Abstract
Transgenic mice expressing stabilized β-catenin in neural progenitors develop enlarged brains resulting from increased progenitor expansion. To more precisely define β-catenin regulation of progenitor fate, we employed a conditional transgenic approach to delete the β-catenin regulatory domain from neural progenitors, resulting in expression of stabilized protein from its endogenous promoter in these cells and their progeny. An increased fraction of transgenic cortical cells express the progenitor markers Nestin and LewisX, confirming a relative expansion of this population. Sustained β-catenin activity expands RC2 and Pax6 expression in the developing cortex while postponing the onset of Tbr2 expression, suggesting a delay in maturation of radial glia into intermediate progenitors. Furthermore, transgenic cortical cells fail to either upregulate ErbB4 or develop a mitogenic response to epidermal growth factor, changes that normally accompany acquisition of an intermediate fate. Likewise, transgenic brains do not develop a distinct subventricular zone or superficial cortical layers, and overexpression of stabilized β-catenin by in utero electroporation caused a relative reduction of upper layer vs. lower layer cortical neurons, indicating that persistent β-catenin activity interferes with the generation of progenitors responsible for the production of upper layer cortical neurons. Collectively, these findings demonstrate that β-catenin functions to maintain the radial glial population, and suggest that downregulation of β-catenin signaling may be critical to facilitate the transition to an intermediate progenitor phenotype.
Keywords: β-catenin, Pax6, subventricular zone, epidermal growth factor, neural progenitor, cerebral cortex
INTRODUCTION
Assembly of the intricate architecture of the mammalian brain requires the ordered specification of a broad variety of cell types (Edlund and Jessell, 1999). The complexity and elegance of brain development is exemplified by the “inside-out” construction of the neocortex, in which neurons fated to reside in the infragranular cortical layers (V-VI) are generated first, while later-born neurons migrate beyond these cells to compose the superficial layers (II-IV) (Takahashi et al., 1999). Deep layer neurons are proposed to originate from the asymmetric division of radial glial ventricular zone (VZ) progenitors at the apical surface (Malatesta et al., 2000; Miyata et al., 2001; Noctor et al., 2004). In contrast, evidence suggests that the upper layers derive primarily from the symmetric division of subventricular zone (SVZ) cells, also referred to as intermediate or basal progenitors (Tarabykin et al., 2001; Haubensak et al., 2004; Noctor et al., 2004; Zimmer et al., 2004). Since alterations in the balance between radial glial and intermediate progenitor proliferation could have a significant impact on ultimate brain architecture, the signals that control the timing and rate of each type of division are critical to normal central nervous system (CNS) function.
Canonical Wnt signaling mediated by β-catenin has been proposed to function in both neural progenitor cell expansion and neuronal lineage choice (Chenn and Walsh, 2002; Zechner et al., 2003; Hirabayashi et al., 2004; Israsena et al., 2004). In the absence of Wnts, cytosolic β-catenin is recruited into a complex with adenomatous polyposis coli (APC) and axin. This association facilitates its N-terminal phosphorylation by glycogen synthase kinase 3β (GSK3β) and its consequent ubiquitination and degradation by the proteasome. Wnt activation of Frizzled/LRP co-receptors increases the stable pool of β-catenin by disrupting this complex. Free β-catenin can then enter the nucleus where it associates with TCF/LEF family members to direct transcription of Wnt target genes (Clevers and van de Wetering, 1997).
Transgenic mice expressing a form of β-catenin resistant to GSK3β-mediated degradation in neural progenitors exhibited a gross horizontal expansion of the cortex resulting from decreased progenitor cell cycle exit (Chenn and Walsh, 2002). Conversely, conditional deletion of β-catenin from cortical progenitors resulted in multiple structural defects, decreased cell proliferation, altered cell migration, and changes in dorsoventral cell identity (Machon et al., 2003; Backman et al., 2005). Moreover, focal inhibition of β-catenin signaling caused cortical precursors to prematurely exit the cell cycle and differentiate into neurons (Woodhead et al, 2006). Collectively, these studies suggest that regulation of β-catenin signaling plays a critical role in controlling the production of cortical neurons by regulating progenitor decisions to proliferate or differentiate.
While these data suggest that altered β-catenin function can influence cell fate and tissue patterning in the developing cortex, whether β-catenin functions to regulate specific cortical progenitor populations during development remains poorly understood. Recent work suggests that β-catenin-mediated signaling is robust in VZ progenitors but appears to be reduced in the SVZ/intermediate zone (IZ), raising the possibility that β-catenin signaling functions primarily in radial glial progenitors and that modulation of signaling may play a role in progenitor maturation (Woodhead et al, 2006). Here, using a stable mouse line that can be used to achieve conditional and persistent expression of stabilized β-catenin from its endogenous promoter, along with in utero electroporation approaches, we examine the role of β-catenin on distinct classes of progenitors during cortical development.
