transgenic mice against working memory impairment, while social housing and/or physical activity do not.
Fig. 4. Radial arm water maze overall and final block escape latencies.
(A) In overall working memory performance, NT had significantly lower escape latencies than IMP, PA, and SH mice on both overall trial 4 (T4) and trial 5 (T5). In contrast, EE mice were indistinguishable from the NT group. * = significant difference for NT vs. IMP, SH, and PA at p<0.02 or higher level of significance. (B, C) Final block escape latencies for working memory trials T4 and T5. For T4, NT mice achieved significantly lower escape latencies compared to SH and PA mice, while EE mice were no different from NT controls. * = significantly different from NT group at p<0.02. During T5, both NT and EE groups had significantly lower latencies compared to all other groups (IMP, SH, PA). ** = significantly different from both NT and EE at p<0.025 or higher level of significance. Abbreviations: as in Figure 1.
In the remaining two cognitive-based tasks evaluated (the Y-maze and circular platform), no impairment was evident in control Tg+/SH mice when compared to NT mice, thus no protective effect of EE could be present. All four transgenic groups performed similar to NT controls on all measures from both tasks (data not shown).
Plasma and Neurohistologic Measures
Plasma corticosterone levels
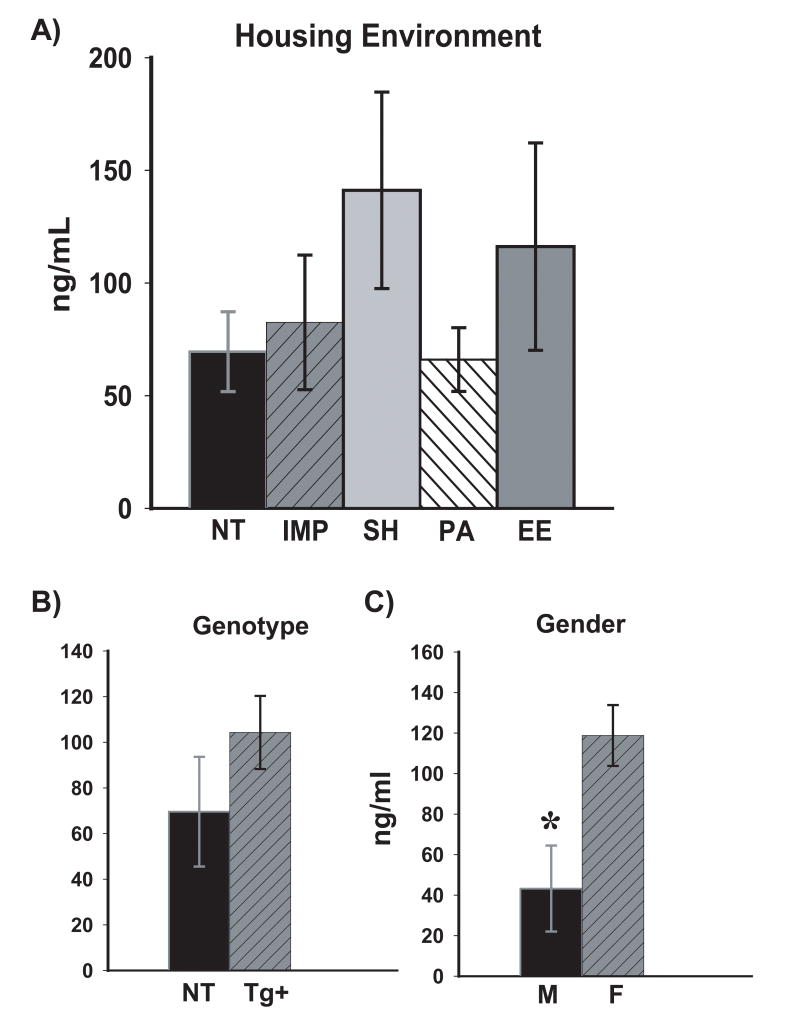
On the final day of behavioral testing and following that testing, a blood sample was collected to measure plasma corticosterone levels of mice living in different housing environments. Statistical analysis revealed no differences in corticosterone levels among animals in the different housing environments (Fig. 5A) and no overall genotype effect (Fig. 5B). The finding that different housing of the four transgenic groups did not affect plasma corticosterone levels suggests that stress did not play a role in the differences in cognitive performance observed among these groups. In contrast, male mice collectively had significantly lower levels of plasma corticosterone levels when compared to females [F(1,37)=8.44; p< 0.01]. Corticosterone levels were almost 3-fold lower in male mice vs. females (Fig. 5C). However, behavioral testing did not reveal any differences in performance between male and female mice in any housing condition or for either genotype.
Fig. 5. Plasma corticosterone levels by housing environment (A), genotype (B), and gender (C).
No housing or transgenic differences were observed in corticosterone levels. In contrast, male mice had almost 3-fold lower levels of corticosterone compared to female mice. * = significantly lower at p<0.01. Abbreviations: Tg+, all transgenic mice combined; M, male; F, female; all other abbreviations as in Figure 1.
Plasma cytokine levels
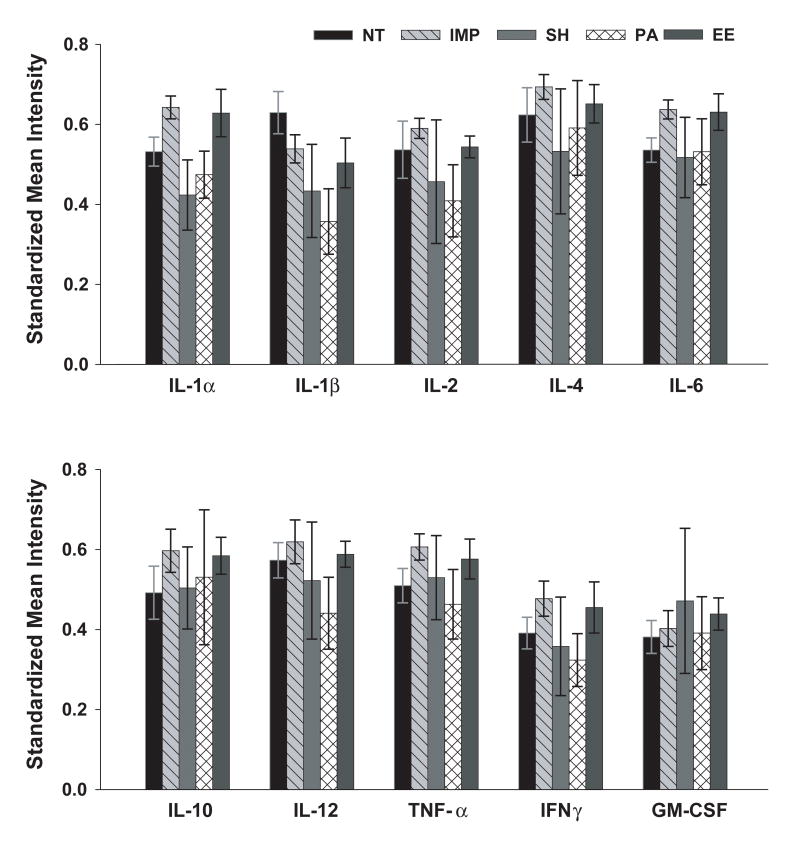
At euthanasia, 3 days following behavioral testing (and approximately 7 months into the various housing environments), a second blood sample was collected from APP+PS1 and NT mice for measurement of plasma cytokine levels. As shown in Fig. 6, no significant differences were observed among the housing groups for any of the 10 pro- and anti-inflammatory cytokines measured. Analysis by genotype revealed no differences in any of these cytokines between APP+PS1 mice collectively and NT mice (data not shown). Thus, APP+PS1 mice did not exhibit an elevated level of inflammation globally and no housing condition induced a change in inflammatory responses among APP+PS1 mice.
