Abstract
Aim
To evaluate the outcome and morbidity after major surgical interventions for inflammatory bowel disease (IBD).
Methods
Retrospective case note analysis of 227 children referred to a tertiary referral centre between 1994 and 2002 for treatment of IBD.
Results
26 of 125 children with Crohn's disease (21%) required surgical management. 13 with disease proximal to the left colon underwent limited segmental resections and primary anastomosis, without significant morbidity. Primary surgery for 13 children with disease distal to the transverse colon included 6 subtotal‐colectomies or panprocto‐colectomies. All seven children undergoing conservative segmental resections (three with primary anastomosis, four with stoma formation), required further colonic resection or defunctioning stoma formation. All three children undergoing primary anastomosis developed a leak or fistula formation. 22 of 102 children with ulcerative colitis (22%) required surgery. Definitive procedures (n = 17) included J‐pouch ileoanal anastomosis (n = 11), ileorectal anastomosis (n = 2), straight ileoanal anastomosis (n = 3), and proctectomy/ileostomy (n = 1). Five children await restorative surgery after subtotal colectomy. Median daily stool frequency after J‐pouch surgery was 5 (range 3–15), and 10 of 11 children reported full daytime continence. All three children with straight ileoanal anastomosis had unacceptable stool frequency and remain diverted.
Conclusion
The complication rate after resectional surgery for IBD was 57% for Crohn's disease, and 31% for ulcerative colitis. In children with Crohn's disease, limited resection with primary anastomosis is safe proximal to the left colon. Where surgery is indicated for disease distal to the transverse colon, subtotal or panproctocolectomy is indicated, and an anastomosis should be avoided. Children with ulcerative colitis had a good functional outcome after J‐pouch reconstruction. However, the overall failure rate of attempted reconstructive surgery was 24%, largely owing to the poor results of straight ileoanal anastomosis.
This study examines the surgical presentation and management of a cohort of children with inflammatory bowel disease (IBD), with particular reference to surgical morbidity in Crohn's disease, functional outcome and quality of life (QOL) after reconstructive surgery in ulcerative colitis.
Materials and methods
The case records of 227 children treated for IBD at the Royal Liverpool Children's Hospital NHS Trust, Liverpool, UK, between January 1994 and December 2002, were reviewed retrospectively. During this period the medical management (AMD, DHC), although individualised, was broadly consistent, and there were no fluctuations in the rate of surgical referral. Children with Crohn's disease were treated with courses of enteral feeding, steroids, azathioprine, 5‐aminosalicylic acid (5‐ASA) and occasionally antibiotics. Infliximab was not used during the study period. Steroids, azathioprine and 5‐ASA were the mainstay for treatment of children with ulcerative colitis. Elective surgical procedures for children with IBD were confined to two surgeons (GLL, CTB). A group of 48 children underwent major surgery for IBD. This group was defined by requirement for laparotomy, and thus excluded children undergoing anaesthesia for gastrostomy or central venous line placement, endoscopy, or drainage of a perianal septic focus. Medical management after resectional surgery for Crohn's disease was continued with maintenance azathioprine and 5‐ASA.
Children undergoing surgery for Crohn's disease were categorised into two groups, based on predominant disease location. In group 1 (13 children), disease was proximal to the left colon (superior mesenteric artery territory). In group 2 (13 children), there was significant disease in the distal colon (inferior mesenteric artery distribution). Postoperative morbidity, symptom‐free interval and requirement for subsequent surgical interventions were recorded. Postoperative morbidity was also recorded for 22 children undergoing colectomy for ulcerative colitis. Children who completed reconstructive surgery were assessed for functional outcome by clinical interview using a simple verbal analogue score for QOL, continence, defaecation sensation and overall satisfaction. The median follow‐up period was 20 (range 4–101) months.
Results
During the 8‐year study period, 125 children (74 male, 51 female) with Crohn's disease, and 102 children (35 male, 67 female) with ulcerative colitis were treated at the Royal Liverpool Children's Hospital, which provides tertiary level care for children with IBD.
