Abstract
Objective
Children with the severe forms of osteogenesis imperfecta have in several studies been treated with intravenous pamidronate, but there are only few reports of the effect of early treatment.
Aim
To evaluate the effect of treatment started in infancy.
Methods
In a prospective observational study, with a historic control group, intravenous disodium pamidronate (APD) was given as monthly infusions to 11 children with osteogenesis imperfecta aged 3–13 (median 3.6) months, who had severe osteogenesis imperfecta with congenital bowing of the femora and vertebral compression fractures.
Results
During treatment of children aged between 3 and 6 (median 4.5) years, dual‐energy x ray absorptiometry measurements of the lumbar spine showed a gradual increase in bone density. Bone metabolism parameters in serum (alkaline phosphatase, osteocalcin, procollagen 1 carboxy‐terminal peptide, collagen 1 teleopeptide) and in urine (deoxypyridinoline) indicated a decrease in bone turnover. An improvement of mobility was seen and at the latest recording, at the age of 3.3–6.5 (median 4.8) years, the children could all walk. Vertebral remodelling was seen, with increased vertebral height, and no child developed scoliosis, kyphosis or basilar impression. All children required femoral intramedullar rods for fractures, and five needed tibial rodding for extreme curvatures that prevented functional standing and walking. No adverse effects were seen on growth, fracture healing or blood chemistry.
Conclusions
APD is an efficient symptomatic treatment for infants with severe osteogenesis imperfecta, but additional orthopaedic surgery is often needed. Early treatment may prevent scoliosis and basilar impression. Long‐term follow‐up is important.
Osteogenesis imperfecta is a disease that shows a large variation in phenotype, ranging from very mild bone fragility (type I) through intermediate severity (types IV and III) to the most severe lethal form (type II). This clinical classification into four major subgroups was designed by Sillence in 19791,2 and describes most cases. Recent modifications encompass additional, even less common, forms of the disease. In most cases, there are mutations in the COLIA1 or COLIA2 genes localised to chromosomes 17 and 7, respectively. This leads either to a reduced production of normal collagen type I or to the synthesis of collagen type I with abnormal structure and function. In general, the genotype is an unreliable predictor of phenotype and severity, and no new classification based on the different mutations has yet been devised.
Intravenous disodium pamidronate (APD) treatment of children with osteogenesis imperfecta has been used since 1991, and we presented the first case at the Fifth International Conference on Osteogenesis Imperfecta in 1993. The rationale for bisphosphonate treatment in osteogenesis imperfecta is that the complex function of bisphosphonate, with a predominantly inhibitory effect on osteoclasts, might lead to a net effect of increased bone mass, especially as growing children with osteogenesis imperfecta have thin bone with few trabeculae, thin cortices and high remodelling rates.3,4,5,6,7 We have previously published long‐term results in 3 adolescents and 28 children aged 0.6–18 years.8,9 The few reports of bisphosphonate treatment of infants with osteogenesis imperfecta are mainly, a mix of toddlers and infants, few under the age of 15 months.9,10,11,12,13,14,15,16 Here we present very promising results of APD treatment in a group of 11 infants with severe but not lethal forms of osteogenesis imperfecta.
Methods
Patients
Eleven consecutive infants with severe forms of osteogenesis imperfecta referred to the Astrid Lindgren Children's Hospital (Stockholm, Sweden) for assessment were invited to participate in the study. All parents accepted treatment after informed consent. The infants all had severe forms of osteogenesis imperfecta, 5 with type III, 4 with type IV and 2 with a severe form of osteogenesis imperfecta type I with congenital skeletal deformities including bowing of the femora (table 1). All had newly acquired vertebral compression fractures at the start of treatment, compared with neonatal images. At the start of treatment, their median age was 3.6 months (range 3–13). At the latest assessment, their median age was 4.8 years (range 3.25–6.5). A historic control group was chosen from 11 untreated age matched (to the age at the latest assessment) children (median age 4.6 years, range 2.7–9) with comparable forms of osteogenesis imperfecta, assessed before the start of APD treatment in our last study (as three infants are also included in this study, patients 4–14 in Astrom and Soderhall's study9 constitute our control group).