Fig. 6. Standardized mean signal intensities for 10 plasma cytokines in APP+PS1 and NT mice.
No group differences were observed for any cytokine measured. Abbreviations: as in Figure 1.
Aβ Histopathology
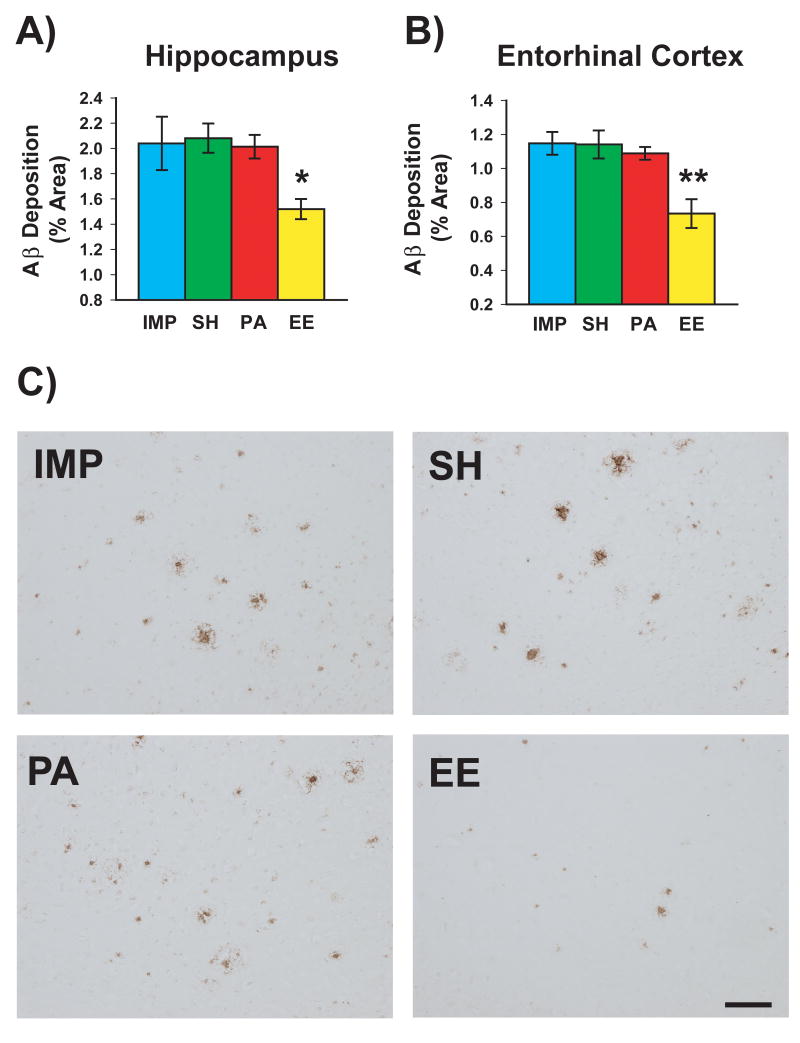
To study the effect of housing condition on Aβ immunoreactive deposits in APP+PS1 mice, total Aβ deposition was analyzed in both hippocampus and entorhinal cortex using rabbit polyclonal anti-pan Aβ antibody. A significant effect of housing on Aβ deposition was present in both hippocampus [F(3,17)=3.59; p<0.05] and entorhinal cortex [F<3,17)=8.24; p<0.002]. Compared to IMP mice, EE mice exhibited significant reductions in total Aβ deposition within both hippocampus (↓28%; p<0.02) and entorhinal cortex (↓36%; p<0.0005, respectively (Fig. 7A and B). By contrast, neither SH or PA mice showed reduced Aβ deposition in either brain area in comparison to IMP mice. Indeed, Aβ deposition in both hippocampus and entorhinal cortex was significantly reduced for EE mice compared to all other housing groups. Fig. 7C depicts Aβ immunostaining in representative hippocampal sections from APP+PS1 mice in each of the four housing conditions.
Fig. 7. Effects of housing environment on total Aβ deposition in APPsw+PS1 mice.
Of the 4 housing environments, only the enhanced cognitive activity provided by “complete” enrichment (EE) was capable of reducing Aβ deposition. Significant reductions in total Aβ deposition were seen in both hippocampus (A) and entorhinal cortex (B) for EE-housed mice. *p<0.25, **p<0.005 versus Tg mice housed in all other environments. (C) Photomicrographic examples of hippocampal Aβ immunostaining from Tg mice raised in impoverished housing (IMP), social housing (SH), physical activity housing (PA), and complete enrichment (EE) housing. Note the reduced Aβ deposition seen only in complete EE-reared mice. Scale bar = 100 μm
Synaptophysin Immunostaining
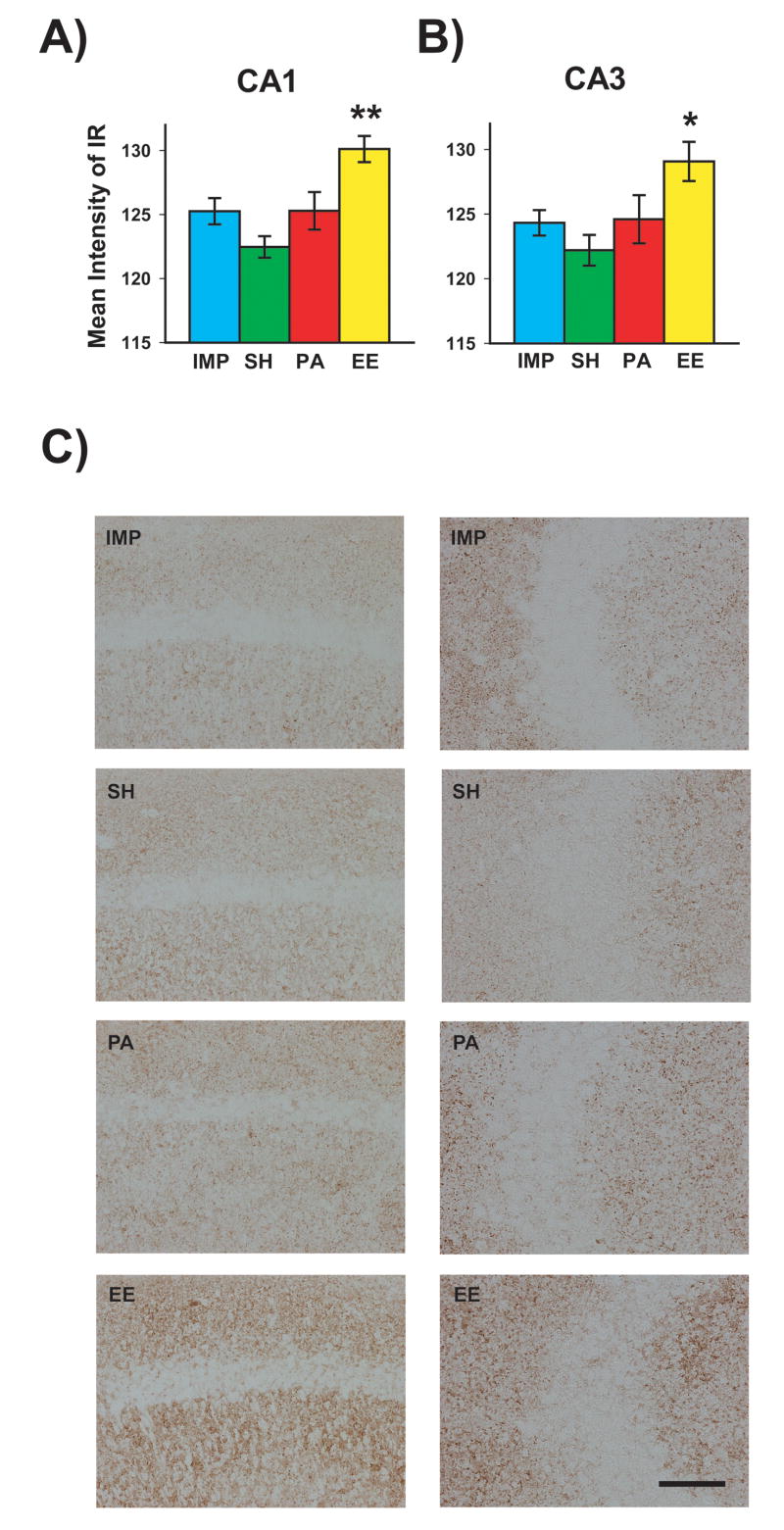
To determine the effect of housing condition on synaptic immunoreactivity in APP+PS1 mice, synaptophysin staining was analyzed in the CA1 and CA3 regions of the hippocampus. A highly significant effect of housing was evident for both CA1 [F(3,17)=7.78; p<0.002] and CA3 [F(3,17)=4.06; p<0.025]. Post hoc analysis revealed that transgenic mice reared in complete EE had significantly greater CA1 and CA3 synaptophysin staining compared to transgenic mice in any of the other three housing conditions (Fig. 8A, B). Thus, EE housing of APP+PS1 mice had induced a selective increase in synaptic immunoreactivity. Neither SH or PA mice exhibited increased synaptic immunoreactivity in CA3 compared to IMP mice. Representative synaptophysin-stained hippocampal sections from APP+PS1 mice in each of the four housing conditions are shown in Fig. 8C for CA1 (left panels) and CA3 (right panels).