Presentation
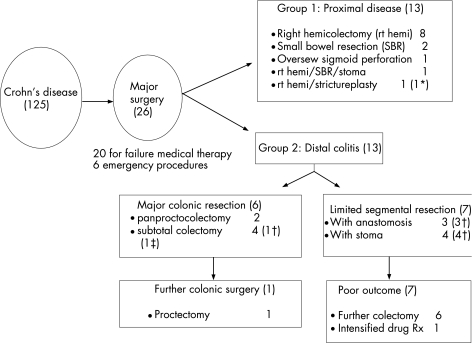
In all, 26 (21%) children required a major surgical intervention for Crohn's disease (fig 1), including four who had an acute surgical presentation (two intestinal obstruction, two suspected appendicitis), and two who required surgery as a result of complications of endoscopic procedures (leakage from percutaneous endoscopic gastrostomy, and colonoscopic perforation). The remaining 20 children required surgery for failure of medical therapy (median duration 27.5 months, range 15–85 months), and included two children in whom the primary indication was severe perianal disease.
Figure 1 Flow diagram for children with Crohn's disease. *Multiple surgical procedures required. †Further colectomy or intensified medical treatment required for colitis. ‡Late death from central line complication.
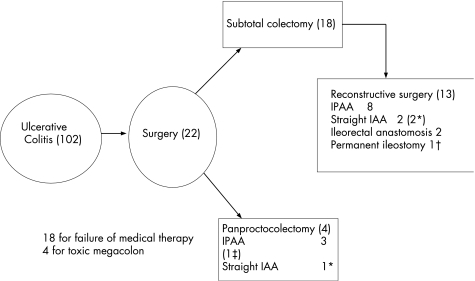
A total of 22 (22%) children required surgery for ulcerative colitis (fig 2). The most common indication for colectomy was failure of medical therapy. In these 18 children, the median duration of medical therapy was 16 (range 1–50) months. In four children the indication for surgery was toxic megacolon. In these children the median duration of medical therapy was only 1.5 (range 1–8) months, suggesting a more aggressive disease course.
Figure 2 Flow diagram for children with ulcerative colitis. *Three children required return to ileostomy for intractable frequency. †Permanent ileostomy due to short vascular pedicle. ‡Marked frequency due to recalcitrant pouchitis.
A positive family history for IBD was reported in 35% of children undergoing surgery for ulcerative colitis and 26% of children for Crohn's disease.
Disease onset and location
The median age at onset of symptoms in the surgical group was 10 (range 1–16) years for children with Crohn's disease, and 11 (range 3–16) years for those with ulcerative colitis. The median time for diagnosis of Crohn's disease was 7 (range 1–36) months, and 4 (range 1–96) months for children with ulcerative colitis. Most children with ulcerative colitis had pancolonic disease at surgical presentation. Inflammation was confined to the left colon in four children, and to the rectosigmoid in a single child. Extensive backwash ileitis was a feature in two children. Macroscopic Crohn's disease was seen in the small bowel (n = 2), ileocaecal region (n = 8), and colon (n = 10), and was panenteric in 6 children.
Surgical procedures—Crohn's disease (group 1)
Primary surgery for children in group 1 (proximal disease; n = 13, fig 1) included eight right hemicolectomies, and two small bowel resections. All procedures were uncomplicated. The median symptom‐free interval was 9 (range 1–52) months, and no further surgical interventions have been required for a median follow‐up period of 13 (range 1–69) months. In all, one child with proximal Crohn's colitis required oversewing of an endoscopic perforation in the sigmoid colon, and has required no further surgical intervention over 69 months follow‐up. Two children with severe panenteric disease had more extensive primary surgery. The first child had right hemicolectomy combined with small bowel resection and stoma formation, and awaits restoration of continuity. The second child had right hemicolectomy combined with small bowel strictureplasty in two areas. This was complicated by leakage from a strictureplasty site, resulting in a total of nine further operations over 48 months, with prolonged total parenteral nutrition support for intestinal failure, before restoration of continuity and full enteral nutrition was finally established.
Surgical procedures—Crohn's disease (group 2)
Children in group 2 (distal colitis; n = 13, fig 1) with significant distal colitis at surgical presentation, were managed either by major colonic resection (MCR; sub‐total or panproctocolectomy), or by more limited segmental colonic resections (SCR).
Limited SCRs were performed in seven children. Further surgery was required in six children, and none enjoyed respite from intensive medical therapy including steroids. A total of three children underwent SCR with anastomosis, of which two developed a leak from the anastomosis, and the third child presented later with a colovesical fistula. The remaining four children had SCR incorporating stoma formation. Conversion to subtotal colectomy was required for failure of medical therapy in two children, and a third child came to panproctocolectomy after two segmental resections, complicated by development of a colocutaneous fistula and persisting severe perianal disease. The other child had two segmental resections, and has persisting symptoms from the retained colon with ongoing requirement for steroid enemas.
