Abstract
In vivo electron paramagnetic resonance (EPR) tooth dosimetry provides a means for non-invasive retrospective assessment of personal radiation exposure. While there is a clear need for such capabilities following radiation accidents, the most pressing need for the development of this technology is the heightened likelihood of terrorist events or nuclear conflicts. This technique will enable such measurements to be made at the site of an incident, while the subject is present, to assist emergency personnel as they perform triage for the affected population. At Dartmouth Medical School this development is currently being tested with normal volunteers with irradiated teeth placed in their mouths and with patients who have undergone radiation therapy. Here we describe progress in practical procedures to provide accurate and reproducible in vivo dose estimates.
Introduction
Following September 11, 2001, and now with heightened international development of nuclear weapons capabilities, there has been a renewed interest in EPR tooth dosimetry and translational development that would enable it to be applied to estimate individuals' absorbed doses, on a large scale, following a terrorist or nuclear attack. It was recognized almost 40 years ago that EPR could be used to measure the existence of radiation-induced long lived radicals produced in various biologic tissues in rats and humans, including teeth, bones, and fingernails. (Brady, et al., 1968) Since this seminal description, substantial progress has been made in EPR dosimetry of in vitro samples and precision as fine as ±2.5 cGy has been reported for low-dose measurements, with precision of ±10% for doses above 25 cGy. (Chumak, et al., 2005)
EPR tooth dosimetry using samples of pure enamel that have been extracted from irradiated subjects are typically performed at an X-band frequency (∼9 GHz) with a standard TE102 cavity resonator and may employ a goniometer to average out spatial anisotropy (El-Faramawy and Wieser, 2006, Gualtieri, et al., 2001b, Hayes, et al., 2000, Hayes, et al., 1998). The high RF frequency and resonator filling factor contribute to the sensitivity of such measurements. These in vitro measurements often employ the “dose-added” technique, where the sample is repeatedly measured after successive irradiation to known doses. This and similar methodologies have proven to be very useful for retrospective radiation dosimetry (see e.g. Hoshi et al. in this volume). There are several preliminary reports of in vitro experiments with whole teeth (Iwasaki, et al., 2005a, Iwasaki, et al., 2005b, Zdravkova, et al., 2003a, Zdravkova, et al., 2002, Zdravkova, et al., 2003b).
It is our goal to develop the instrumentation and methodology required to enable EPR dosimetry to be performed in vivo, using intact whole teeth, for large numbers of people. The resulting information then could be used to screen the affected population to provide guidance for entrance into medical care following a radiation incident (Swartz, et al., (In Press), Swartz, et al., 2005). Swartz et al. (In Press) describe several of the challenges involved with this translational research. In order to be useful, these measurements must provide dose estimates with appropriate precision (∼±100 cGy) for doses as small as 100 cGy, with rapid data acquisition and analysis (5-10 min per individual), and the device must be transportable so it can be deployed at the site of the incident. The resulting dose estimate must be supplied immediately so subjects can be directed for further care or dismissed, enabling effective decision-making in an organized manner in the midst of potential chaos following the event. It is expected that the majority of the population will have received doses that are unlikely to result in the acute radiation syndrome, so it is crucial that high specificity can be achieved without severely compromising the sensitivity of the measurement. These combined considerations call for the development of a non-invasive means to perform EPR dosimetry. This development is underway at Dartmouth Medical School (Iwasaki, et al., 2005a, Iwasaki, et al., 2005b, Salikhov, et al., 2005, Swartz, et al., 2006, Swartz, et al., 2005, Swartz, et al., 2004) in normal volunteers with irradiated teeth placed in their mouths and initial measurements with patients who have undergone radiation therapy have begun.
Description of clinical facility
In vivo EPR dosimetry is currently performed using the whole-body clinical EPR spectrometer at the Dartmouth EPR Center (Salikhov, et al., 2005, Swartz, et al., 2006, Swartz, et al., 2005, Swartz, et al., 2004). This continuous wave (CW) spectrometer operates at an L-Band frequency (1.2 GHz) and the main magnetic field is produced by a 420 G permanent magnet with 50 cm pole separation. Noninvasive measurements of the in situ teeth are made using external surface loop resonators (Hirata, et al., 2000, Salikhov, et al., 2003) which have been adapted specifically for intra-oral measurements of molar teeth. These resonators are equipped with automatic tuning control (ATC) circuitry to minimize effects of subject motion. Detailed descriptions of this clinical spectrometer are given in the published reports (Salikhov, et al., 2005, Swartz, et al., 2004) and specific modifications to the instrument for tooth dosimetry are given here. Descriptions of another dedicated transportable spectrometer for tooth dosimetry have also been published (Swartz, et al., 2006, Swartz, et al., 2005).