Fig. 8. Synaptophysin immunoreactivity in CA1 and CA3 hippocampal areas for APPsw+PS1 mice raised in various housing environments.
Transgenic mice raised in “complete” EE (enhanced cognitive activity) had increased synaptophysin immunoreactivity in hippocampal CA1 (A) and CA3 regions (B) compared to transgenic mice raised in all other housing environments. *p<0.05, **p<0.005 vs. all other groups. (C) Photomicrographic examples of synaptophysin immunoreactivity in hippocampal CA1 (left panels) and CA3 (right panels) for transgenic mice raised in IMP, SH, PA, or EE housing. Note the enhanced synaptophysin immunoreactivity seen exclusively for Tg mice raised in complete EE housing (i.e., enhanced cognitive activity). Scale bar = 50 μm.
Correlation Analysis
Behavior vs. Plasma Corticosterone Levels
With all animals (NT and Tg+) included, no correlations were present between any of the 15 behavioral measures and plasma corticosterone levels. Similarly, for all Tg mice or individual Tg mouse housing groups, no correlations were evident.
Behavior vs. Plasma Cytokine Levels
For all APP+PS1 mice collectively, correlation analyses were performed between the 15 behavior measures and plasma levels of the 10 cytokines measured. A total of 18 significant correlations were present, with 17 of these involving either Morris water maze measures (14) or Y-maze percent alternation (3). Correlations between cognitive performance and plasma levels of both TNF-α and IFNγ were most prevalent. For all significant correlations, higher plasma cytokine levels were associated with better cognitive performance. A very similar pattern of correlations was present when all animals (NT and Tg+) were included in the correlation analysis.
Behavior vs. Hippocampal/Entorhinal Cortex Aβ Levels
For all APP+PS1 mice combined or for each of the housing groups separately, significant correlations were observed between behavioral measures and Aβ deposition in hippocampus and entorhinal cortex. These correlations largely involved the three water-based cognitive tasks, with poorer performance being associated with higher Aβ deposition. For example, poorer RAWM performance on Trial 5 overall was correlated with Aβ deposition in entorhinal cortex when all APP+PS1 animals were included (r=0.503; p<0.02). For EE mice alone, performance in all four RAWM measures was correlated with hippocampal Aβ deposition. As an example, superior RAWM performance on Trial 5 overall was highly correlated with lower hippocampal Aβ deposition (r=0.929; p<0.01).
Hippocampal Synaptophysin Staining vs. Behavior/Pathology
For all APP+PS1 mice collectively, consistent correlations were present between CA3 synaptophysin immunoreactivity and both Morris maze acquisition and RAWM performance. CA3 synaptophysin immunoreactivity was negatively correlated with both overall and final day Morris maze acquisitional latency (r= -0.62; p<0.005 and r= -0.47; p<0.05, respectively). Similarly, CA3 synaptophysin immunoreactivity was negatively correlated with both overall and final block RAWM latency during Trial 5 (r= -0.58; p<0.01 and r= -0.533; p<0.02, respectively). Thus, higher synaptophysin immunoreactivity in CA3 was associated with better spatial learning and better working memory in APP+PS1 mice. Essentially no correlations were present between CA1 synaptophysin immunoreactivity and behavioral measures. For the two correlations involving hippocampal Aβ deposition and either CA1 or CA3 synaptophysin immunoreactivity, only the correlation involving CA3 was significant (r= -0.49; p<0.05); higher hippocampal Aβ deposition was associated with lower CA3 synaptophysin immunoreactivity.
Factor Analysis
FA of behavioral measures with and without pathologic measures was performed to determine the underlying relationships between behavioral tasks and neurohistologic/neurochemical measures (Table 1). Including all NT and Tg+ mice, FA involving all 15 behavioral measures resulted in 12 of those measures loading on four principle factors. Collectively, these four factors accounted for over 65% of the total variance. All measures for RAWM and platform recognition loaded heavily under Factor 1, which was the primary cognitive-based factor; this factor provided more variance (32.5%) than any other factor.
Table 1.
Factor loadings of behavioral measures, with and without pathologic measuresa
| Factor | Behavioral
Measures (Both Tg+ and NT) |
Behavioral and
Neurohistologic/Plasma Measures (Only Tg+) |
|---|---|---|
| 1 | (32.55) | (29.01) |
| RM-T5-Fin | AB-EC | |
| RM-T5 | AB-H | |
| RM-T4-Fin | SYN-CA1 | |
| RM-T4 | RA-OA4 | |
| PR-Fin | RA-OA5 | |
| PR-Avg | RA-B3T4 | |
| RA-B3T5 | ||
| WM-Fin | ||
| 2 | (15.47) | (16.99) |
| YM-Ent | CORT | |
| CPL-Fin | CPE-Fin | |
| CPE-OA | ||
| CPL-Fin | ||
| WM-OA | ||
| 3 | (10.51) | (13.47) |
| No Significant | PR-Fin | |
| Loadings | ||
| 4 | (9.85) | (10.27) |
| CPE-Fin | CPL-OA | |
| WM-Ret | ||
| 5 | (8.26) | (7.24) |
| CPE-Fin | YM-Alt | |
| YM-Alt | ||
| 6 | (5.72) | |
| WM-Ret |
Percent of total variance explained by a given factor is indicated in bold type within parentheses.
SYN-CA1, synaptic area within the CA1; SYN-CA3, synaptic area within the CA3; AB-Hipp, area of Aβ within hippocampus; AB-EC, area of Aβ within enterinal cortex; CORT, levels of corticosterone; YM-Ent, Y-maze entries; YM-Alt, Y-Maze % alternations; WM-Fin, water maze latency on last day; WM-Avg, water maze latency over all days; WM-Ret, water maze % time spent in Q2 during probe trial; CPE-Fin, circular platform errors on last day; CPE-Avg, circular platform errors over all days; CPL-Fin, circular platform latency on last day; CPL-Fin, circular platform latency over all days; PR-Fin, platform recognition latency on final day; PR-Avg, platform recognition latency over all days; RM-T4-Fin, RAWM latency for trial 4 of final block; RM-T5-Fin, RAWM latency for trial 5 of final block; RM-T4-Avg, RAWM latency over all blocks for trial 4; RM-T5-Avg, RAWM latency over all blocks for trial 5.