MCRs were performed in six children. After primary panproctocolectomy, two children were completely asymptomatic on minimal medical therapy (16 and 24 months follow‐up). Two children had subtotal colectomy and ileostomy formation. One remains asymptomatic (11 months follow‐up). The second developed symptoms from the retained rectum after 30 months, and also required revision of the ileostomy. After proctectomy, the child has remained symptom‐free for 36 months. In two children the initial diagnosis made was ulcerative colitis, and both had subtotal colectomy with ileostomy. One required revisional surgery for an ileostomy stricture at which time the diagnosis of Crohn's disease was made. The second had proctectomy and restorative surgery complicated by anastomotic leakage at closure of ileostomy. At this time, the diagnosis was revised to Crohn's disease. Subsequent adhesion obstruction episodes and ileostomy complications for >9 years required several operations. Sadly, the patient died following an acute complication of central line insertion.
Surgical procedures—ulcerative colitis (n = 22)
The most common primary surgical intervention was subtotal colectomy and ileostomy (18 children), leaving the rectal stump oversewn just above the peritoneal reflection (fig 2). The alternative option, used in four children, was panproctocolectomy with primary reconstructive “pouch”‐ or “straight”‐ileoanal anastomosis, protected by a temporary ileostomy. Staged reconstructive surgery was performed in 13 of the 18 children who underwent subtotal colectomy. The median delay to reconstructive surgery was 17 (range 7–57) months. Reconstructive surgery has been deferred indefinitely in five children.
Reconstructive surgery, whether primary or delayed, comprised ileal pouch anal anastomosis (IPAA, 11), straight ileoanal anastomosis (n = 3) and ileorectal anastomosis (n = 2). An ileoanal anastomosis was attempted and abandoned for permanent ileostomy in one child because of excessive tension on the terminal ileal vascular pedicle. With the exception of two children undergoing ileorectal anastomosis, reconstructive surgery involved abdominal proctectomy combined with transanal sphincter‐preserving mucosectomy, and was completed with a handsewn anastomosis. In all cases formation of a J‐pouch was the ideal, but where tension on the vascular pedicle was severe, a straight ileoanal anastomosis was performed. All ileoanal anastomoses were covered by a temporary diverting ileostomy, which was closed at a median period of 3 months.
Functional outcome after reconstructive surgery for ulcerative colitis
The two children with ileorectal anastomosis continue to require maintenance therapy, report a stool frequency of 4.5 per day and will require life‐long cancer surveillance assuming that their rectal disease remains quiescent.
Reconstructive surgery was not technically possible in a single child after proctectomy. All three children with straight ileoanal anastomoses required stoma reformation for intractable frequency and poor control. Thus, 4 of 17 children (24%) have a permanent stoma after failure of reconstructive surgery.
J‐pouch ileo‐anal anastomosis was performed in 11 children (3 one‐stage, and 8 two‐stage procedures). Transient pouch‐anal strictures were successfully dilated in two children, and deteriorating pouch function due to pouchitis has been observed in a further child. The median daily stool frequency is 5 (range 3–15), with 10 of 11 children reporting full daytime continence. In all, 10 of 11 children reported a significantly improved QOL after surgery, with a single child reporting no change.
Surgical complications and recurrence of Crohn's disease
Children undergoing surgery for Crohn's disease had a 57% complication rate, mainly from sepsis, adhesions and stomal complications. Secondary surgery was required for recurrent Crohn's disease in 38% of the children in this series. In children with ulcerative colitis, 21 of 67 operations (31%) were for complications of definitive surgery. Adhesion obstruction and stomal complications were excessively common. One child had a lower limb compartment syndrome and required fasciotomies and extensive muscle debridement resulting in considerable functional impairment.