MATERIALS AND METHODS
Animals
All animals were used in accordance with a protocol approved by the Northwestern University Institutional Animal Care and Use Committee. Construction of the Ctnnb1tm1Mmt (Catnblox(Ex3)) mice has been described previously (Harada et al., 1999). Tg(Nes-cre)1Wmz (NesCre8) mice (Petersen et al., 2002) were a gift from Susan McConnell (Stanford University), Tg(Nes-cre)1Kln (NesCre) animals (Tronche et al., 1999) were obtained from Jackson Laboratories (Bar Harbor, ME). ROSA26;NesCre mice were a gift from Yuanyi Feng (Northwestern University), generated by crossing NesCre and B6;129-Gt(ROSA)26Sortm1Sho/J (Jackson Laboratories) animals. Female mice homozygous for an allele of β-catenin with loxP sites flanking the third exon (Catnblox(Ex3)/Catnblox(Ex3)), which spans the GSK3β regulatory domain, were crossed with NesCre8, NesCre, or ROSA26;NesCre transgenic males. Pregnant females were euthanized at embryonic day (E) 14.5 to E17.5 (plug date = E0.5) and primary cortical cells were separately isolated from each embryo as previously described (Murphy et al., 1989). Data represent the analysis of multiple embryos per genotype from at least two separate litters. Presence of the Cre transgene was detected by PCR amplification and expression of truncated β-catenin confirmed by Western blot. All electroporation studies were conducted on timed-pregnant Swiss-Webster mice (Charles River Laboratories, Wilmington, MA).
Plasmid DNA
The N-terminal truncated β-catenin construct (Δ90β-cateninGFP) used has been described previously (Chenn and Walsh 2002). It was subcloned into a pCAG electroporation vector designed to allow expression in both neural progenitors and mature cell types (Niwa, et al., 1991). pCAG-EGFP was used in control experiments.
Neurosphere assay
Neurospheres were formed by seeding E14.5 or E17.5 primary cortical cells at clonal density (1 × 104 cells/mL; Seaberg et al., 2005) in suspension culture in clonal density media (CDM): DMEM + N2, B27, 2 mM L-glutamine, 1 mM sodium pyruvate, and 1 mM N-acetyl cysteine (Invitrogen, Carlsbad, CA) supplemented with FGF2 alone or EGF and FGF2 at 20 ng/mL each (Sigma, St. Louis, MO). Methylcellulose (0.5%, Sigma) was included to ensure clonality (Gritti et al., 1999). After 6 days in culture, a representative collection (n ≥ 30) of cell clusters with a compact spheroid morphology and a minimum diameter of 40 μm were measured using MetaMorph Version 6.3r1 software (Molecular Devices, Sunnyvale, CA).
Luciferase assay
E14.5 primary cortical cells cultured overnight on poly-D-lysine (Sigma) in CDM + 20 ng/mL FGF2 were transfected using Lipofectamine 2000 (Invitrogen) with a TCF/LEF firefly luciferase reporter (Super(8x)TOPFLASH) or a mutated control reporter (Super(8x)FOPFLASH) to measure background activity (Veeman et al., 2003). An RNA polymerase III renilla luciferase reporter was included as a transfection control, and cotransfection of pCDNA3 ICAT was used to demonstrate that activation was β-catenin-dependent (Gottardi and Gumbiner, 2004). Luciferase activity was measured using the Dual-Luciferase Assay System (Promega, Madison, WI) and a Veritas microplate luminometer (Turner Biosystems, Sunnyvale, CA). Each condition was assayed in triplicate, and firefly values were normalized to corresponding renilla values.
Immunoblotting
E14.5 and E17.5 primary cortical cells (8 × 106/mL) were lysed at 4°C in RIPA buffer (20 mM Tris pH 7.5, 150 mM NaCl, 1% Triton, 1% sodium deoxycholate, 0.1% SDS) supplemented with protease inhibitor cocktail set III (Calbiochem, La Jolla, CA). Total protein concentrations of cleared lysates were determined by BCA assay (Pierce, Rockford, IL), and 15-30 μg protein was resolved on 8-10% SDS-PAGE gels. Blots were incubated overnight in monoclonal β-catenin (BD Biosciences, San Diego, CA), monoclonal actin (Chemicon, Temecula, CA), or polyclonal ErbB4 (Santa Cruz, Santa Cruz, CA) antibodies diluted 1:500 in 5% milk. After 1 hour exposure to HRP-conjugated goat secondary anti-mouse anti-rabbit Abs (1:3000, Bio-Rad, Hercules, CA), blots were developed using enhanced chemiluminescence (GE Healthcare, Waukesha, WI).