When the 4 neurohistologic measures (2 Aβ deposition measures, 2 synaptophysin measures) and plasma corticosterone levels were included in FA involving only APP+PS1 mice, six factors were evident (Table 1). Factor 1 was mixed in containing both cognitive-based and neurohistologic measures. Cognitive measures, particularly from the RAWM task of working memory, were included in Factor 1, along with both entorhinal cortex and hippocampal Aβ deposition plus synaptophysin staining in hippocampal CA1. Thus, there was an underlying relationship between hippocampal Aβ deposition, hippocampal synaptic area, and working memory performance. Factor 2 was also mixed in loading plasma corticosterone levels along with most circular platform measures. Interestingly, final platform recognition performance, overall circular platform latency, Y-maze percent alternation, and Morris maze memory retention all loaded alone in Factors 3, 4, 5, and 6, respectively.
In a final FA that involved behavioral measures and plasma cytokine levels in all APP+PS1 mice, 4 factors results (data not shown). Factor 1 loaded all 10 cytokines and both Morris maze acquisition measures, while factor 2 contained all RAWM and PR measures. Thus, with the exception of Morris maze acquisition, blood cytokine levels were largely not related to behavioral measures.
Discriminant Function Analysis
Discriminant function analysis (DFA) was utilized to determine if behavioral performance of the five housing groups (NT, IMP, SH, PA, and EE) or the three main housing groups (NT, SH, and EE) could be distinguished from one another (Table 2). For both the 5 and the 3 group analyses, direct entry DFAs could not discriminate between the housing groups based on their behavioral performance. In sharp contrast, stepwise-forward DFAs could effectively discriminate NT from IMP, SH, and PA groups (p<0.005), but not the NT and EE groups. Four behavioral measures (including RAWM overall T5 latency and all 3 Morris water maze measures) were retained as providing maximal discrimination. When only 3 groups were included, stepwise-forward DFA distinguished NT and EE from the SH group (p<0.01). Two measures (one from RAWM and one from MWM) provided maximal discrimination.
Table 2.
Summary of discriminant functional analyses of behavioral measures.
| Measures | # of Groups | Direct Entry Method | Stepwise-forward Method
|
|
|---|---|---|---|---|
| Significance | Measures Retained | |||
| All 15 | 5 | N.S. | p<0.005 | RM-T5 |
| NT vs IMP, SH, PA | WM-Avg | |||
| WM-Fin | ||||
| WM-Ret | ||||
| All 15 | 3 | N.S. | p<0.001 | RM-T5-Fin |
| NT, EE vs SH | WM-Fin | |||
| Factor 1 (6 cognitive measures) | 5 | N.S. | p<0.05 | RM-T5-Fin |
| EE vs IMP, SH, PA | ||||
| Factor 1 (6 cognitive measures) | 3 | N.S. | p=0.07 | RM-T5-Fin |
| EE vs SH | ||||
“p”values are from Wilks’s λ. N.S. = not significant; All other abbreviations defined in Table 1.
Additional DFAs were performed utilizing only the 6 behavioral measures that had loaded on Factor 1, the primary cognitive-based factor in factor analysis (see Table 1). For all five groups or the main three groups, the direct entry method was again unsuccessful in discriminating between groups (Table 2). In contrast, stepwise-forward DFA’s nicely discriminated between housing groups. With all five groups included, the stepwise-forward method separated EE mice from IMP, SH, and PA groups (p<0.01), but could not distinguish EE from NT mice (Table 2). When stepwise-forward DFA was repeated using only the three main groups, EE and SH groups were nearly separated (p=0.07), but not EE and NT groups. Trial 5 latency on the final block of RAWM testing was the sole measure retained for these stepwise-forward DFAs. Thus, for all behavioral measures included, or with inclusion of only the cognitive measures in Factor 1, stepwise-forward DFA’s were able to discriminate the comparably-performing NT and EE groups from the poorer performing IMP, SH, and PA groups.
Discussion
Although social, physical, and cognitive activities have each been suggested to reduce the risk of AD, epidemiologic human studies cannot determine which activity is most important or what combination of activities may be most effective for risk reduction. Here, we utilized mutant APP transgenic mice in a well-controlled longitudinal study to determine the potential of “complete” environmental enrichment (EE) versus it’s components to: 1) protect against cognitive impairment, and 2) provide beneficial changes to a variety of brain measures. Our results indicate that enhanced cognitive activity, over and above that provided by social and/or physical activity, is required to protect against cognitive impairment in AD transgenic mice. Moreover, only the enhanced cognitive activity provided by complete EE reduces brain Aβ deposition and increases synaptic immunoreactivity in the hippocampus, underscoring involvement of both Aβ-dependent and independent mechanisms for EE-induced cognitive protection. These cognitive and neurohistologic benefits of complete EE occurred without any changes in blood cytokine or corticosterone levels, suggesting that the enrichment-dependent mechanisms do not involve inflammation or stress levels. Our results indicate that, of all activities, a high lifelong level of cognitive activity is critical for protecting against AD-like cognitive impairment and providing associated neurohistologic benefits.
In humans, both retrospective and prospective studies have proposed that a lifelong high level of cognitive activity reduces the risk of AD (Stern et al., 1994; Letenneur et al., 1999; Friedland et al., 2001; Wilson et al., 2002; Verghese et al., 2003; Smyth et al., 2004; Valenzuela & Sachdev, 2006). The robust cognitive protection and neurohistologic benefits of complete EE reported in the present study provide substantial support for that premise, while calling into question the protective value of social and physical activities against AD. Nonetheless, this study does involve a genetically-manipulated mouse model for the disease that may or may not accurately gauge the ability of various activities to protect against AD. It has been suggested that the interaction between cognitive, social, and physical activities in complete EE may be essential for cognitive/neurologic benefits and that no single one of these activities alone is sufficient (van Praag et al., 2000). This possibility cannot be eliminated in the present study, although there is no direct evidence for it in either the human or rodent scientific literature. Recent epidemiologic/retrospective studies have tried to separate the components of enrichment and have reported that enhanced physical activity (Abbott et al., 2004 ; Podewils et al., 2005; Larson et al., 2006) or enhanced social activity (Scarmeas et al., 2001; Wang et al., 2002) are associated with decreased incidence of AD, leading to the assertion that physical and/or social activities are protective against AD. However, epidemiologic studies are observational and not controlled, so causation cannot be established. Moreover, the complete separation of physical or social activity in either a retrospective or longitudinal prospective human study would be impossible to accomplish in that varying degrees of all three components are always present. For example, individuals leading a lifestyle involving high physical activity may also lead cognitively stimulating lives.
Although physical activity/fitness training appears to sustain general cognitive function during aging (Barnes et al., 2003; Colcombe & Kramer, 2003), that is not the same as protecting against AD. Along this line, physical activity (i.e., wheel running) has been shown in numerous studies to provide “normal” rodents with enhanced cognitive function and a variety of neurologic benefits (see Kramer et al., 2006 for review). By contrast, AD transgenic mice destined to develop cognitive impairment are quite different in that their brains experience ever increasing Aβ levels, neuritic plaque development, and other AD characteristics. Effects of enhanced physical activity through voluntary wheel running activity in AD transgenic mice have resulted in divergent findings, with one study reporting decreased brain Aβ levels/deposition and faster Morris maze acquisition (Adlard et al. (2005), while another study found no effects of wheel running on brain Aβ deposition or Morris maze acquisition (Wolf et al., 2006). In another relevant study (Lazarov et al., 2006), wherein APP+PS1 mice were given enrichment “sessions” several times weekly and evaluated retrospectively in terms of physical activity level, 3 mice having high activity had lower brain Aβ deposition than 3 mice having lower activity. However, whether the increased physical activity lead to decreased Aβ deposition or whether the increased activity was a result of decreased Aβ deposition could not be determined. Moreover, mice in the the study by Lazarov et al. (2006) were not cognitively evaluated.