Discussion
The requirement for major surgical intervention in reported series of paediatric Crohn's disease1,2,3,4 ranges from 21% (this study) to 79%.4 This discrepancy may reflect varying lengths of follow‐up, or improving medical management over time. A more useful indicator is likelihood of surgery in relation to symptom duration. Suggested figures are 29% after 3 years,1 and 47–50% after 5 years.1,4
Surgical treatment of Crohn's disease may relieve symptoms in the short term and optimise growth potential if appropriately timed,5 but it does not prevent disease recurrence quoted as 50% at 2 years in one paediatric series.5 In our experience the median symptom‐free interval was only 9 months, and 38% of children had required secondary surgery for recurrent disease. This possibly reflects a high incidence of colonic disease in this series. Surgery carries a high reoperative complication rate (36% in this study), with septic intrabdominal complications particularly associated with steroid therapy, surgery for intestinal fistulae, abdominal abscess at presentation and low serum albumin.6
Macroscopic disease is conventionally subdivided into small bowel, ileocaecal and colonic sites, with panenteric disease incorporating multiregional involvement. This descriptive classification has prognostic significance. Unsurprisingly, children with panenteric disease have the worst relapse rate.7,8 Children undergoing small bowel and ileocaecal resections can have prolonged asymptomatic intervals, with one study reporting a 4‐year median recurrence‐free period.8 Colonic resection is associated with a less favourable outcome. The same study reported only a 1‐year recurrence‐free interval,8 and others confirm a higher reoperation rate in Crohn's colitis.3 A large adult series of colonic Crohn's disease suggests a 50% 10‐year resection rate with half eventually requiring an ileostomy.9 These observations concerning colonic disease deserve careful scrutiny as the choice of operation may influence outcome as much as disease severity or natural history.
We chose to classify patients into two groups based on the presence or absence of significant distal colitis. This was based on an impression that limited bowel resections with primary anastomosis up to and including the right colon were associated with minimal surgical morbidity. The results of group 1 patients confirm this. A single patient had significant morbidity after a leak from a strictureplasty site. There is little experience in the paediatric surgical literature regarding strictureplasty, although good results have been reported.10
Group 2 patients with distal colonic involvement were subclassified depending on the nature of their colonic resection (MCR vs SCR). Despite small numbers, our results suggest a better prognosis after MCR and avoidance of anastomosis. Davies et al7 similarly suggest an increased relapse rate after segmental resection or loop ileostomy alone. Support for the concept of MCR is also given by other paediatric series.1,3,11 However, Andersson et al12 have expressed a contrary opinion arguing in favour of SCR, although from a predominantly adult experience.
Two patients in our series had colostomies fashioned primarily for severe perianal disease in association with left‐sided colitis. Conservative surgery is indicated for perianal disease,13,14 except in situations of uncontrolled sepsis, recalcitrant anal stricture or sphincter destruction.15
Surgery was required in 22% of the children treated for ulcerative colitis in this series. The indications for surgery included toxic megacolon and failure of medical therapy (principally steroid‐resistant colitis and growth impairment). The surgical treatment of ulcerative colitis has been revolutionised by the description of the IPAA by Parks and Nichols in 1978.16 The IPAA combines acceptable stool frequency with near normal continence, and now represents the gold standard in surgical management. Straight ileaoanal anastomosis was described in 1947,17 and is still advocated by some experts.18,19 In this series, straight ileoanal anastomosis was performed where an IPAA was considered to be under excessive tension. In the light of the dismal outcome with this technique, we feel that the risk of tension on the anastomosis should be accepted to achieve an IPAA with a covering ileostomy. The opinion from the literature also favours the IPAA in most comparative series in children.20,21,22 The ileal J‐pouch anal anastomosis is recommended by virtue of its simplicity, and its durable functional outcome in the larger paediatic series.21,22,23,24,25,26
As an alternative to proctectomy, the ileorectal anastomosis provides an excellent functional outcome, but long‐term treatment may be required for proctitis and regular endoscopic surveillance for dysplasia/cancer. The IPAA is associated with reduced female fertility,27 although pouch function itself seems to be unaffected by pregnancy.28 Fertility considerations in females represent the main reason for continuing use of the ileorectal anastomosis.
Most children and their parents have time to consider the implications of surgical treatment for ulcerative colitis, as colectomy is rarely required as an emergency procedure. Advising the family on surgical options is paramount. It is important to stress the high overall complication rate of both initial and reconstructive surgery, with a figure of 50% being both reasonable and honest.23 Most complications relate to wound sepsis and are probably partly attributable to long‐term steroid use. However, the risk of adhesion obstruction is approximately 30%,23,24 and complications related to the ileostomy are also frequent, especially if a loop ileostomy is used.29
The cumulative rate of pouch failure is 15% at 10–15 years.30 Causes of failure include sepsis, pouch‐vaginal fistula, unsuspected Crohn's disease, anastomotic stricture, retained rectum, small volume reservoir and pouchitis. Failure may necessitate excision of the pouch and possibly permanent ileostomy. Given the occasional need for long‐term ileostomy due to pouch failure, it can be argued that living with and adapting to life with a stoma for a period of time is important before undergoing reconstructive surgery. This represents an important argument for staging restorative surgery. Despite this some families will want to have single‐stage IPAA, to reduce both the number of procedures and time spent with a stoma. This approach is perfectly reasonable provided the nutritional condition of the child is good, and the colitis is well controlled in the short term.