Table 1 Descriptive data of the patients at the start of treatment and at the latest recording, and untreated controls age matched to the patient's age at the latest recording.
| Patientno | OI type | Sex | Age (years) at start | Height/weight at start, SD | Age (years) latest record | Height/weight latest record, SD |
|---|---|---|---|---|---|---|
| 1 | IV | F | 1.1 | −6.5/−6.1 | 6.1 | −4.0/−2.7 |
| 2 | IV | F | 1.1 | −6.0/−5.3 | 6.2 | −4.8/−2.8 |
| 3 | IV | M | 0.5 | −11.0/−5.5 | 6.5 | −7.2/−3.8 |
| 4 | III | M | 0.4 | −11.5/−2.5 | 6.4 | −5.0/−3.7 |
| 5 | I | M | 0.25 | −0.6/0.2 | 5.8 | −3.2/−2.1 |
| 6 | I | M | 0.3 | 0.5/0.6 | 4.3 | −3.0/−0.6 |
| 7 | IV | F | 0.3 | −2.0/1.0 | 3.3 | −3.0/−1.0 |
| 8 | III | M | 0.24 | −7.0/−3.6 | 4.8 | −7.0/−5.0 |
| 9 | III | F | 0.3 | −1.2/−1.3 | 3.3 | −3.2/−2.5 |
| 10 | III | F | 0.3 | −5.5/−6.0 | 3.3 | −5.5/−4.1 |
| 11 | III | F | 0.25 | −0.8/−2.0 | 3.3 | −5.0/−3.2 |
| Mean | 0.5 | −4.7/−2.8 | 4.8 | −4.6/−2.9 | ||
| Median | 0.3 | −5.5/−2.5 | 4.8 | −4.8/−2.8 |
| Controlno | OI type | Sex | Age (years) at early record | Height/weight at early record, SD | Age (years) before treatment | Height/weight before treatment, SD |
|---|---|---|---|---|---|---|
| c 4 | III | M | 1.5 | −9.0/−3.5 | 2.7 | −9.5/−3.0 |
| c 5 | III | M | 0.7 | −9.5/−3.8 | 3.1 | −9.0/−3.5 |
| c 6 | I | M | 0.25 | −2.0/−2.2 | 3.7 | −4.0/−2.5 |
| c 7 | IV | F | 1.2 | −4.6/−3.1 | 3.9 | −5.0/−3.5 |
| c 8 | III | F | 0.75 | −3.5/−3.0 | 3.9 | −5.0/−2.5 |
| c 9 | IV | F | * | * | 4.6 | −2.7/−2.4 |
| c 10 | IV | M | 0.25 | −2.0/−1.0 | 6.4 | −3.2/−0.5 |
| c 11 | III | F | 0.7 | −9.3/−6.0 | 7.0 | −12.0/−5.8 |
| c 12 | I | F | * | * | 7.3 | −4.5/−1.3 |
| c 13 | IV | F | 0.5 | −2.8/−3.3 | 8.6 | −8.7/−5.0 |
| c 14 | I | M | 0.25 | −6.0/−5.2 | 9.0 | −3.7/0.3 |
| Mean | 0.7 | −5.4/−3.5 | 5.5 | −6.1/−2.7 | ||
| Median | 0.7 | −4.6/−3.3 | 4.6 | −5.0/−2.5 |
f, female; m, male; OI osteogenesis imperfecta.
The SD scores are age‐adjusted.
*No early data available.
Treatment
After hydration, APD was given as monthly infusions of 10 mg/m2 for 3 months and 20 mg/m2 for 3 months, followed by 30 mg/m2 on further treatment. After 1–2 years of treatment, six children showed less bone mineral gain and reported skeletal pain during the week preceding the next treatment. Their dose was then increased to 40 mg/m2. This treatment protocol was maintained for 3–6 years (median 4.5 years), and postponed to 6 weeks after fractures or osteotomies. The intravenous route was chosen mainly to prevent differences in bioavailability from interfering with the interpretation of results. Oral intake of tablets is also very difficult for infants and toddlers. Seven of the children received an intravenous port without vascular complications.
No patient needed compensation for an expected generalised decrease in serum Ca levels after the infusion. There were no dietary restrictions but we made sure that nobody avoided dairy products.
One child (patient 10) was treated with stem cells intrauterine in gestation week 22.17
Study design
In a prospective observational study, APD was given monthly and the children assessed every 6 months during and after 3–6 years of treatment. The latest recorded data were compared with those of our own historic control group of untreated patients. The study was approved by the local ethics committee and by the Swedish Medical Products Agency, Uppsala, Sweden.
Assessments
Bone turnover variables and blood chemistry were assayed every 6 months using the method recently described.9 Bone density was assessed every 6 months, by dual‐energy x ray absorptiometry (DXA) measurements of the lumbar spine (L1–L4) using the Hologic QDR 4500 system (Hologic, Bedford, Masachussets, USA).18,19 Conventional x ray of the spine in frontal and side projections was taken before the start of and yearly during treatment, every second year combined with a lateral projection of the base of the skull. Each lumbar vertebra was measured by a single observer (HJ) in side projection; the superior‐caudal height divided by the anterior–posterior width gave a quotient from which the mean of all lumbar quotients was calculated. Bone age was determined according to the method of Greulich and Pyle20 before and after 2 and 4 years of treatment.
Parents were asked for the motor milestones every 6 months, and ambulation was assessed using the Wilson and Bleck scales.9,21 Stature was measured, in the supine position, by the same two people, every 6 months and plotted in standard growth charts of healthy Swedish children. Ultrasound of kidneys was done before treatment and after 1 and 3 years.