In the present study, multiple cognitive tasks and measures therein were utilized in determining that physical activity, even in combination with social activity, could not protect AD transgenic mice against cognitive impairment or limit Aβ deposition. In a follow-up study, we have found that animals in both physical activity and complete EE housing spend the same amount of time on physical activity (i.e., wheeling running, exploring, running), particularly at night when 100% of animals in both housing environments are always active (Arendash et al., unpublished observations). Thus, PA and complete EE mice spend the same amount of time on physical activity events, yet only the enhanced cognitive activity provided by complete EE results in cognitive protection and neurologic benefits. Reasons for the behavioral and/or Aβ deposition discrepancies among Adlard et al. (2005), Lazarov et al. (2006), and the present study are unclear, but may involve different transgenic lines and different enrichment protocols being utilized, as well as Adlard et al.’s housing of all animals in isolation. The behavioral findings in AD transgenic mice of the present study, showing wide-spread protective benefits of EE across multiple cognitive domains, are somewhat consistent with a recent study reporting that long-term enrichment housing of APP-23 mice improves learning in a single task (Morris maze), but does not decrease brain Aβ deposition (Wolf et al., 2006). A faster swim speed of enriched mice and/or single housing of all animals in that study could explain these later results. Nonetheless, both Wolf et al. (2006) and the present study conclude that physical activity (either for their mice raised in isolation or our mice raised socially) was insufficient to impact brain Aβ deposition.
Both Aβ dependent and independent mechanisms would appear to be involved in the ability of complete EE to provide cognitive protection, while social and/or physical activity does not. First, complete EE reduced Aβ deposition in both hippocampus and entorhinal cortex, leading to the possibility that complete EE decreases the process of Aβ deposition. Although an early study reported that non-transgenic (normal) rats raised in an enriched environment exhibited an increase in brain APP levels (Huber et al., 1997), more recent studies involving mutant APP transgenic mice reported that enrichment housing does not affect APP expression or processing (Lazarov et al., 2005; Ambree et al., 2006). Thus, EE most likely induces increased brain clearance and/or Aβ sequestration in AD transgenic models. Regarding the later possibility, we recently reported that complete EE in AD transgenic mice increases hippocampal expression of transthyretin, an Aβ sequestering protein (Costa et al., 2006). Second, complete EE in the present study increased synaptic immunoreactivity in both hippocampal CA1 and CA3 regions, suggesting that increased synaptic formation/function had occurred. Finally, complete EE’s benefits may involve enhanced neuronal health through up-regulation of genes important for neuroprotection and neuritic growth, as we have previously reported (Costa et al., 2006). Detailed discussions of the behavioral, neurohistologic/plasma, and multimetric analyses, as well as general conclusions, are presented below.
Behavioral Analysis
Our past work has shown the RAWM task to be an extremely sensitive test of working memory (Arendash et al., 2001a, b; Austin et al., 2003; Nilsson et al., 2004; Jensen et al., 2005; Ethell et al., 2006), which is the earliest cognitive symptom of AD. In our laboratory, APPsw+PS1 mice are impaired in this task by 5-6 months of age (Jensen et al., 2005). Along that line, SH transgenic controls in the present study showed impaired RAWM working memory. By contrast, performance of EE-housed transgenic mice was equivalent to NT mice. Our earlier study also reported on this working memory protection afforded to APP transgenic mice by EE housing (Costa et al., 2006), as have Jankowsky et al. (2005) in a similar AD transgenic line. Importantly, the working memory protection in the present study was not observed in IMP, PA, or SH groups, who performed identical to one another in this task. As well, the superior performance of EE-housed transgenic mice cannot be attributable to faster swim speed since there were no group differences in swim speed. Thus, only the enhanced cognitive activity provided by complete EE protected working memory, with social and/or physical activity being insufficient.
Also noteworthy in the present study is that transgenic mice living in an impoverished (IMP) environment (e.g., individually housed in standard-sized cages) were not cognitively more impaired than transgenic mice raised in social activity (SH) or physical activity (PA) housing. As well, these three groups did not differ in any of the blood/brain measures analyzed. These results indicate that any of these three housing environments could be an appropriate control for APP transgenic studies and that neither social nor physical activity housing is a form of behavioral enrichment. Interestingly, Dong et al. (2004) reported that raising immature Tg2576 mice in isolation stress (e.g., individually in cages only one-third normal size) resulted in greater Aβ deposition and poorer performance (less freezing) in a contextual memory task. Blood corticosterone levels were not measured in that study and the decreased freezing observed in isolation stress mice could have simply reflected increased exploratory behavior due to chronic isolation (not cognitive impairment). Nonetheless, it is possible that chronic stress associated with an abnormally reduced housing environment could have deleterious behavioral and neuropathologic effects, as Dong et al. (2004) suggest. Then again, the similar group levels of blood corticosterone in the current study underscore that none of our housing conditions involved increased stress to any transgenic group.
In Morris maze acquisition, complete EE mice showed superior performance compared to several other housing groups, including the physical activity group. However, the relatively poor acquisitional performance of NT mice resulted in no transgenic effect being present (i.e., NT vs. SH groups not different). Nonetheless, EE mice were able to improve their Morris maze learning at a faster rate than other groups in the present study. The protective effects of EE on Morris maze acquisition are in harmony with our earlier study (Costa et al., 2006) and Jankowsky et al. (2005), wherein mutant APP transgenic mice reared in complete EE had superior Morris maze acquisition compared to control transgenic mice. Also consistent with these two prior studies, complete EE did not provide benefit in the memory retention phase of Morris maze testing. In the present study, the inability of a physical activity environment to improve Morris maze acquisition is contrary to Adlard et al. (2005), who showed an improved rate of Morris acquisition in TgCRND8 transgenic mice after 5 months of voluntary wheel running. As mentioned earlier, our housing paradigm results in EE and PA mice being equally active - most notably at night. Therefore, it is difficult to reconcile this earlier study’s findings involving a single task with the present study, wherein no cognitive benefits were observed across multiple tasks/measures following 7 months of physical activity (voluntary wheel running).
By performing the platform recognition task after the Morris maze task, mice must switch from the spatial strategy of the Morris maze to a recognition/identification strategy (Arendash et al., 2001a, b; Austin et al., 2003; Nilsson et al., 2004; Jensen et al., 2005). Overall platform recognition performance revealed that IMP, SH, and PA mice were significantly impaired in comparison to NT mice; however, EE mice were identical to the NT group. This demonstrates that only the enhanced cognitive activity provided by complete EE protected APPsw+PS1 mice from recognition/identification impairment - social and/or physical environments were insufficient for doing so. The present results showing protection of “strategy switching” by complete EE are consistent with our earlier study involving PDAPP+PS1 mice (Costa et al., 2006), wherein the same protection was provided by complete EE. Widely documented in AD patients is impairment in a variety of attention-related tasks, resulting in an inability to “search” or to shift attention from one item to another (Tales et al., 2004). Our platform recognition results involving such strategy switching could thus be of considerable clinical significance.
Neurohistologic and Plasma Measures
The robust cognitive protection provided to APP+PS1 mice by EE was accompanied by a significant reduction in Aβ deposition within both hippocampus and entorhinal cortex - social and/or physical activity housing provided no such reductions in brain Aβ deposition. An important link between Aβ deposition and cognitive impairment was further underscored by the numerous correlations found between higher Aβ levels and poorer cognitive performance in individual APP+PS1 mice irrespective of housing environment. Other studies investigating the effects of EE on brain Aβ levels in APP+PS1 mice are inconsistent, with EE being reported to either increase (Jankowsky et al.,2003, 2005) or decrease (Lazarov et al., 2005) brain Aβ deposition/levels. In our own recent study (Costa et al., 2006), no change in Aβ deposition resulted from housing PDAPP+PS1 in EE; however, when EE was combined with extensive behavioral testing (a form of EE itself), decreases in brain Aβ deposition occurred, indicating that behavioral testing can synergize with EE housing to lower brain Aβ levels. Interestingly, in that same study we found that EE induces a 5-fold increase in transthyretin gene expression within the hippocampus. Inasmuch as transthyretin binds to and sequesters Aβ (Schwarzman et al., 1994), this represents a viable “Aβ-dependent” mechanism through which EE limited Aβ deposition and protected cognition in the present study. Despite the incongruent reports of EE effects on brain Aβ levels (increased, decreased, or no change), our current findings and both other behavioral studies investigating complete EE in APP transgenic mice (Jankowsky et al., 2005; Costa et al., 2006) all showed cognitive protection irrespective of brain Aβ levels, thus also underscoring that “Aβ-independent” mechanisms are contributing to the cognitive protection.