Dose Quantitation using EPR
The amplitude of the CW EPR signal is proportional to the magnetic susceptibility of the sample, the filling factor, the quality factor of the resonator, the square root of the microwave power, and the square root of the impedance of the transmission line. The detected signal amplitude also depends on the Zeeman modulation amplitude and the combined amplification, losses, and averaging that occur in the detection system. The term of interest is the magnetic susceptibility, which is proportional to the number of spins in the sample and, after accounting for the native signal, proportional to the absorbed dose. A relative measurement of the magnetic susceptibility can be inferred from the measured signal only if each of the other terms are accurately accounted for or are constant across subjects. The filling factor of the resonator depends on the relative locations and shapes of the detection loop and the teeth of interest. The quality factor depends on the physical construction of the resonator as well as the amount of lossy tissue within its sensitive volume. The modulation amplitude depends on the relative position of the modulation coils with respect to the teeth, the geometry of the coils, and the amount of current flowing through the coils. Finally, all of these terms depend on the mechanical and electrical stability of the spectrometer. We have developed a measurement procedure designed to provide stable and uniform measurement conditions across subjects and over time so that sensitive and reliable estimation of the absorbed dose can be performed.
Measurement Approach
Potentially the most critical experimental consideration for the acquisition of reproducible measurements of radiation induced radical density is the positioning of the resonator relative to the tooth. The effect of the resonator position is reflected in both the filling factor and quality factor terms. The filling factor reflects the overlap of the B1 magnetic field established by the resonator with the locations of radicals in the tooth sample. The B1 magnetic field distribution, which defines the sensitive volume of the resonator, is nonuniform for surface loop resonators (He, et al., 2002). Experimental measurements of the EPR signal amplitude recorded for a tooth sample with variable position relative to the resonator are shown in Figure 1. Measurements were performed with a single molar tooth that had been irradiated to a dose of 30 Gy, as the center of the biting surface was translated vertically and horizontally with respect to the center of a 12 cm diameter detection loop. For each measurement, nine 10 second scans were averaged and were collected with an incident power of 40 mW, 22 G scan width, and a 4.8 G modulation amplitude. These data show a strong dependence of the EPR signal amplitude on the vertical position of the detection loop with a maximum at Δx = -4 mm, where the tooth is placed within the resonator and the biting surface is slightly above the central plane of the loop. There is a weaker dependence of the amplitude on the horizontal position of the resonator, with a broad maximum occurring at roughly +3 mm, where the tooth is under the detection loop with its center slightly biased toward the distal tip of the detection loop.
Figure 1.
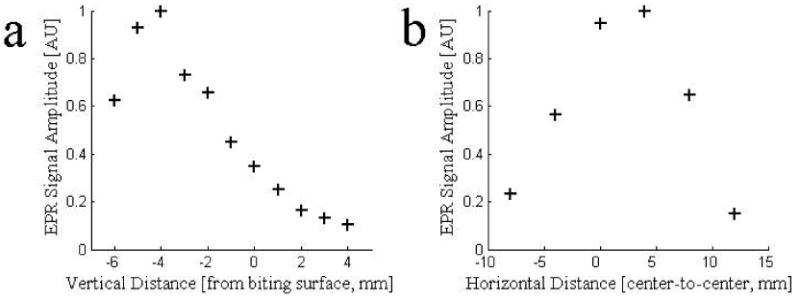
The EPR signal amplitude of a tooth irradiated to 30 Gy was measured as the location of the center of the resonator detection loop was varied both (a) vertically and (b) horizontally from the center of the biting surface of the tooth. The maximal signal amplitude was recorded when the detection loop was lowered to encompass the upper portion of the tooth (Δy ∼ -0.4 mm) and when the loop is slightly displaced horizontally such that the distal edge of the loop is positioned over the edge of the tooth (Δx ∼ 3 mm).