Statistical analysis
Wilcoxon matched‐pairs signed‐ranks tests were used to evaluate the difference between pretreatment levels and those at 2 years and endpoint levels for DXA, alkaline phosphatase (ALP), osteocalcin, procollagen 1 carboxy‐terminal peptide (P1CP), collagen 1 teleopeptide (1CTP) and deoxypyridinoline, as well as changes in mobility and the vertebral height/width quotient. Mann–Whitney U tests were used to compare patient's endpoint levels with those of controls. As the results were not normally distributed, and to facilitate comparison with other studies, results were reported as both medians and means. The tests were two‐tailed and p<0.05 was chosen to indicate significant deviation from the null hypothesis.
Results
Biochemical changes
Before treatment, five infants had slightly raised serum Ca values of 2.62–2.72 mmol/l (normal range 2.2–2.6). Of these, three had compensatory decreased parathyroid hormone levels of 4.4–6.2 ng/l (normal range 10–65). In the days after APD infusion, the levels of serum Ca fell in 5 of 11 patients, usually to values within the normal range. The decrease normalised within 7 days. In six infants the serum calcium values stayed around the upper range even after treatment and they were recommended to discontinue the vitamin AD drops (retinol and cholecalciferol) routinely given to all Swedish infants. No difference in the therapeutic effect of treatment (assessed by biochemical markers, DXA, ambulatory change and growth rate) was seen between these groups. The parathyroid hormone levels gradually increased but stayed within normal ranges. Serum ALP, osteocalcin, P1CP, 1CTP and urine deoxypyridinoline decreased over time, indicating a reduction in bone turnover (table 2). Untreated controls had significantly higher levels except for 1CTP. No abnormal values or changes over time were observed for serum white cell count, platelets, haemoglobin, Na, K, PO4, Mg, alanine aminotransferase, aspartate aminotransferase, albumin and creatinine.
Table 2 Median (and mean (SD)) values of serum alkaline phosphatase (μkat/l)), osteocalcin (μg/l), procollagen 1 carboxy‐terminal peptide (μg/l), collagen 1 teleopeptide (μg/l) and urine deoxypyridinoline/creatinine (nmol/mmol) before and during intravenous disodium pamidronate treatment and in untreated controls age matched to the latest recorded values.
| Patients | Controls | |||
|---|---|---|---|---|
| At start | After 2 years | Latest recorded | Before treatment | |
| ALP | 10.9 (11.1) | 6.3 (6.0)* | 6.0 (4.7)* | 9.4 (9.6)† |
| Osteocalcin | 46 (55) | 45 (52) | 42 (41) | 60 (57) |
| P1CP | 312 (381) | 100 (105)‡ | 88 (94)‡ | 98 (132) |
| 1CTP | 26 (27) | 14 (13)§ | 13 (13)§ | 10 (10) |
| u‐DPD/crea | 51.7 (49.3) | 26.2 (26.0) | 18.1 (19.4) | 150 (93.4) |
ALP, alkaline phosphatase; P1CP, procollagen 1 carboxy‐terminal peptide; 1CTP, collagen 1 teleopeptide; u‐DPD/crea, urine deoxypyridinoline.
*Significant change during treatment (p⩽0.001).
†When patient's latest recording values were compared with untreated controls, only alkaline phosphatase was found to be significantly lower (p⩽0.01).
‡Significant change during treatment (p⩽0.01).
§Significant change during treatment (p⩽0.04).
Bone density
Before treatment, all infants had low lumbar bone density (median z = 3.7). During treatment a gradual significant increase was observed, and after 1–4 years all children had achieved a lumbar bone density of z>−2.0 (table 3). In four children with z>0 the treatment continued with decreased dose to promote further vertebral regeneration. At the latest recording after a median of 4.5 years of treatment, the median z value was 0.20 compared with −6.59 in the control group. The improvement cannot be explained as an effect of growth.10 When the treatment, after 3–5 years, was ended in three patients, the bone density in all of them remained within normal limits and no skeletal pain was reported during 1.5–2 years of follow‐up.