Also accompanying the cognitive protection provided to APP+PS1 mice by complete EE was a significant increase in hippocampal synatophysin immunoreactivity for both the CA1 and CA3 regions. Since neither social- nor physical activity-housed mice exhibited this increase in synaptic immunostaining, the enhanced cognitive activity provided by EE housing was necessary. More specifically, our results indicate that raising APP+PS1 mice in EE housing provides increased synaptic immunostaining of Mossy fiber terminals in CA3 projecting from granule cell, as well as for Schaffer collateral terminals of CA3 pyramidal cells in CA1. The enhanced CA3 synaptic immunoreactivity provided by EE appears to be of particular importance since greater synaptic immunostaining in CA3 (but not CA1) was associated with better cognitive performance and lower hippocampal Aβ deposition. Several earlier studies have shown the ability of EE (or behavioral over-training) to enhance synaptophysin levels/staining in hippocampus of “normal” rodents (Ramirez-Amaya et al., 2001; Levi et al., 2003; Frick & Fernandez, 2003). The present study shows that even with Aβ production/deposition occurring in the APP+PS1 mouse’s hippocampus, the enhanced cognitive activity of EE was capable of favorably impacting synaptic immunoreactivity there. This increased synaptic immunoreactivity provided by EE housing is consistent with our earlier study in APP transgenic mice (Costa et al., 2006) showing that EE housing induces increased hippocampal gene expression of proteins related to synaptic plasticity and pre-synaptic function. This again underscores an involvement of Aβ-independent mechanisms in the cognitive protection provided to APP+PS1 mice by EE.
Across all 10 plasma cytokines measured, there were no differences between APP+PS1 mice collectively and NT mice, nor were there any effects of housing condition on plasma cytokine levels among the transgenic groups. Thus, neither presence of the mutant APP transgene or housing environment induced a global, sustained change in the immune system. These findings are consistent with Marashi et al. (2004), who reported no differences in spleen cytokine levels of normal mice raised in EE. In a recent study (Ethell et al., 2006), we reported no differences in cytokine levels between NT and APP+PS1 mice in both plasma and brain. Results from that and the present study indicate that APP+PS1 mice are “immune tolerant” to the presence of human Aβ in plasma, which would be expected for any peptide produced endogenously at high levels throughout life. In the present study, it is noteworthy that plasma cytokine levels correlated almost exclusively with Morris maze acquisition (spatial learning) - higher cytokine levels were associated with better spatial learning. Consistent with an underlying relationship between plasma cytokine levels and spatial learning was the factor analysis showing that all 10 cytokines load with both Morris maze acquisition measures in factor 1.
In view of the possibility that different levels of stress associated with the four housing environments could impact behavioral and neurohistologic measures, plasma corticosterone levels were determined following completion of behavioral testing. No group differences were observed, either between housing groups or between genotypes. Thus, all four housing environments were associated with the same level of stress, eliminating the possibility that stress contributed to differences in cognitive performance of APP+PS1 mice in the various environments. Also underscoring this assertion, corticosterone levels were not correlated with cognitive performance in individual animals, irrespective of housing environment. In contrast to our findings, Belz et al. (2003) reported that normal mice housed individually in EE had lower blood stress hormones (corticosterone and ACTH) than mice housed individually in standard cages, suggesting that EE reduces stress by relieving boredom. Irrespective of genotype, male mice of the present study had almost 3-fold lower plasma corticosterone levels compared to females. This is consistent with several studies involving normal mice, which reported significantly lower corticosterone levels in males than in females (Galea et al., 1997; Finn et al., 2004)
Multimetric analyses (Factor and Discriminant Function Analyses)
A benefit to utilizing an extensive behavioral battery and multiple neurohistologic measures is the ability to employ higher level statistical analysis to characterize and distinguish between genotypic or treatment groups. One such analysis is factor analysis, which determines the underlying relationships between measures. Including all animals, factor analysis performed on behavioral measures alone revealed one primary factor, which was comprised of all measures from the radial arm water maze and platform recognition tasks - the two tasks wherein EE’s effects on cognitive performance were most evident. Thus, there is some commonality between these two tasks, which is very consistent with our prior studies (Leighty et al., 2004; Jensen et al., 2005) showing these same measures loading on factor 1 and underscoring that this is the primary “cognitive-based” factor. An additional factor analysis, including behavioral, neurohistologic, and plasma corticosterone measures from APP+PS1 mice revealed a close underlying relationship between RAWM working memory, brain Aβ deposition, and hippocampal synaptophysin measures. We have previously reported factor analyses showing a relationship between working memory and brain Aβ deposition (Leighty et al., 2004; Jensen et al., 2005). Correlation analyses in the present paper further underscore this relationship between greater Aβ deposition and working memory impairment, as well as between greater hippocampal synaptic immunostaining and better working memory performance. Also in factor analysis, plasma corticosterone levels loaded with only a few (circular platform) cognitive measures, again consistent with our finding no correlations between plasma corticosterone levels and cognitive performance irrespective of housing environment or genotype.
Discriminant functional analysis (DFA) was used to determine if behavioral performance across multiple measures could distinguish between any of the transgenic groups raised in four different housing environments. The “stepwise-forward” DFA method was consistently able to discriminated EE (and NT) mice from all other transgenic groups (IMP, SH, and PA). This was consistent with our analysis of individual measures wherein EE mice were identical to NT mice in having excellent cognitive performance, while the IMP, SH, and PA groups were impaired. Interestingly, the one cognitive measure unfailingly retained for providing this discrimination was the delayed retention trial (Trial 5) during the final block of RAWM testing. This is perhaps the most sensitive of all RAWM measures to working memory impairment and Aβ deposition (Leighty et al., 2004; Jensen et al., 2005). It is important to note that our DFA results extend and complement traditional single-measure assessments by indicating that the protective effects of complete EE occurred across multiple cognitive measures from multiple tasks evaluated as a group. Much like the indexing of multiple behavioral measures/domains of elderly humans or AD patients (Altepeter et al., 1990; Whelihan et al., 1997), DFA of AD transgenic models provides a valuable tool in assessing cognitive performance across various cognitive domains.
Conclusions
In summary, this study clearly shows the necessity of enhanced cognitive activity provided by complete environmental enrichment housing for protecting cognition in AD transgenic mice. By contrast, physical and/or social activity housing cannot provide this protection, suggesting that humans who emphasize life-long cognitive activities (in addition to social and physical activities) will have the greatest environmental risk-reduction against AD.