The ethical question of whether paediatric or adult surgeons should be performing reconstructive surgery is clearly important. A recent audit undertaken by the authors on behalf of the British Association of Paediatric Surgeons has shown that arrangements for joint operating with adult surgeons are established in 55% of paediatric surgical centres. The median experience with IPAA for responding paediatric surgeons was 0.7/year of consultant practice (unpublished data). These data support the concept of shared care and well organised transition between paediatric and adult services.
In our experience of childhood IBD, a multi‐disciplinary approach is invaluable in making decisions regarding surgical management. A conservative surgical strategy serves well where Crohn's disease is located proximal to the left colon. In this situation anastomosis is safe. A case for more aggressive colonic resection and avoidance of anastomosis is made where there is significant distal colitis. Similarly, excellent functional and QOL outcomes can be obtained in ulcerative colitis from J‐pouch IPAA in childhood.
What is already known on this topic
Surgery for inflammatory bowel disease is associated with a high complication rate, and a significant recurrence rate for Crohn's disease.
Indications for surgery include failure of medical therapy (including delayed puberty and drug intolerance), toxic megacolon, bowel perforation, obstruction, enteric fistula and abdominal abscess.
What this study adds
Subtotal or panproctocolectomy is appropriate in refractory distal Crohn's colitis, rather than conservative segmental resections.
Ileal pouch anal anastomosis is associated with an acceptable outcome in children and is the preferred reconstructive surgical option.
Abbreviations
5‐ASA - 5‐aminosalicylic acid
IBD - inflammatory bowel disease
IPAA - ileal pouch anal anastomosis
MCR - major colonic resection
SCR - segmental colonic resection
QOL - quality of life
Footnotes
Competing interests: None declared.
References
- 1.Patel H I, Leichtner A M, Colodny A H.et al Surgery for Crohn's disease in infants and children. J Pediatr Surg 1997321063–1068. [DOI] [PubMed] [Google Scholar]
- 2.Dokucu A I, Sarnacki S, Michel J L.et al Indications and results of surgery in patients with Crohn's disease with onset under 10 years of age: a series of 18 patients. Eur J Pediatr Surg 200212180–185. [DOI] [PubMed] [Google Scholar]
- 3.Aronson D C, Van Coevorden F, Heijmans H S.et al Surgical treatment of Crohn disease in children and adolescents; how conservative can the paediatrician be? Eur J Pediatr 1993152727–729. [DOI] [PubMed] [Google Scholar]
- 4.Sedgwick D M, Barton J R, Hamer‐Hodges D W.et al Population‐based study of surgery in juvenile onset Crohn's disease. Br J Surg 199178171–175. [DOI] [PubMed] [Google Scholar]
- 5.Besnard M, Jaby O, Mougenot J F.et al Postoperative outcome of Crohn's disease in 30 children. Gut 199843634–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto T, Allan R N, Keighley M R. Risk factors for intra‐abdominal sepsis after surgery in Crohn's disease. Dis Colon Rectum 2000431141–1145. [DOI] [PubMed] [Google Scholar]
- 7.Davies G, Evans C M, Shand W S.et al Surgery for Crohn's disease in childhood: influence of site of disease and operative procedure on outcome. Br J Surg 199077891–894. [DOI] [PubMed] [Google Scholar]
- 8.Baldassano R N, Han P D, Jeshion W C.et al Paediatric Crohn's disease: risk factors for postoperative recurrence. Am J Gastroenterol 2001962169–2176. [DOI] [PubMed] [Google Scholar]
- 9.Lapidus A, Bernell O, Hellers G.et al Clinical course of colorectal Crohn's disease: a 35‐year follow up study of 507 patients. Gastroenterology 19981141151–1160. [DOI] [PubMed] [Google Scholar]
- 10.Di Abriola G F, De Angelis P, Dall'oglio L.et al Strictureplasty: an alternative approach in long segment bowel stenosis Crohn's disease. J Pediatr Surg 200338814–818. [DOI] [PubMed] [Google Scholar]
- 11.Fonkalsrud E W, Ashcraft K W, Holder T M. Inflammatory bowel disease. In: eds. Pediatric Surgery (2nd edn), Philadelphia: PA: Saunders 1993440–452.