Table 3 Relative vertebral height (mean height/width quotient) of the lumbar vertebrae and bone density (z values of dual energy x ray absorptiometry lumbar spine L1–L4) in patients and controls.
| Patient number | Relative vertebral height | DXA z score | Control number | Relative vertebral height | DXA z score | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| At start | Latest recording | At start | After 2 years | Latest | Early recording (age) | Before treatment | Before treatment | |||
| 1 | 0.32 | 0.55 | −3.7 | −2.63 | −1.85 | c4 | 0.63 (0.003) | 0.14 | −6.68 | |
| 2 | 0.33 | 0.57 | −3.1 | 0.7 | 0.88 | c5 | 0.21 | −7.33 | ||
| 3 | 0.16 | 0.74 | −4.52 | −2.97 | −1.34 | c6 | 0.5 | −5.9 | ||
| 4 | 0.28 | 0.8 | −2.91 | −1.57 | 0.6 | c7 | 0.91 (0.003) | 0.29 | −6.6 | |
| 5 | 0.7 | 0.51 | −3.5 | −2.14 | −0.75 | c8 | 0.13 | −6.59 | ||
| 6 | 0.84 | 0.81 | −3.45 | 1.27 | 2.04 | c9 | 0.69 (2.2) | 0.53 | −4.79 | |
| 7 | 0.84 | 0.84 | −4.48 | 2.17 | 3.91 | c10 | 0.74 (1) | 0.39 | −5.76 | |
| 8 | 0.18 | 0.51 | −4.1 | −1.59 | −1.5 | c11 | 0.06 | −7.26 | ||
| 9 | 0.72 | 0.77 | −3.12 | −0.59 | 0.6 | c12 | 0.11 | −5.09 | ||
| 10 | 0.59 | 0.75 | −4.92 | −2.46 | −1.2 | c13 | 0.17 (3.5) | 0.05 | −6.65 | |
| 11 | 0.62 | 0.62 | −5.66 | −0.8 | 0.2 | c14 | 0.76 | −3.62 | ||
| Mean | 0.51 | 0.68* | −3.95 | −0.96† | 0.14† | 0.63 (1.3) | 0.29* | −6.02† | ||
| Median | 0.59 | 0.74* | −3.7 | −0.57† | 0.2† | 0.69 (1) | 0.21* | −6.59† | ||
DXA, dual‐energy x ray absorptiometry.
*Significant difference between patient's latest recording and control group (p<0.001).
† Significant difference between patient's latest recording compared with pretreatment recording (p⩽0.003) and control group (p⩽0.001).
Radiological findings
There was no significant difference between the relative vertebral height at the early registrations of patients and controls. The vertebral height increased after 1–2 years of treatment when compared with vertebral width (fig 1). This gradual improvement continued during 3–6 years of treatment to a median of 0.74, whereas in the control group there was a significant (p<0.043) progression to more severe vertebral compressions to a median of 0.21 (table 3). In three children (two with type IV and one with type III), a pre‐existing gibbus disappeared after 1–2 years of treatment. One infant had a mild scoliosis before treatment that remained unchanged and no child developed scoliosis, kyphosis or basilar impression.
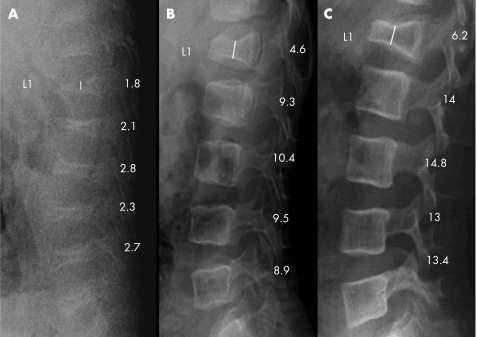
Figure 1 Radiograms of the lumbar spine, lateral projection of a boy with osteogenesis imperfecta type IV (patient no. 3). (A) Before treatment at 0.4 years of age. (B) After 1.5 years of treatment. (C) After 3 years of treatment. Vertebral height measurement is indicated by the vertical line in L1. A successive increase of mineralisation and vertebral height was seen.
In seven children, spondylolysis was detected after they had learned to walk, and in five children spondylolisthesis of 1–3 mm of the 5th lumbar vertebra was also detected at 2.3–6.4 (median 3.3) years of age after 2–6 (median 3.0) years of treatment. No spondylolysis was seen in the control group.
Only one girl has reached the 2 years post‐treatment assessment. Radiographs show normal vertebral growth but lower mineral content in the newly formed bone (fig 2).
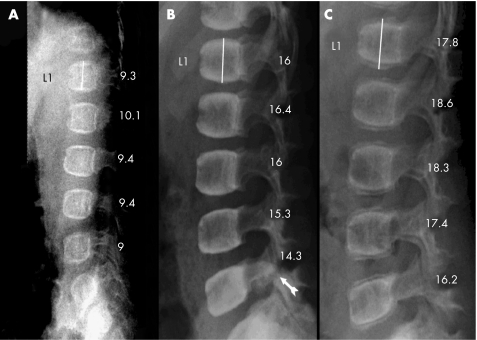
Figure 2 Radiograms of the lumbar spine, lateral projection of a girl with osteogenesis imperfecta type IV (patient no. 7) who, at the start of the treatment, had only compressions of the thoracic vertebrae. (A) Before start of treatment at 0.3 years of age. (B) After 3 years of treatment with increased mineralisation and vertebral height. Spondylolysis of the 5th lumbar vertebral arch was seen (arrow). (C) Two years after the end of treatment, lower mineral content was detected in the newly formed bone than in the older bone. The spondylolysis was healed.