Acknowledgments
This research was supported by grants to G.A. and H.P. within the NIA-designated Florida Alzheimer’s Disease Research Center (P50AG025711), a grant to G.A. from the Alzheimer’s Disease and Related Diseases Association, and funds from the Byrd Alzheimer’s Center and Research Institute. We also gratefully acknowledge the assistance of Alexander Dickson in preparation of graphs/tables.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott R, White L, Ross G, Masaki K, Curb J, Petrovitch H. Walking and dementia in physically capable elderly men. Journal of the American Medical Association. 2004;292:1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- Adlard P, Perreau V, Pop V, Cotman C. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s Disease. Journal of Neuroscience. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altepeter T, Adams R, Buchana W, Buck P. Luria memory words test and Wechsler memory scale: comparison of utility in discriminating neurologically impaired from controls. Journal of Clinical Psychology. 1990;46:190–193. doi: 10.1002/1097-4679(199003)46:2<190::aid-jclp2270460211>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Ambree O, Leimer U, Herring A, Gortz N, Sachser N, Heneka M, Paulus W, Keyvani K. Reduction of amyloid angiopathy and Aβ plaque burden after enriched housing in TgCRND8 mice: Involvement of multiple pathways. American Journal of Pathology. 2006;169:544–552. doi: 10.2353/ajpath.2006.051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B, Rapp D, Baek D, McCloskey D, Coburn-Litvak P, Robinson J. Exercise influences spatial learning in the radial arm maze. Physiology and Behavior. 2000;70:425–429. doi: 10.1016/s0031-9384(00)00282-1. [DOI] [PubMed] [Google Scholar]
- Arendash G, King D, Gordon M, Morgan D, Hatcher J, Hope C, Diamond D. Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Research. 2001a;891:42–53. doi: 10.1016/s0006-8993(00)03186-3. [DOI] [PubMed] [Google Scholar]
- Arendash G, Gordon M, Diamond D, Austin L, Hatcher J, Jantzen P, Dicarlo G, Wilcock D, Morgan D. Behavioral assessment of Alzheimer’s transgenic mice following long-term Aβ vaccination: Task specificity and correlations between Aβ deposition and spatial memory. DNA and Cell Biology. 2001b;20:737–744. doi: 10.1089/10445490152717604. [DOI] [PubMed] [Google Scholar]
- Arendash G, Lewis J, Leighty R, McGowan E, Cracchiolo J, Hutton M, Garcia M. Multi-metric behavioral comparison of APPsw and P301L models for Alzheimer’s Disease: linkage of poorer cognitive performance to tau pathology in forebrain. Brain Research. 2004a;1012:29–41. doi: 10.1016/j.brainres.2004.02.081. [DOI] [PubMed] [Google Scholar]
- Arendash G, Garcia M, Costa D, Cracchiolo J, Wefes I, Potter H. Environmental enrichment improves cognition in aged Alzheimer’s transgenic mice despite stable β-amyloid deposition. NeuroReport. 2004b;15:1751–1754. doi: 10.1097/01.wnr.0000137183.68847.4e. [DOI] [PubMed] [Google Scholar]
- Arendash G, Schleif W, Rezai-Zadeh K, Jackson E, Zacharia L, Cracchiolo J, Shippy D, Tan J. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain β-amyloid production. Neuroscience. 2006;142:941–952. doi: 10.1016/j.neuroscience.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Austin L, Arendash G, Gordon M, Diamond D, DiCarlo G, Dickey C, Ugen K, Morgan D. Short-term β-amyloid vaccinations do not improve cognitive pereformance in cognitively impaired APP+PS1 mice. Behavioral Neuroscience. 2003;117:478–484. doi: 10.1037/0735-7044.117.3.478. [DOI] [PubMed] [Google Scholar]
- Belz E, Kennell J, Czambel R, Rubin R, Rhodes M. Environmental enrichment lowers stress-responsive hormones in singly housed male and female rats. Pharmacology, Biochemistry, and Behavior. 2003;76:481–486. doi: 10.1016/j.pbb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Barnes D, Yaffee K, Satariano W, Tager I. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. Journal of the American Gerontologic Society. 2003;51:459–465. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Chen K, Know J, Inglis J, Bernard A, Martin S, Justice A, McConlogue L, Games D, Freedman S, Morris R. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer’s disesase. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer A. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychologic Sciences. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Costa D, Cracchioilo J, Bachstetter A, Hughes T, Bales K, Paul S, Mervis R, Arendash G, Potter H. Enrichment improves cognition in AD mice by amyloid-related and unrelated mechanisms. Neurobiology of Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Cotman C, Berchtold N. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in Neuroscience. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Davis R, Massman P, Doody R. Cognitive intervention in Alzheimer disease: a randomized placebo-controlled study. Alzheimer’s Disease and Associated Disorders. 2001;15:1–9. doi: 10.1097/00002093-200101000-00001. [DOI] [PubMed] [Google Scholar]
- Dong H, Goico B, Martin M, Csernansky C, Bertchume A, Csernansky J. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127:601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Ethell D, Shippy D, Cao C, Cracchiolo J, Runfeldt M, Blake B, Arendash G. Aβ-specific T-cells reverse cognitive decline and synaptic loss in Alzheimer’s mice. Neurobiology of Aging. 2006;23:351–361. doi: 10.1016/j.nbd.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Farina E, Fioravanti R, Chiavari L, Imbornone E, Alberoni M, Pomati S, Pinardi G, Pignatti R, Mariani C. Comparing two programs of cognitive training in Alzheimer’s disease: a pilot study. ACTA Neurologica Scandanavia. 2002;105:365–371. doi: 10.1034/j.1600-0404.2002.01086.x. [DOI] [PubMed] [Google Scholar]
- Finn D, Sinnott R, Ford M, Long S, Tanchuck M, Phillips T. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004;123:813–819. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Frick K, Fernandez S. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiology of Aging. 2003;24:615–626. doi: 10.1016/s0197-4580(02)00138-0. [DOI] [PubMed] [Google Scholar]
- Friedland R, Fritsch T, Smyth K, Koss E, Lerner A, Chen C, Petot G, Debanne S. Patients with Alzheimer’s disease have reduced activities in midlife compared with healthy control-group members. Proceedings of the National Academy of Sciences. 2004;98:3440–3445. doi: 10.1073/pnas.061002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea L, McEwen B, Tanapat P, Deak T, Spencer R, Dhabhar F. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Huber G, Bailly Y, Martin J, Mariani J, Brugg B. Synaptic β-amyloid precursor proteins increasewith learning capacity in rats. Neuroscience. 1997;80:313–320. doi: 10.1016/s0306-4522(97)00120-6. [DOI] [PubMed] [Google Scholar]
- Jankowsky J, Zu G, Fromholt D, Gonzales V, Borchelt D. Environmental enrichment exacerbates amyloid plaque formation in a transgenic mouse model of Alzheimer disease. Journal of Neuropathology and Experimental Neurology. 2003;62:1220–1227. doi: 10.1093/jnen/62.12.1220. [DOI] [PubMed] [Google Scholar]
- Jankowsky J, Melnikova T, Fadale D, Zu G, Slunt H, Gonzales V, Younkin L, Younkin S, Borchelt D, Savonenko A. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer’s Disease. Journal of Neuroscience. 2005;25:5217–5224. doi: 10.1523/JNEUROSCI.5080-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M, Mottin M, Cracchiolo J, Leighty R, Arendash G. Lifelong immunization with human β-amyloid (1-42) protects Alzheimer’s transgenic mice against cognitive impairment throughout aging. Neuroscience. 2005;130:667–684. doi: 10.1016/j.neuroscience.2004.09.055. [DOI] [PubMed] [Google Scholar]
- Johnson R, Rhodes J, Jeffrey L, Garland T, Mitchell G. Hippocampal brain-derived neurotrophic factor but not neurotrophin-3 increases more in mice selected for increased voluntary wheel running. Neuroscience. 2003;121:1–7. doi: 10.1016/s0306-4522(03)00422-6. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Mishina M, Sugiyama H. Enhancement of neurogenesis by running wheel exercises is suppressed in mice lacking NMDA receptor ε1 subunit. Neuroscience Research. 2003;47:55–63. doi: 10.1016/s0168-0102(03)00171-8. [DOI] [PubMed] [Google Scholar]