Dosimetric measurements with the whole body clinical spectrometer are made with the subject in a lying position on a dedicated custom-made stretcher (Fig.2a and 2b). It is crucial that subjects are comfortably immobilized during the measurements, as any motion adds noise to the spectrum and can alter the position of the resonator on the tooth surface. Importantly, this setup allows all of the positioning procedures for placing the resonator on the teeth of the subject to be carried out with the subject outside of the magnet, where there is ample visual and tactile access. In addition to allowing precise positioning, this would facilitate the throughput of these measurements by limiting the time in the magnet to that needed for the actual measurements.
Figure 2.
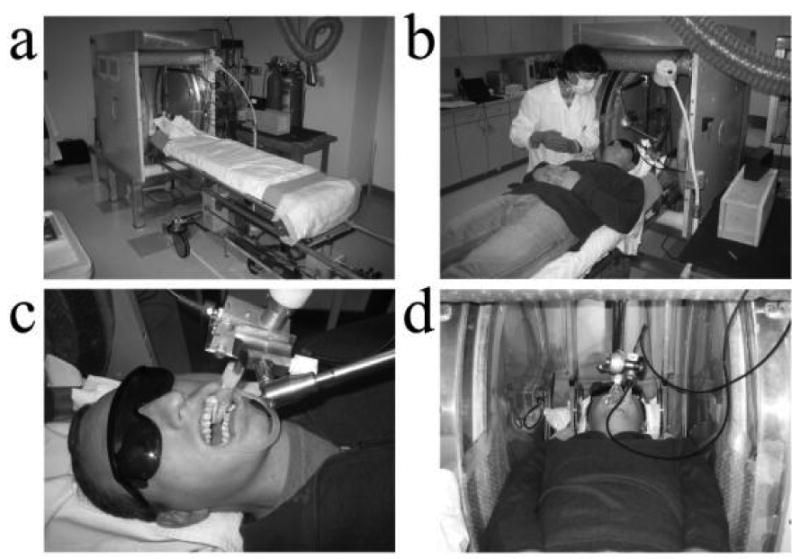
Measurements are made with the subject in a lying position on a stretcher that can be placed within the magnet. The resonator is mounted on a lockable articulating arm that is fixed to the subject bed.
Once the subject is lying comfortably in the desired position, the resonator is positioned on the surface of the tooth. A double ended cheek retractor (Hager & Werken GmbH & Co. Duisburg, Germany) is used to hold the lips and cheeks out of the way (Fig 2c). Prior to positioning the resonator, the maximal width of the tooth of interest and the width along the orthogonal dimension are measured using a set of dental calipers and a standard intra-oral dental mirror and recorded for use in normalizing for the tooth size prior to dose estimation. One or more small absorptive pads (Cotton Rolls No. 2 Medium, Crosstex International, Hauppauge, NY)(Dry Tips, Molnlycke Health Care AB, Goteborg, SWE) are positioned in the mouth to absorb saliva during the experiment (Fig. 2c and 2d). The surface loop resonator is affixed to the end of an articulating arm that is mounted to the subject bed. (Fig. 2c and 2d). The articulating arm (Fisso, Baitella AG, Zurich), was customized in house to replace several ferrous parts. It allows full 3D translation and rotation of the resonator so the detection loop can be precisely placed on the tooth of interest and robustly locked in position using a unified single-handed locking mechanism.
The placement of the resonator is further guided and stabilized using an individualized pair of dental casts (Fig. 3). These casts are constructed using commercial Exafast dental putty (GC America Inc., Alsip, IL). This putty requires approximately 5 minutes to harden and has been observed to have no confounding EPR signal or lead to significant reduction in the quality factor (Q) of the resonator. One piece of the cast is placed over the tooth of interest, mating with the row of teeth on one of its sides and with the detection loop on the other. A hole is cut in the center of this piece between the tooth and the detection loop allowing placement of the resonator directly on the tooth surface. A second cast piece is placed over the detection loop and couples with the first piece. The subject holds the resonator and the pair of casts in place by lightly biting on the combination (Fig. 2c). This arrangement has several advantages in addition to the stability it confers in the placement of the detection loop on the tooth surface. The casts push both the tongue and cheek away from the resonator, which reduces the amount of lossy tissue near the resonator. This prevents the Q of the resonator from being further reduced and decreases the amount of variability in this factor during measurements and in between subjects. This system increases the comfort of the subject by reducing fatigue associated with keeping the mouth open. If repeated measurements are to be made for a given subject, these casts are re-usable and enable the resonator to be placed in precisely the same position. Measurements made with volunteers with single irradiated teeth have used casts that isolate the resonator from the opposing teeth, but measurements made with subjects with complete sets of irradiated teeth may use casts that position the detection loop in close proximity to both upper and lower teeth for simultaneous measurement and increased sensitivity.