Figure 3 shows the difference in the back of a 3.4‐year‐old boy and his untreated mother, both with type IV osteogenesis imperfecta.
Figure 3 Photograph of the back of a 3.4‐year‐old boy and his untreated mother, both with osteogenesis imperfecta type IV. The boy (patient no. 3) had received intravenous disodium pamidronate treatment for 3 years, from 0.4 years of age, whereas the mother was untreated. The mother has a severe kyphoscoliosis and basilar impression. The boy has a straight back and no basilar impression. Informed consent was obtained for publication of this figure.
Bone age followed the normal chronological development.
Ultrasound examination of the kidneys was done after 1 year of treatment in the first three patients. The first patient had microcalcifications. The patients included from that time on underwent ultrasound assessments before treatment. Three of these eight infants, at median age of 3 months, had microcalcifications before the start of treatment. Repeated ultrasound examinations every 6 months showed a decrease in the size and number of microcalcifications during treatment in two, and disappearance in one patient. No patient was shown to develop microcalcifications during treatment.
Clinical changes
Table 4 shows the motor milestones. Two controls were not even unsupported able to sit. All treated children improved their ambulatory ability (table 5). Notably, 9 of the 11 infants were under 6 months of age at the start of treatment. At this age the scale is not informative, as the lowest score is normal. All treated children learned to walk, and at the latest recording five had normal mobility for their age. In the control group, only two could walk and six had lost previous abilities (tables 4 and 5).
Table 4 Patients and controls; years of age at achieving the motor milestones of unsupported sitting, crawling, standing, supported walking and free walking.
| Patient no | Sitting | Crawling | Standing | Walking with support | Walking without support | Treatment start* | Femur rods* | Tibia rods* |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.4 | 1.5 | 1.6 | 1.8 | 2.2 | 1.1 | 1.9 | 1.9 |
| 2 | 0.9 | 1.3 | 1.3 | 1.3 | 1.4 | 1.1 | 1.5 | – |
| 3 | 1.1 | 1.5 | 3 | 3 | 6 | 0.5 | 3.7 | 4.6 |
| 4 | 0.8 | 1.9 | 1.5 | 5.3 | 5.5 | 0.4 | 2.3 | 3.3 |
| 5 | 0.7 | 1.1 | 1.4 | 1.4 | 1.6 | 0.25 | 2.4 | 3.1 |
| 6 | 0.6 | 0.9 | 1 | 1 | 1.1 | 0.3 | 2 | – |
| 7 | 0.4 | 0.7 | 1 | 1.3 | 1.5 | 0.3 | 1.1 | – |
| 8 | 1.5 | 1.9 | 2.5 | 2.9 | 3.9 | 0.24 | 3 | 3.5 |
| 9 | 0.7 | 0.7 | 0.8 | 0.8 | 1.2 | 0.3 | 2.3 | – |
| 10 | 1.1 | 1.6 | 1.6 | 1.7 | 2.6 | 0.3 | 2.3 | – |
| 11 | 0.8 | 1.2 | 1.2 | 1.4 | 1.4 | 0.25 | 1.4 | – |
| Mean | 0.9 | 1.3 | 1.5 | 2 | 2.6 | 0.5 | 2.2 | 3.3 |
| Median | 0.8 | 1.3 | 1.4 | 1.4 | 1.6 | 0.3 | 2.3 | 3.3 |
| Control | ||||||||
| c4 | – | – | – | – | – | 2.7 | 2.5 | 2.5 |
| c5 | 1 | – | – | – | – | 3.1 | 3 | 3 |
| c6 | 1.1 | 1.6 | 1.7 | 2.5 | 3.3 | 3.7 | – | – |
| c7 | 1.2 | 2.3 | – | – | – | 3.9 | 1.3 | – |
| c8 | 1.3 | – | – | – | – | 3.9 | 2.4 | – |
| c9 | 0.75 | 0.9 | 1.25 | 1.5 | 2.5 | 4.6 | – | – |
| c10 | 1.1 | 4.5 | 5 | 5.5 | – | 6.4 | 0.9 | – |
| c11 | – | – | – | – | – | 7 | – | – |
| c12 | 0.7 | 0.8 | 1 | 1.2 | 1.5 | 7.3 | 5.9 | – |
| c13 | 1.2 | 1.25 | 1.5 | 1.5 | – | 8.6 | 5.5 | 5.5 |
| c14 | 2.5 | 2 | 1 | 5.8 | – | 9 | 3 | 4 |
| Mean | 1.2 | 1.9 | 1.9 | 3 | 2.4 | 5.5 | 3.1 | 3.8 |
| Median | 1.1 | 1.6 | 1.4 | 2 | 2.5 | 4.6 | 2.8 | 3.5 |
The sign “—” indicates that the motor milestone was never achieved, or that no rodding was done at the latest recording/age of assessment.