Immunohistochemistry
E14.5 and E17.5 primary cells were seeded overnight or for 2 hours where noted on poly-D-lysine in CDM + 20 ng/mL FGF2 prior to fixation in 4% paraformaldehyde (PFA) and staining. For preparation of tissue sections, E14.5-E19.5 embryonic brains were fixed 6 hours to overnight in 4% paraformaldehyde. After fixation, brains were sunk in 30% sucrose solution and then flash frozen in O.C.T. Compound (Tissue-Tek, Torrance, CA), and sectioned in the coronal plane at 14 μm thickness. Primary antibodies used were monoclonal Nestin (1:700, BD Biosciences), TuJ1 (1:500, Covance, Princeton, NJ), RC2 and Pax6 (1:1600, 1:200, Developmental Studies Hybridoma Bank, Iowa City, IA); and polyclonal β-galactosidase (1:1000, a gift from Tom Glaser, University of Michigan), ErbB4 (1:500, Santa Cruz), Tbr1 (1:500, Chemicon), and Tbr2 and FoxP2 (1:250, 1:500, Abcam, Cambridge, MA). Primary antibodies were detected with goat secondary anti-mouse Alexa 488 and anti-rabbit Alexa 488 or 555 (1:250, Invitrogen), and DNA was stained with Hoechst 33342. Images were captured using a Nikon (San Diego, CA) TE2000-U inverted fluorescence microscope and MetaMorph Version 6.3r1 software. Sections from brains subject to in utero electroporation were incubated with the following primary antibodies: anti-GFP (Invitrogen, rabbit polyclonal, and Abcam, chicken polyclonal), anti-Brn1 (Santa Cruz Biotechnologies, goat polyclonal) and anti-FoxP2, and secondary antibodies from Invitrogen. DNA was counterstained with DAPI (source?). One micrometer optical sections were obtained using the 40x objective on a Zeiss (Oberkochen, Germany) confocal microscope.
Cell Cycle Exit Analysis
To label proliferating cells, embryos were exposed to bromodeoxyuridine (BrdU) by intraperitoneal injection of the mother with 50mg/kg body weight (Sigma). Embryos were fixed and embedded and 10 μm coronal sections prepared as above. Antigen retrieval was performed by incubation in 2.4N hydrochloric acid for 1 hour at room temperature prior to overnight incubation with rat monoclonal BrdU (1:200, Serotec, Raleigh, NC) and rabbit polyclonal phospho-histone H3 (1:200, Millipore, Billerica, MA). For each randomly chosen field of view, 50 BrdU-positive cells were identified, then the percentage of those cells that persisted in the mitotic fraction was determined by pHH3 expression.
In situ analysis
In situ hybridization was performed on coronal sections from three independent sets of wild-type and Catnblox(Ex3)/+;NesCre transgenic E14.5 and E17.5 littermates as previously described (Chenn and Walsh, 2002). Heads were frozen in isopentane at -30°C. The Svet1 and Cux2 probes have been previously described (Tarabykin et al., 2001; Nieto et al., 2004).
In Utero Electroporation Studies
Our protocol for in utero electroporation of plasmid DNA constructs has been described previously (Woodhead, et al. 2006). Briefly, timed pregnant mice were anesthetized with a 10:1 ketamine-xylazine cocktail. Plasmid DNA solution (2.5 ul at 0.5 ug/ul) was injected into the lateral ventricle of the embryonic brain through the uterine wall using a pulled capillary pipette. After injection, five 50 ms square pulses of 45 mV at 950 ms intervals were delivered by an electroporator (BTX 830, BTX, Holliston, MA). Afterward, the timed pregnant females were sutured and allowed to recover. Mice were then sacrificed at predetermined intervals and embryonic brains were recovered and fixed with 4% PFA.
Statistical analysis
Labeled cell counts were analyzed by Student’s t-test using Prism 4.0 software (GraphPad, San Diego, CA). Neurosphere sizes were analyzed by ANOVA followed by Tukey’s pairwise comparison t-test.