- Kramer A, Erickson K, Colcombe S. Exercise, cognition and the aging brain. Journal of Applied Physiology. 2006 doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- Larson E, Wang L, Bowen J, McCormick W, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk of incident dementia among persons 65 years of age and older. Annals of Internal Medicine. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- Lazarov O, Robinson J, Tang YP, Hairston I, Korade-Mirnics Z, Lee V, Hersh L, Sapolsky R, Mirnics K, Sisodia S. Environmental enrichment reduces Aβ levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Leighty R, Nilsson L, Potter H, Costa D, Low M, Bales K, Paul S, Arendash G. Use of multimetric statistical analysis to characterize and discriminate between the performance of four Alzheimer’s transgenic mouse lines differing in Aβ deposition. Behavioral Brain Research. 2004;153:107–121. doi: 10.1016/j.bbr.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Letenneur L, Gilleron V, Commenges D, Helmer C, Orgogozo J, Dartigues J. Are sex and educational level independent predictors of dementia and Alzheimer’s disease? Incidence data from the PAQUID project. Journal of Neurology, Neurosurgery, and Psychiatry. 1999;66:177–183. doi: 10.1136/jnnp.66.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi O, Jongen-Relo A, Feldon J, Roses A, Michaelson D. ApoE4 impairs hippocampal plasticity isoform-specifically and blocks the environmental stimulation of synaptogenesis and memory. Neurobiology of Disease. 2003;13:273–282. doi: 10.1016/s0969-9961(03)00045-7. [DOI] [PubMed] [Google Scholar]
- Loewenstein D, Acevedo A, Czaja S, Duara R. Cognitive rehabilitation of mildly impaired Alzheimer disease patients on cholinesterase inhibitors. American Journal of Geriatric Psychiatry. 2004;12:395–402. doi: 10.1176/appi.ajgp.12.4.395. [DOI] [PubMed] [Google Scholar]
- Marashi V, Barnekow A, Sachser N. Effects of environmental enrichment on males of a docile inbred strain of mice. Physiology and Behavior. 2004;82:765–776. doi: 10.1016/j.physbeh.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Newman M, Nazian S, Sanberg P, Diamond D, Shytle R. Corticosterone-attenuating and anxiolytic properties of mecamylamine in the rat. Prog Neuropsychopharmacol BIol Psychiatry. 2001;25:609–620. doi: 10.1016/s0278-5846(00)00178-0. [DOI] [PubMed] [Google Scholar]
- Nilsson L, Arendash G, Leighty R, Costa D, Low M, Garcia M, Cracciolo J, Rojiani A, Wu X, Bales K, Paul S, Potter G. Cognitive impairment in PDAPP mice depends on ApoE and ACT-catalyzed amyloid formation. Neurobiology of Aging. 2004;25:1153–1167. doi: 10.1016/j.neurobiolaging.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Podewils L, Gualler E, Kuller L, Fried L, Lopez O, Carlson M, Lyketsos C. Physical activity, ApoE genotype, and dementia risk: Findings from the cardiovascular health cognition study. American Journal of Epidemiology. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- Ramierz-Amaya V, Balderas I, Sandoval J, Escobar M, Bermudez-Rattoni F. Spatial long-term memory is related to mossy fiber synaptogenesis. Journal of Neuroscience. 2001;21:7340–7348. doi: 10.1523/JNEUROSCI.21-18-07340.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Levy G, Tang M-X, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer’s Disease. Neurology. 2001;57:2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijver N, Pallier P, Brown V, Wurbel H. Double dissociation of social and environmental stimulation on spatial learning and reversal learning in rats. Behavioral Brain Research. 2004;152:307–314. doi: 10.1016/j.bbr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Schwarzman A, Gregori L, Vitek M, Lyubski S, Strittmatter W, Enghilde J, Bhasin R, Silverman J, Weisgraber K, Coyle P. Transthyretin sequesters amyloid beta protein and prevents amyloid formation. Proceedings of the National Academy of Sciences. 1994;91:8368–8372. doi: 10.1073/pnas.91.18.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitzer D, Twamley E, Jeste D. Cognitive training in Alzheimer’s disease: a meta-analysis of the literature. ACTA Psychiatry Acandanavia. 2006;114:75–90. doi: 10.1111/j.1600-0447.2006.00789.x. [DOI] [PubMed] [Google Scholar]
- Smyth K, Fritsch T, Cook M, McClendon M, Santillan C, Friedland R. Worker functions and traits associated with occupations and the development of AD. Neurology. 2004;63:498–503. doi: 10.1212/01.wnl.0000133007.87028.09. [DOI] [PubMed] [Google Scholar]
- Stern Y, Gurland B, Tatemichi T, Tang M, Wilder D, Mayeuz R. Influence of education and occupation on the incidence of Alzheimer’s disease. Journal of the American Medical Association. 1994;271:1004–1010. [PubMed] [Google Scholar]
- Tales A, Muir J, Jones R, Bayer A, Snowden R. The effects of saliency and task difficulty on visual search performance in ageing and Alzheimer’s disease. Neuropsychologia. 2004;42:335–345. doi: 10.1016/j.neuropsychologia.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Tan J, Town T, Crawford F, Mori T, DelleDonne A, Crescentini R, Obregon D, Flavell R, Mullan M. Role of CD40 ligand in amyloidosis in transgenic Alzheimer’s mice. Nature Neuroscience. 2002;5:1288–1293. doi: 10.1038/nn968. [DOI] [PubMed] [Google Scholar]
- Valenzuela M, Sachdev P. Brain reserve and dementia: a systematic review. Psychological Medicine. 2006;36:441–454. doi: 10.1017/S0033291705006264. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage F. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage F. Neural consequences of environmental enrichment. Nature Reviews in Neuroscience. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage F. Exercise enhances learning and hippocampal neurogenesis in aged mice. Journal of Neuroscience. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. European Journal of Neuroscience. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Verghese J, Lipton R, Katz M, Hall C, Derby C, Kuslansky G, Ambrose A, Sliwinski M, Buschke H. Leisure activities and the risk of dementia in the elderly. New England Journal of Medicine. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- Voikar V, Polus A, Vasar E, Rauvala H. Long-term individual housing in C57BL/J6 and DBA/2 mice: Assessment of behavioral consequences. Genes, Brain, and Behavior. 2005;4:240–252. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- Wang H-X, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: A longitudinal study from the Kungsholmen project. American Journal of Epidemiology. 2002;155:1081–1087. doi: 10.1093/aje/155.12.1081. [DOI] [PubMed] [Google Scholar]
- Westerman M, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin L, Carlson G, Younkin S, Ashe K. The relationship between A-beta and memory in the Tg2576 mouse model of Alzhiemer’s disease. Journal of Neuroscience. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelihan W, Thompson J, Piatt A, Caron M, Chung T. The relationship of neuropsychological measures to levels of cognitive functioning in elderly subjects: A discriminant analysis approach. Applied Neuropsychology. 1997;4:160–164. doi: 10.1207/s15324826an0403_3. [DOI] [PubMed] [Google Scholar]
- Williams B, Luo Y, Waard C, Redd K, Gibson R, Kuczaj S, McCoy J. Environmental enrichment: Effects on spatial memory and hippocampal CREB immunoreactivity. Physiology and Behavior. 2001;73:649–658. doi: 10.1016/s0031-9384(01)00543-1. [DOI] [PubMed] [Google Scholar]
- Wilson R, Bennet D, Bienias J, Aggarwal N, Mendes de Leon C, Morris M, Schneider J, Evans D. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59:1910–1914. doi: 10.1212/01.wnl.0000036905.59156.a1. [DOI] [PubMed] [Google Scholar]
- Wolf S, Kronenberg G, Lehmann K, Blankenship A, Overall R, Staufenbiel M, Kempermann G. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer’s disease. Biol Psychiatry. 2006;60:1314–1323. doi: 10.1016/j.biopsych.2006.04.004. [DOI] [PubMed] [Google Scholar]