*Age at the start of intravenous disodium pamidronate ‐treatment and age at orthopaedic surgery.
Table 5 Change in ambulation during treatment and comparison with untreated controls using the Wilson scale of nine levels: 1, functional walking without aid in all surroundings, to 9, sitting with support but no locomotion9.
| Patient number | At start | After 1 year | After 2 years | Latest recording | Control number | Before treatment |
|---|---|---|---|---|---|---|
| 1 | 9 (0) | 6 (1) | 2 (3) | 6 (3) | c 4 | 9 (0) |
| 2 | 8 (0) | 5 (2) | 2 (3) | 2 (3) | c5 | 8 (0) |
| 3 | 9 (0) | 7 (0) | 7 (0) | 2 (2) | c6 | 7 (0) |
| 4 | 9 (0) | 9 (0) | 7 (0) | 6 (2) | c7 | 7 (0) |
| 5 | 9 (0) | 7 (0) | 2 (2) | 2 (3) | c8 | 8 (0) |
| 6 | 9 (0) | 2 (1) | 2 (3) | 1 (4) | c9 | 6 (1) |
| 7 | 9 (0) | 7 (0) | 2 (3) | 2 (3) | c10 | 7 (1) |
| 8 | 9 (0) | 8 (0) | 7 (0) | 6 (2) | c11 | 9 (0) |
| 9 | 9 (0) | 2 (2) | 2 (3) | 1 (4) | c12 | 9 (0) |
| 10 | 9 (0) | 8 (0) | 7 (1) | 2 (3) | c13 | 8 (0) |
| 11 | 9 (0) | 7 (0) | 2 (3) | 1 (4) | c14 | 4 (2) |
| Mean | 9 (0) | 6 (1) | 4 (2) | 3 (3)* | 8 (0) | |
| Median | 9 (0) | 7 (0) | 2 (3) | 2 (3)* | 8 (0) |
The Bleck scale of five levels is shown in parenthesis: 0 non‐walker to 4 community walker.
*The improvement seen at the latest recording in treated patients is significant (p⩽0.001) compared to both pretreatment values and the untreated age‐matched controls.
At the start of treatment, the standard deviation (SD) from the median body height was −5.5. During treatment a slight improvement was seen to −5 after 1 and 2 years, and −4.8 at the latest recording, compared with a change from −4.6 to −5 in the control group.
Side effects
Five infants had a short episode of fever after the first APD infusion. No adverse respiratory events or hyperphosphatasaemia were seen.22,23
Discussion
The prognosis of severe types of osteogenesis imperfecta without treatment is poor with repeated fractures and fissures leading to progressive skeletal deformation with severe kyphoscoliosis, skeletal pain and immobilisation. Thus, we found it unethical not to treat infants with early and progressive vertebral fractures. Whether early treatment can prevent development of kyphosis and scoliosis is not clear, but our results seem promising. Treatment given after the development of major skeletal deformities may have less effect in preventing further scoliosis as this condition itself leads to a harmful load on the compressed vertebrae. Our treated group shows a great improvement in bone density and ambulation compared with untreated controls.
Engelbert et al24,25,26 studied mobility and motor development in untreated children with osteogenesis imperfecta. In children with type III and IV osteogenesis imperfecta aged 2–12 years (mean 6.7 years), after 1.25 years there was increased immobilisation in type III and a decrease of household walkers in type IV. At 5.5 years of age, 4 of 11 children with osteogenesis imperfecta type III could walk supported and none unsupported. The type of osteogenesis imperfecta was the most important predictor of walking ability.24,25,26 Munns et al16 reported significantly greater PEDI gross motor score and mobility after 3 years of pamidronate treatment in infants aged 0.5–23 months (median 6) compared with untreated controls. Our treated groups were all household walkers or better at the latest recording at a median age of 4.8 years. In our control group with a median age of 4.6 years, only two could walk (table 5).
Owing to the young age at the start of treatment, most parents could not estimate the amount of pain or degree of well‐being according to the scales that we have used in our previous studies,8,9 thus these parameters were not assessed.
Despite the early APD treatment, all children needed intramedullary rodding of the femora, eight with bilateral and three with unilateral rods. Rodding has generally been performed at the first fracture of a very curved femur. The improvement of mobility during APD treatment results in increased activity and weight‐bearing loading on the bone, which increases the risk of curvatures and fractures (table 4). Because of the extreme and increasing tibial curvatures preventing unsupported standing, five children needed tibial rodding. A secondary shortening of the Achilles tendons made a unilateral tendon lengthening operation necessary in three of these to achieve functional walking ability. Close clinical observation with earlier osteotomies and rodding can probably lead to gain in bone length and earlier walking which strengthens the skeleton and favours growth.27
Fracture rate is difficult to estimate and therefore not assessed. No adverse effects on fracture healing were seen.