RESULTS
Conditional deletion of the Catnb third exon stimulates β-catenin signaling
Transient expression of stabilized β-catenin in neural progenitors under the direction of a Nestin enhancer (Nestin-ΔN90-β-catenin) results in dramatic horizontal expansion of the cerebral cortex (Chenn and Walsh, 2002). The N-terminal portion of β-catenin containing the GSK3β phosphorylation sites required for its degradation is encoded by exon 3 of the β-catenin locus, and removal of this region results in the production of a stabilized protein (Barth et al., 1997). To further examine the effects of increased levels of β-catenin while preserving its normal expression pattern, we crossed animals homozygous for an allele of β-catenin with loxP sites flanking the third exon (Catnblox(Ex3)) (Harada et al., 1999) with two different nestin-Cre driver lines. The NesCre8 transgene (Petersen et al., 2002) results in widespread activation of the ROSA26 reporter in the neuroepithelium by E8.5, whereas NesCre (Tronche et al., 1999) expression led to mosaic lacZ expression in the E10.5 telencephalon which became widespread by E11.5 (Backman et al., 2005). Both strains have been employed previously to activate the Catnblox(Ex3) locus at these respective ages (Backman et al., 2005).
In contrast with animals used in the previous study, where Nestin regulatory elements drove the expression of truncated β-catenin only in progenitors (Chenn and Walsh, 2002), the current strategy facilitated the persistent expression of stabilized β-catenin in both Nestin-expressing progenitors and their lineage. Furthermore, it allowed us to circumvent the perinatal lethality of the Nestin-ΔN90-β-catenin transgene in order to maintain stable parental lines prior to introduction of Cre expression. While Catnblox(Ex3)/+;NesCre transgenic animals still die perinatally, this nonetheless facilitated an analysis of a statistically more powerful number of embryos at multiple stages of development, and further provided the advantage of preserving β-catenin expression under control of its endogenous regulatory elements. Immunoblotting of primary cortical lysates from Cre-positive, Catnblox(Ex3) heterozygous embryos invariably demonstrated expression of truncated β-catenin at levels in excess of the full-length protein expressed from the remaining wild-type allele by E14.5 (Fig. 1A).
Figure 1.
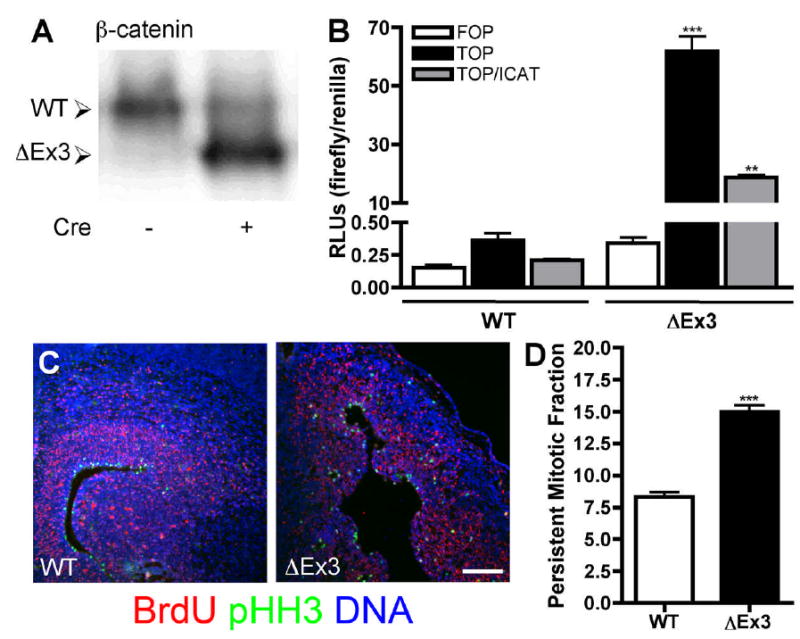
Cre-mediated deletion of β-catenin exon 3 results in high level expression of the stabilized protein and increased transcriptional activity. (A) E14.5 primary cortical lysates were probed for β-catenin. The truncated protein was detected in Cre-positive embryos at levels in excess of the wild-type protein. (B) E14.5 wild-type or ΔEx3 transgenic primary cortical cells were cotransfected with Super(8x)TOPFLASH or Super(8x)FOPFLASH firefly luciferase reporters, an RNA polymerase III renilla luciferase reporter, and pCDNA3-ICAT or vector control, and relative luciferase activity was determined. Error bars represent ± s.e.m. p=0.0003, WT v. ΔEx3; p=0.0012, ΔEx3 + vector v. ΔEx3 + ICAT. (C) Fluorescence images of E16.5 sections stained for BrdU (red), pHH3 (green) and Hoescht (blue). Bar = 150 μm. (D) The fraction of cortical cells labeled with BrdU that remained positive for pHH3 24 hours after injection was quantified (p<0.0001).