- 12.Andersson P, Olaison G, Bodemar G.et al Surgery for Crohn's colitis over a twenty‐eight‐year period: fewer stomas and the replacement of total colectomy by segmental resection. Scand J Gastroenterol 20023768–73. [DOI] [PubMed] [Google Scholar]
- 13.Vogelsang H, Granditsch G, Binder C.et al Consensus of the Chronic Inflammatory Bowel Disease Study Group of the Austrian Society of Gastroenterology and Hepatology on the topic of “diagnosis and therapy of chronic inflammatory bowel diseases in adolescence”. Z Gastroenterol 200038791–794. [DOI] [PubMed] [Google Scholar]
- 14.Palder S B, Shandling B, Bilik R.et al Perianal complications of pediatric Crohn's disease. J Pediatr Surg 199126513–515. [DOI] [PubMed] [Google Scholar]
- 15.Tolia V. Perianal Crohn's disease in children and adolescents. Am J Gastroenterol 199691922–926. [PubMed] [Google Scholar]
- 16.Parks A G, Nicholls R J. Proctocolectomy without ileostomy for ulcerative colitis. BMJ 1978ii85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravitch M M, Sabiston D J., Jr Anal ileostomy with preservation of the sphincter. A proposed operation in patients requiring total colectomy for benign lesions. Surg Gynecol Obstet 1947841095–1099. [PubMed] [Google Scholar]
- 18.Dodero P, Magillo P, Scarsi P L. Total colectomy and straight ileo‐anal soave endorectal pull‐through: personal experience with 42 cases. Eur J Pediatr Surg 200111319–323. [DOI] [PubMed] [Google Scholar]
- 19.Coran A G. A personal experience with 100 consecutive total colectomies and straight ileoanal endorectal pull‐throughs for benign disease of the colon in children and adults. Ann Surg 1990212242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rintala R J, Lindahl H. Restorative proctocolectomy for ulcerative colitis in children—is the J‐pouch better than straight pull‐through? J Pediatr Surg 199631530–533. [DOI] [PubMed] [Google Scholar]
- 21.Telander R L, Spencer M, Perrault J.et al Long‐term follow‐up of the ileoanal anastomosis in children and young adults. Surgery 1990108717–723. [PubMed] [Google Scholar]
- 22.Durno C, Sherman P, Harris K.et al Outcome after ileoanal anastomosis in pediatric patients with ulcerative colitis. J Pediatr Gastroenterol Nutr 199827501–507. [DOI] [PubMed] [Google Scholar]
- 23.Rintala R J, Lindahl H G. Proctocolectomy and J‐pouch ileo‐anal anastomosis in children. J Pediatr Surg 20023766–70. [DOI] [PubMed] [Google Scholar]
- 24.Wewer V, Hesselfeldt P, Qvist N.et al J‐pouch ileoanal anastomosis in children and adolescents with ulcerative colitis:functional outcome, satisfaction and impact on social life. J Pediatr Gastroenterol Nutr 200540189–193. [DOI] [PubMed] [Google Scholar]
- 25.Fonkalsrud E W. Long‐term results after colectomy and ileoanal pull‐through procedure in children. Arch Surg 1996131881–885. [DOI] [PubMed] [Google Scholar]
- 26.Chew S S, Kerdic R I, Yang J L.et al Functional outcome and quality of life after ileal pouch‐anal anastomosis in children and adults. ANZ J Surg 200373983–987. [DOI] [PubMed] [Google Scholar]
- 27.Johnson P, Richard C, Ravid A.et al Female infertility after ileal pouch‐anal anastomosis for ulcerative colitis. Dis Colon Rectum 2004471119–1126. [DOI] [PubMed] [Google Scholar]
- 28.Hahnloser D, Pemberton J H, Wolff B G.et al Pregnancy and delivery before and after ileal pouch‐anal anastomosis for inflammatory bowel disease: immediate and long‐term consequences and outcomes. Dis Colon Rectum 2004471127–1135. [DOI] [PubMed] [Google Scholar]
- 29.Fonkalsrud E W, Thakur A, Roof L. Comparison of loop versus end ileostomy for fecal diversion after restorative proctocolectomy for ulcerative colitis. J Am Coll Surg 2000190418–422. [DOI] [PubMed] [Google Scholar]
- 30.Tulchinsky H, Cohen C R G, Nicholls R J. Salvage surgery after restorative proctocolectomy. Br J Surg 200390909–921. [DOI] [PubMed] [Google Scholar]