Figure 3.
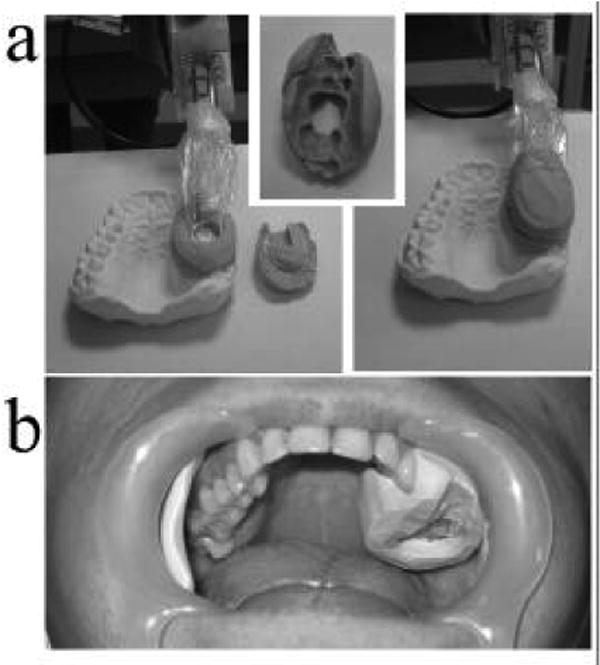
Dental casts are constructed to allow for accurate and reproducible positioning of the resonator on the tooth or teeth of interest. Panel (a) shows the base of a cast coupled to the dentition with the resonator in place, the tooth-mating surface of the base, and the resonator held in position by the complete cast with cap in place. Image (b) shows the base of the cast installed in the mouth of a volunteer.
Once the resonator has been installed, the subject bed is rolled into the magnet so the tooth of interest and the detection loop of the resonator are positioned near the center of the magnet (Fig 2d). A pair of modulation coils that are fixed on arms that mount to the magnet frame are positioned such that the tooth lies along the axis defined by the coils. These coils are capable of providing modulation amplitudes of up to 5 G peak-to-peak. As the current coils are far from the Helmholtz configuration, due to geometrical constraints, they are significantly nonuniform. The modulation amplitude increases as the region of interest gets closer to either coil, increasing by up to 25% within a 4 in diameter central volume. Such nonuniformity can lead to substantial variation in the signal amplitude as the location of tooth varies from side-to-side or between subjects. In order to address this potential source of error, we have implemented a reference standard measurement which provides the modulation amplitude at the base of the detection loop.
The reference standard consists of a single small crystal of lithium phthalocyanine (LiPc) with a natural Lorentzian linewidth of 40 mG contained in an evacuated capsule. The amount of LiPc is large enough that its signal is much larger than that of an irradiated tooth, but spectra can be acquired with all the same instrumental settings (e.g., sensitivity and gain) as those used for measurements of the tooth alone. This capsule is fixed at the end of retractable rod that runs parallel to the transmission lines of the resonator. At the onset of a tooth measurement the rod is pushed forward such that the LiPc sample is at the proximal edge of the detection loop. After installing and tuning the resonator, a small set of spectra are acquired with 20 dB attenuation of the 100 mW of incident power used for tooth measurements and a nominal modulation amplitude of 4.0 G. These spectra are then analyzed using a least-squares fit with a spectral model that incorporates the effects of modulation amplitude and frequency (Robinson, et al., 1999). With a known fixed natural linewidth, the modulation amplitude is treated as an adjustable parameter during the spectral fitting, resulting in a direct measurement of the modulation amplitude at the detector loop. Using this measurement, the amplitude of the low frequency source can be adjusted to set the modulation amplitude at the tooth to the desired value of 4.0 G. With this adjustment completed, the LiPc sample is removed from the sensitive volume of the resonator by retracting the supporting rod and the RF power is returned to 100 mW for tooth measurement. The removal of the LiPc standard does not affect the position of the resonator detection loop or significantly affect the loaded Q of the resonator. In addition to providing the modulation amplitude, development is underway to also use the LiPc reference standard to provide a measurement of the amplitude of the B1 magnetic field generated by the resonator. With online fitting of the LiPc spectra, this measurement and adjustment of the modulation amplitude can be completed in approximately 1 min.