To determine whether expression of stabilized β-catenin results in an increase in transcriptional activation in primary cortical cells, we employed a dual luciferase assay to monitor β-catenin transcriptional activity. Cells were isolated from E14.5 Catnblox(Ex3)/+;NesCre transgenic (hereafter referred to as ΔEx3 transgenic) and wild-type littermates and transfected with Super(8x)TOPFLASH, which contains 8 copies of the optimal TCF/LEF consensus motif (CCTTTGATC) upstream of the firefly luciferase gene (Veeman et al., 2003). Super(8x)FOPFLASH, which contains 8 mutated binding sites (CCTTTGGCC), was used as a specificity control. Cortical cells expressing stabilized β-catenin demonstrated a greater than 150-fold increase in TOPFLASH activation compared with wild-type cells, confirming an increase in signaling (Fig. 1B; WT, 0.36 ± 0.06 relative luciferase units (RLUs), ΔEx3, 61.81 ± 5.18 RLUs). This elevated signaling was dependent upon β-catenin interaction with TCF factors, as coexpression of ICAT, an inhibitor of β-catenin-TCF association (Gottardi and Gumbiner, 2004), led to a 69% reduction in luciferase activity.
Expression of the Nestin-ΔN90-β-catenin transgene led to a two-fold increase in progenitor cell cycle re-entry as determined by the fraction of cells that remained positive for the cell cycle antigen Ki67 24 hours after pulse labeling with BrdU. To determine whether persistent expression of stabilized β-catenin results in a similar decrease in cell cycle exit, we exposed embryos to BrdU in utero 24 hours prior to sacrifice and determined the fraction of labeled cells that continued to divide by phospho-histone H3 (pHH3) immunostaining (Fig. 1C). We found that the persistent mitotic fraction in E16.5 ΔEx3 transgenic embryos was likewise nearly doubled (Fig. 1D; WT, 8.34 ± 0.37%, ΔEx3, 14.97 ± 0.52%), suggesting that expression of stabilized β-catenin using this conditional strategy has an effect of similar magnitude on progenitor decisions to re-enter the cell cycle rather than differentiate.
Continued expression of stabilized β-catenin causes a disruption of cortical architecture during mid-to-late neurogenesis
Because our current approach allowed us to generate transgenic animals consistently, we were able for the first time to characterize cortical morphogenesis at multiple time points during embryonic development. As described previously (Chenn and Walsh, 2002), ΔEx3 transgenic brains were grossly enlarged compared with brains from wild-type littermates. Similar to Nestin-ΔN90-β-catenin transgenic animals, embryos expressing this form of stabilized β-catenin in neural progenitors exhibited a considerable increase in cortical surface area and demonstrated cortical folding at mid-neurogenesis (Fig. 2A). E14.5 ΔEx3 transgenic cortices stained more broadly than wild-type for the progenitor marker Nestin, and staining for the pan-neuronal antigen β-III-tubulin (TuJ1) revealed a decrease in the absolute thickness of the developing cortical plate (Fig. 2B).
Figure 2.
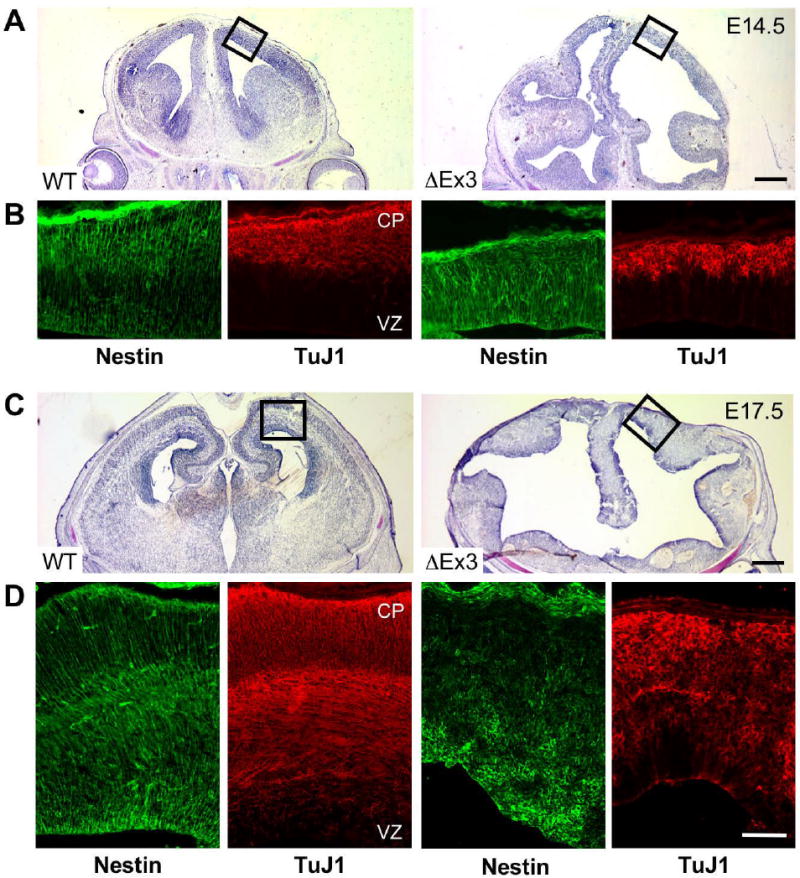
Continued expression of stabilized β-catenin leads to gross enlargement of cortical surface area and perturbs cortical organization. E14.5 and E17.5 coronal sections were stained with cresyl violet (A,C), or labeled for the progenitor marker Nestin or the neuronal antigen TuJ1 (B,D). Boxes in (A,C) indicate regions depicted in (B,D). Bars = 250 μm (A), 500 μm (C), 100 μm (B,D).