Whether spondylolysis of the 5th lumbar vertebra in seven children is related to the APD treatment is unclear. Perhaps it is an effect of the change in mechanical load due to the standing and walking posture in these infants and toddlers, or is undiagnosed owing to low mineralisation in untreated children with severe osteogenesis imperfecta. When reassessing the spinal radiograms taken 1–2 years previously, a spondylolysis almost undetectable owing to low mineralisation was already present in two of the seven children. Only bipedal ambulators develop spondylolysis, mostly during early childhood, which is found in 4–5% of children at 6 years of age. It is more frequent in athletes who repeatedly have to hyperextend and rotate their lumbar spine, and is correlated to increased lumbar lordosis and ligament flexibility. It is also seen in a few children with osteopetrosis.28,29,30,31,32
In our previous studies, we have not used z values when reporting the results of DXA measurements, as they are calculated from a normal population, although we now present them to enable comparison with other studies. Plotkin et al10 reported a significant decrease in lumbar DXA values and increased vertebral compressions over a 1 year period in his historical control group of six untreated controls, with osteogenesis imperfecta type III and IV, with a mean age of 10.7 months at the start of observation. Munns et al16 reported better preserved vertebral shape and higher lumbar bone mineral density in treated infants.16
The girl treated with intrauterine stem cell transplantation (patient no. 10) differs in no way from the other patients before and during APD treatment, but as each individual has a unique mutation, the effect of ADP and stem cell treatment is difficult to estimate.17
Further long‐term follow‐up is important, both during and after treatment, to identify yet unknown side effects and to develop criteria to determine the optimal duration of treatment. Probably, the latter is individual and age dependent. Studies of combinations of treatments are also important to optimise the beneficial effects for the patients.
What is already known on this topic
The bisphosphonates are effective as symptomatic treatment of children with osteogenesis imperfecta.
Studies with intravenous treatment seem to show more pronounced beneficial effects than studies with oral treatment.
What this study adds
Treatment with intravenous disodium pamidronate is beneficial in infants with severe osteogenesis imperfecta.
Intravenous treatment in this age group improves mobility and vertebral height.
Treated infants achieved their motor milestones earlier and more completely than untreated controls.
Acknowledgements
This study was supported by grants from the Läkarförbundet, Norrbacka Eugenia Stiftelsen, RBU, Sunnerdahls Handikappfond and Stiftelsen Frimurare Barnhuset. A grant from the Socialstyrelsen supported the establishment of the multidisciplinary specialist team for osteogenesis imperfecta at the Astrid Lindgren Children's Hospital. We specially thank our collaborators in this team.
Abbreviations
ALP - alkaline phosphatase
APD - intravenous disodium pamidronate
DXA - dual‐energy x ray absorptiometry
P1CP - procollagen 1 carboxy‐terminal peptide 1CTP, collagen 1 teleopeptide
Footnotes
Informed consent was obtained for publication of figure 3.
Competing interests: None.
References
- 1.Sillence D O, Rimon D L, Danks D M. Clinical variability in osteogenesis imperfecta. Variable expressivity or genetic heterogeneity. Birth Defects 197915113–129. [PubMed] [Google Scholar]
- 2.Sillence D O, Senn A, Danks D M. Genetic heterogeneity in osteogenesis imperfecta [abstract]. J Med Genet 197916101–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boonekamp P M, van der Wee‐Pals L J, van Wijk‐van Lennep M M.et al Two models of action of bisphosphonates on osteoclastic resorption of mineralized matrix. Bone Miner 1986127–39. [PubMed] [Google Scholar]
- 4.Allgrove J. Bisphosphonates. Arch Dis Child 19977673–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srivastava T, Alon U S. Bisphosphonates: from grandparents to grandchildren. Clin Pediatr (Phila) 199938687–702. [DOI] [PubMed] [Google Scholar]
- 6.Lindsay R. Modeling the benefits of pamidronate in children with osteogenesis imperfecta. J Clin Invest 20021101239–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rauch F, Travers R, Plotkin H.et al The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. J Clin Invest 20021101293–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Åström E, Söderhäll S. Beneficial effect of bisphosphonate during five years of treatment of severe osteogenesis imperfecta. Acta Paediatr 19988764–68. [DOI] [PubMed] [Google Scholar]