Finally, a larger set of spectra are acquired of the dosimetric tooth signal. Typically, 60 scans are acquired with a 3-sec sweep time, 25 Gauss scan range, 100 mW of incident power, with 1024 points and a 30 ms time constant. Including the delays between scans, these scans are completed within 4 minutes. Longer measurements, with increased averaging, can be performed to increase the signal-to-noise ratio; this might be especially useful after an initial screening has placed most subjects into appropriate categories, enabling measurements to be made more precisely when the initial result was borderline. Using the data acquisition methodology described here and the data analysis techniques described by Demidenko et al. (Demidenko, et al., (In Press)) in vivo dosimetry measurements have been performed in normal volunteers with irradiated single-tooth dentures with standard error of prediction equal to ±184 cGy. Averaging similar measurements made on 3 days reduced this standard error of prediction to ±46 cGy. Refinements to this methodology are continuous, and it is expected that the error of prediction can be reduced substantially. The most straightforward improvement is the simultaneous measurement of multiple irradiated teeth to increase the signal to noise ratio. This approach is currently being investigated in measurements of volunteers who have completed courses of radiation therapy that resulted in significant doses to the teeth.
Conclusion
As noted throughout the papers of this special issue of Radiation Measurements there is currently intense interest in the development of the technology and methodology for using electron paramagnetic resonance spectroscopy of tooth enamel for retrospective radiation dosimetry. The most important role for this technique will be to make measurements in the field that are available for decision-making while the subject is present. While the basic requirements for this capability have been shown to be achievable, very significant improvements are likely using approaches that seem quite feasible. While the current system could be deployed in the field almost immediately, it has some significant limitations. It seems likely that the next generation system, which will be more versatile and with greater capabilities, will be available within 1 – 2 years for widespread field deployment.
Acknowledgments
This study was supported in part by NIH grant U19 AI067733, by a Dept. of Defense grant, DA905-02-011 (DTRA) and used the facilities of the EPR Center for the Study of Viable Systems (NIH grant P41 EB002032). We wish to thank the National Diseases Research Interchange (NDRI) for procurement of the teeth used in our experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brady JM, Aarestad NO, Swartz HM. In vivo dosimetry by electron spin resonance spectroscopy. Health Physics. 1968;15:43–7. doi: 10.1097/00004032-196807000-00007. [DOI] [PubMed] [Google Scholar]
- Chumak V, Sholom S, Bakhanova E, Pasalskaya L, Musijachenko A. High precision EPR dosimetry as a reference tool for validation of other techniques. Appl Radiat Isot. 2005;62:141–6. doi: 10.1016/j.apradiso.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Demidenko E, Williams BB, Sucheta A, Dong R, Swartz HM. Radiation dose reconstruction from L-band in vivo EPR spectroscopy of intact teeth: Comparison of methods. Radiation Measurements. doi: 10.1016/j.radmeas.2007.05.025. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers M. In vivo assessment of radiation exposure. Health Physics. 1991;61:859–861. doi: 10.1097/00004032-199112000-00018. [DOI] [PubMed] [Google Scholar]
- El-Faramawy N, Wieser A. The use of deciduous molars in EPR dose reconstruction. Radiation and Environmental Biophysics. 2006;44:273–277. doi: 10.1007/s00411-005-0023-2. [DOI] [PubMed] [Google Scholar]
- Gualtieri G, Colacicchi S, Sgattoni R, Giannoni M. EPR spectroscopy of tooth enamel: the tooth radicals and the microcrystal alignment. Research on Chemical Intermediates. 2001b;27:945–956. [Google Scholar]