At later ages, ΔEx3 transgenic cortices exhibited highly dilated ventricles and showed marked variability in thickness from ventricular to pial surfaces as previously reported (Chenn and Walsh, 2003)(Fig. 2C). It was likewise evident at this stage that the increase in cortical area was accompanied by a reduction in the size of ventral telencephalic structures, consistent with previous reports suggesting that activation of Wnt signaling leads to the acquisition of dorsal identity in telencephalic progenitors (Gunhaga, et al., 2003). In striking contrast to cortices from early embryos, by late neurogenesis, the normal radial organization of Nestin-expressing progenitors and the maturing domains of postmitotic neurons became progressively disordered (Fig. 2D). ΔEx3 transgenic cortical germinal zones were characterized by irregular accumulations of cells staining strongly for Nestin. Catnblox(Ex3);NesCre8 embryos displayed a nearly identical albeit somewhat more severe cortical phenotype and correspondingly exhibited reduced viability at E17.5 (Fig. 6 and data not shown).
Figure 6.
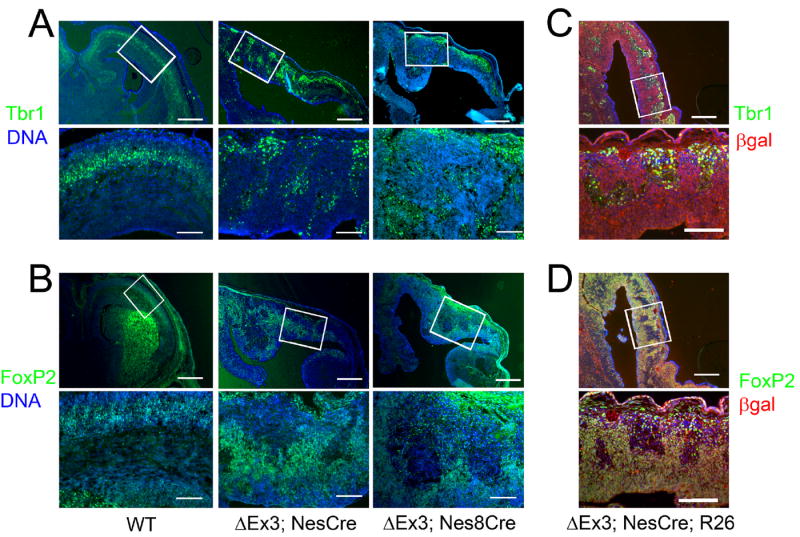
Persistent expression of stabilized β-catenin does not interfere with the generation of deep layer neurons. (A,B) Fluorescence images of coronal sections from E17.5 NesCre (left and center panels) or NesCre8 (right panels) embryos stained for Tbr1 (A) or FoxP2 (B, green) and Hoescht (blue). Boxes in the upper panels indicate the regions depicted at higher magnification in the lower panels. Bars = 500 μm (upper panels), 100 μm (lower panels) (C, D) Coronal sections from E16.5 ROSA26NesCre transgenic embryos stained for Tbr1 (C) or FoxP2 (D, green), β-galactosidase (red), and Hoescht (blue). Expression of deep layer markers can be detected in cells in which recombination has occurred. Bars = 300 μm (upper panels), 100 μm (lower panels).