- 9.Åström E, Söderhäll S. Beneficial effect of long term intravenous bisphosphonate treatment of osteogenesis imperfecta. Arch Dis Child 200286356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plotkin H, Rauch F, Bishop N J.et al Pamidronate treatment of severe osteogenesis imperfecta in children under 3 years of age. J Clin Endocr Metab 2000851846–1850. [DOI] [PubMed] [Google Scholar]
- 11.Rauch F, Plotkin H, Travers R.et al Osteogenesis imperfecta types I, III and IV: effect of pamidronate therapy on bone and mineral metabolism. J Clin Endocr Metab 200388984–985. [DOI] [PubMed] [Google Scholar]
- 12.Rauch F, Plotkin H, Zeitlin L.et al Bone mass, size, and density in children and adolescents with osteogenesis imperfecta: effect of intravenous pamidronate therapy. J Bone Miner Res 200318610–614. [DOI] [PubMed] [Google Scholar]
- 13.DiMeglio L A, Ford L, McClintock C.et al Intravenous pamidronate treatment of children under 36 months of age with osteogenesis imperfecta. Bone 2004351038–1045. [DOI] [PubMed] [Google Scholar]
- 14.Zeitlin L, Rauch F, Plotkin H.et al Height and weight development during four years of therapy with cyclical intravenous pamidronate in children and adolescents with osteogenesis imperfecta Types I, III and IV. Pediatrics 20031111030–1036. [DOI] [PubMed] [Google Scholar]
- 15.Forin V, Arabi A, Giugonis V.et al Benefits of pamidronate in children with osteogenesis imperfecta: an open prospective study. Joint Bone Spine 200572313–318. [DOI] [PubMed] [Google Scholar]
- 16.Munns C, Rauch F, Rose T.et al Effects of intravenous pamidronate treatment in infants with osteogenesis imperfecta: clinical and histomorphometric outcome. J Bone Miner Res 2005201235–1243. [DOI] [PubMed] [Google Scholar]
- 17.Le Blanc K, Götherström C, Ringden O.et al Fetal mesenchymal stem‐cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation 2005791607–1614. [DOI] [PubMed] [Google Scholar]
- 18.Pepper W W, Mazess R B. Total body bone mineral and lean body mass by dual‐photon absorptiometry. I. Theory and measurement procedure. Calcif Tissue Int 198133353–359. [DOI] [PubMed] [Google Scholar]
- 19.Kröger H, Heikkinen J, Laitinen K.et al Dual‐energy x‐ray absorptiometry in normal women: a cross‐sectional study of 717 Finnish volunteers. Osteoporos Int 19922135–140. [DOI] [PubMed] [Google Scholar]
- 20.Greulich W W, Pyle S I.Radiographic atlas of skeletal development of the hand and wrist. Stanford, CA: Stanford University Press, 1959
- 21.Bleck E E. Nonoperative treatment of osteogenesis imperfecta: orthotic and mobility management. Clin Orthop 1981159111–122. [PubMed] [Google Scholar]
- 22.Munns C F, Rauch F, Mier R J.et al Respiratory distress with pamidronate treatment in infants with severe osteogenesis imperfecta. Bone 200435231–234. [DOI] [PubMed] [Google Scholar]
- 23.Whyte M P, Wenkert D, Clements K L.et al Bisphosphonate‐induced osteopetrosis. N Engl J Med 2003349457–463. [DOI] [PubMed] [Google Scholar]
- 24.Engelbert R H, van Empelen R, Scheurer N D.et al Osteogenesis imperfecta in childhood: impairment and disability—a follow up study. Arch Phys Med Rehabil 199980896–903. [DOI] [PubMed] [Google Scholar]
- 25.Engelbert R H, Uiterwaal C S P, Gulmans V A.et al Osteogenesis imperfecta: profiles of motor development as assessed by a postal questionnaire. Eur J Pediatr 2000159616–620. [DOI] [PubMed] [Google Scholar]
- 26.Engelbert R H, Uiterwaal C S, Gulmans V A.et al Osteogenesis imperfecta in childhood: prognosis for walking. J Pediatr 2000137397–402. [DOI] [PubMed] [Google Scholar]
- 27.Frost H M. Vital biomechanics: proposed general concepts for skeletal adaptations to mechanical usage. Calcif Tissue Int 198842145–156. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg N J, Bargar W L, Friedman B. The incidence of spondylolysis and spondylolisthesis in nonambulatory patients. Spine 1981635–38. [DOI] [PubMed] [Google Scholar]
- 29.Hensinger R N. Spondylolysis and spondylolisthesis in children. Instr Course Lect 198332132–151. [PubMed] [Google Scholar]
- 30.Logroscino G, Mazza O, Aulisa G.et al Spondylolysis and spondylolisthesis in the pediatric and adolescent population. Childs Nerv Syst 200117644–655. [DOI] [PubMed] [Google Scholar]
- 31.Hasler C, Dick W. Spondylolysis and spondylolisthesis during growth. Orthopade 20023178–87. [DOI] [PubMed] [Google Scholar]
- 32.Martin R P, Deane R H, Colett V. Spondylolysis in children who have osteopetrosis. J Bone Joint Surg Am 1997791685–1689. [DOI] [PubMed] [Google Scholar]