- Hayes R, Haskell E, Barrus J, Kenner G, Romanyukha A. Accurate EPR radiosensitivity calibration using small sample masses. Nuclear Inst and Methods in Physics Research A. 2000;441:535–550. [Google Scholar]
- Hayes R, Haskell E, Romanyukha A. Technique for increasing reproducibility in EPR dosimetry of tooth enamel. Measurement Science and Technology. 1998;9:1994–2006. [Google Scholar]
- He G, Evalappan SP, Hirata H, Deng Y, Petryakov S, Kuppusamy P, Zweier JL. Mapping of the B1 field distribution of a surface coil resonator using EPR imaging. Magn Reson Med. 2002;48:1057–62. doi: 10.1002/mrm.10302. [DOI] [PubMed] [Google Scholar]
- Hirata H, Walczak T, Swartz HM. Electronically tunable surface-coil-type resonator for L-band EPR spectroscopy. Journal of Magnetic Resonance. 2000;142:159–67. doi: 10.1006/jmre.1999.1927. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Grinberg O, Walczak T, Swartz HM. In vivo measurements of EPR signals in whole human teeth. Appl Radiat Isot. 2005a;62:187–90. doi: 10.1016/j.apradiso.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Walczak T, Grinberg O, Swartz HM. Differentiation of the observed low frequency (1200MHz) EPR signals in whole human teeth. Appl Radiat Isot. 2005b;62:133–9. doi: 10.1016/j.apradiso.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Robinson BH, Mailer C, Reese AW. Linewidth analysis of spin labels in liquids. I. Theory and data analysis. Journal of Magnetic Resonance. 1999;138:199–209. doi: 10.1006/jmre.1999.1737. [DOI] [PubMed] [Google Scholar]
- Salikhov I, Hirata H, Walczak T, Swartz HM. An improved external loop resonator for in vivo L-band EPR spectroscopy. J Magn Reson. 2003;164:54–9. doi: 10.1016/s1090-7807(03)00175-7. [DOI] [PubMed] [Google Scholar]
- Salikhov I, Walczak T, Lesniewski P, Khan N, Iwasaki A, Comi R, Buckey J, Swartz HM. EPR spectrometer for clinical applications. Magn Reson Med. 2005;54:1317–20. doi: 10.1002/mrm.20689. [DOI] [PubMed] [Google Scholar]
- Swartz HM, Burke GC, Coey M, Demidenko E, Dong R, Grinberg OY, Hilton J, Iwasaki A, Lesniewski P, Kmiec M, Lo KM, Nicolalde RJ, Ruuge A, Sakata Y, Sucheta A, Walczak T, Williams BB, Mitchell C, Romanyukha A, Schauer DA. In Vivo EPR For Dosimetry. Radiation Measurements. doi: 10.1016/j.radmeas.2007.05.023. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz HM, Iwasaki A, Walczak T, Demidenko E, Salikhov I, Khan N, Lesniewski P, Thomas J, Romanyukha A, Schauer D, Starewicz P. In vivo EPR dosimetry to quantify exposures to clinically significant doses of ionising radiation. Radiat Prot Dosimetry. 2006;120:163–70. doi: 10.1093/rpd/nci554. [DOI] [PubMed] [Google Scholar]
- Swartz HM, Iwasaki A, Walczak T, Demidenko E, Salikov I, Lesniewski P, Starewicz P, Schauer D, Romanyukha A. Measurements of clinically significant doses of ionizing radiation using non-invasive in vivo EPR spectroscopy of teeth in situ. Appl Radiat Isot. 2005;62:293–9. doi: 10.1016/j.apradiso.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Swartz HM, Khan N, Buckey J, Comi R, Gould L, Grinberg O, Hartford A, Hopf H, Hou H, Hug E, Iwasaki A, Lesniewski P, Salikhov I, Walczak T. Clinical applications of EPR: overview and perspectives. NMR Biomed. 2004;17:335–51. doi: 10.1002/nbm.911. [DOI] [PubMed] [Google Scholar]
- Zdravkova M, Crokart N, Trompier F, Asselineau B, Gallez B, Gaillard-Lecanu E, Debuyst R. Retrospective dosimetry after criticality accidents using low-frequency EPR: a study of whole human teeth irradiated in a mixed neutron and gamma-radiation field. Radiat Res. 2003a;160:168–73. doi: 10.1667/rr3026. [DOI] [PubMed] [Google Scholar]
- Zdravkova M, Wieser A, El-Faramawy N, Gallez B, Debuyst R. An in vitro L-band electron paramagnetic resonance study of highly irradiated whole teeth. Radiat Prot Dosimetry. 2002;101:497–502. doi: 10.1093/oxfordjournals.rpd.a006036. [DOI] [PubMed] [Google Scholar]
- Zdravkova M, Wieser A, El-Faramawy N, Ivanov D, Gallez B, Debuyst R. An in vitro L-band EPR study with whole human teeth in a surface coil resonator. Radiation Measurements. 2003b;37:347–353. [Google Scholar]