Persistent expression of stabilized β-catenin expands the radial glial cell fraction
To quantify the effect of expression of stabilized β-catenin on cortical progenitor populations, we determined the percentage of primary cortical cells expressing Nestin, a global progenitor marker (Lothian and Lendahl, 1997), Lewis X (LeX), an antigen reported to enrich for neural stem-like cells versus intermediate progenitors (Capela and Temple, 2002), and RC2, which recognizes a modified isoform of Nestin expressed in radial glial cells (Chanas-Sacre et al., 2000) using immunocytochemistry. The fraction of cortical cells expressing Nestin and LeX was significantly enhanced by expression of β-catenin in E14.5 and E17.5 primary cells, confirming a relative expansion of the progenitor population (Fig. 3A,B,C,D; E14.5 Nestin: WT, 41.6 ± 2.2%, ΔEx3, 61.6 ± 3.6%; LeX: WT, 34.9 ± 3.0%, ΔEx3, 60.6 ± 3.3%; E17.5 Nestin: WT, 26.5 ± 1.7%, ΔEx3, 53.2 ± 2.3%; LeX: WT, 25.3 ± 5.6%, ΔEx3, 40.2 ± 2.1%). We also observed a significant increase in the percentage of cells derived from ΔEx3 transgenic brains positive for RC2 at both ages (Fig. 3E,F; E14.5: WT, 28.2 ± 0.5%, ΔEx3, 36.8 ± 0.3%; E17.5: WT, 17.2 ± 1.2%, ΔEx3, 28.9 ± 0.5%), suggesting the hypothesis that β-catenin expands the radial glial progenitor fraction.
Figure 3.
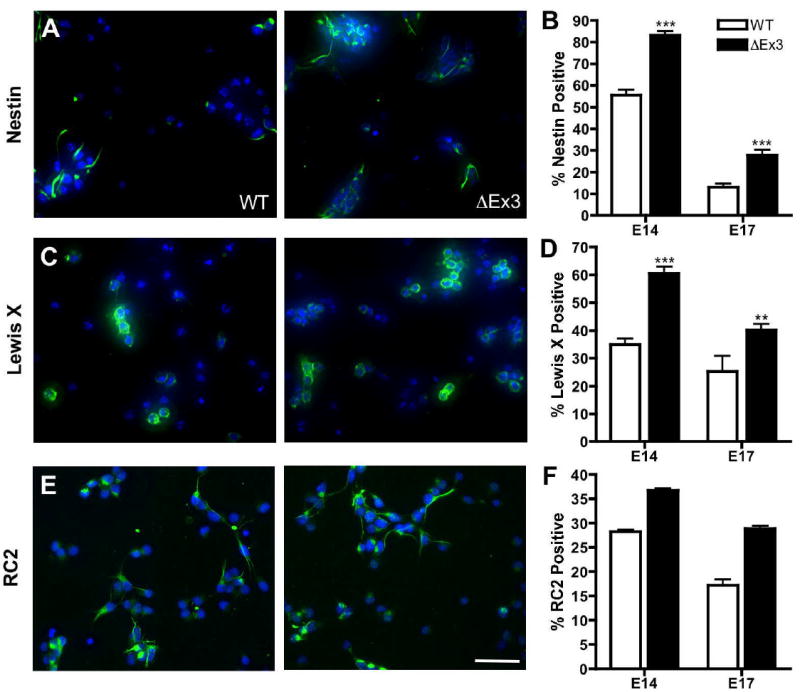
β-catenin stabilization increases the fraction of cells expressing markers of progenitor and radial glial cells. E14.5 primary cortical cells were seeded on poly-D-lysine in CDM + FGF2 overnight and stained with the progenitor markers Nestin (A), LeX (C), or RC2 (E, green) and Hoechst (blue). Bar = 50 μm. (B,D,F) The fraction of E14.5 and E17.5 cells that stained for each marker was quantified. N ≥ 300 cells per embryo. Error bars represent ± s.e.m. E14.5: p<0.0001, Nestin, LeX, RC2. E17.5: p<0.0001, Nestin; p=0.0025, LeX; p=0.0009, RC2.
Stabilized β-catenin impairs the production and development of intermediate progenitors
Our observation that persistent β-catenin activity causes a progressive architectural disorganization of cortical germinal zones and increases the relative fraction of radial glial progenitors led us to examine the development of specific cortical progenitor populations and the formation of the subventricular zone (SVZ) in ΔEx3 transgenic mice. The developmental progression from a radial glial phenotype, characteristic of the VZ, to an intermediate progenitor phenotype, predominant in the SVZ, is accompanied by the downregulation of the homeodomain transcription factor Pax6 and a concomitant induction of the T-box transcription factor Tbr2 (Englund et al., 2005). We therefore examined the size and spatial distribution of these populations in the ΔEx3 cortex.
In the wild-type E14.5 cortex, low-level Pax6 expression was confined to the VZ, and Tbr2 was detected in a broad band demarcating the SVZ (Fig. 4A). In contrast, ΔEx3 transgenic cortex exhibited an expansion of Pax6 expression, and only scattered cells stained for Tbr2. At E17.5, while the layers defined by each marker were relatively thinner in wild-type cortex, ΔEx3 transgenic brains demonstrated widespread disorganized expression of Pax6 and Tbr2 throughout the developing cortex (Fig. 4B). Corresponding age-related alterations in the expression of Pax6 and Tbr2 were observed in dissociated cortical cells (Fig. 4C,D; E14.5 (20h) Pax6: WT, 16.5 ± 0.6%, ΔEx3, 22.8 ± 0.8%; Tbr2: WT, 13.1 ± 0.6%, ΔEx3, 5.0 ± 0.5%; E17.5 Pax6: WT, 5.4 ± 0.8%, ΔEx3 15.2 ± 3.1%; Tbr2: WT, 7.1 ± 1.8%, ΔEx3, 12.6 ± 2.6%). Pax6 and Tbr2 were each expressed in a similar fraction of E14.5 cortical cells immediately after attachment (Fig. 4C,D; E14.5 (2h) Pax6: WT, 16.8 ± 1.2%, ΔEx3, 21.4 ± 1.8%; Tbr2: WT, 13.8 ± 2.0%, ΔEx3, 6.1 ± 1.0%). These findings suggest that the notable absence of Tbr2-positive cells at this age is likely not due to a selective increase in the rate of intermediate progenitor apoptosis as would be detectable during the 20 hour culture period. Collectively, these observations suggest that β-catenin plays a role in maintaining the Pax6-expressing progenitor population while delaying the onset of Tbr2 expression.
Figure 4.
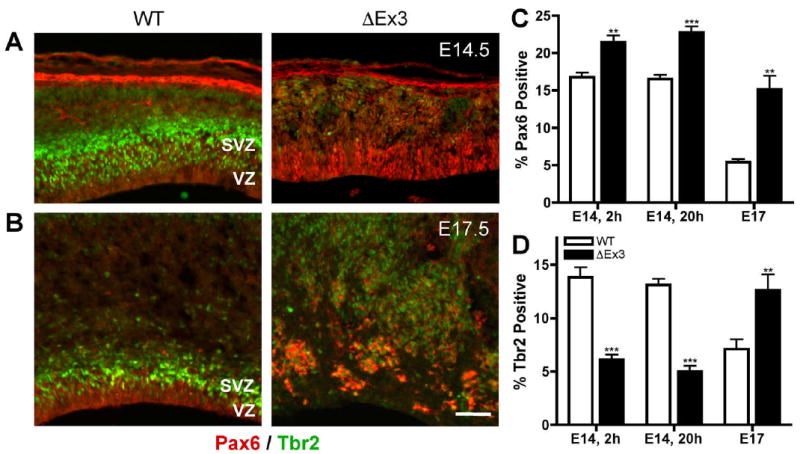
Stabilized β-catenin expands expression of the radial glial marker Pax6 and delays the appearance of Tbr2-positive intermediate progenitors. Fluorescence images of E14.5 (A) and E17.5 (B) sections stained for Pax6 (red) and Tbr2 (green). Bar = 100 μm. Primary cortical cells were seeded on poly-D-lysine in CDM + FGF2 overnight and the fraction of cells that stained for Pax6 (C) or Tbr2 (D) was quantified. N ≥ 300 cells per embryo. Error bars represent ± s.e.m. E14.5, 3h: p=0.005, Pax6; p=0.0004, Tbr2. E14.5, 20h: p<0.0001, Pax6 and Tbr2. E17.5: p=0.0061, Pax6; p=0.0395, Tbr2.
In a previous study, it was shown that the cortical region normally populated by SVZ progenitors in adult animals was expanded in thickness and aberrantly occupied by large neuronal heterotopia distinct from the cortical plate (Chenn and Walsh, 2003). The altered composition of this enlarged “SVZ” suggested that the normal SVZ progenitor population may be reduced or absent in these animals, and further implied a possible defect in neuronal migration. To examine more specifically the development of the SVZ, we used in situ hybridization to characterize the expression of the homeodomain transcription factor Cux2 and the noncoding transcript Svet1, normally restricted to the SVZ and upper cortical layers (II-IV)(Tarabykin et al., 2001; Nieto et al., 2004). At E14.5, while both transcripts were expressed in the wild-type SVZ, they were completely absent from ΔEx3 transgenic brains, corresponding with the absence of Tbr2-positive progenitors at this age (Fig. 5A). Similarly at E17.5, both Svet1 and Cux2 transcripts were detected in the wild-type SVZ; in contrast, neither transcript was detected in transgenic brains (Fig. 5B). These findings demonstrate that despite the eventual induction of Tbr2, persistent expression of stabilized β-catenin interferes with the acquisition of further hallmarks of an intermediate progenitor phenotype. Together with the observation that β-catenin signaling is normally downregulated at the VZ to SVZ transition (Woodhead, et al., 2006), these data suggest that a suppression of β-catenin activity may be critical for the development of the SVZ as a secondary germinal zone.
Figure 5.
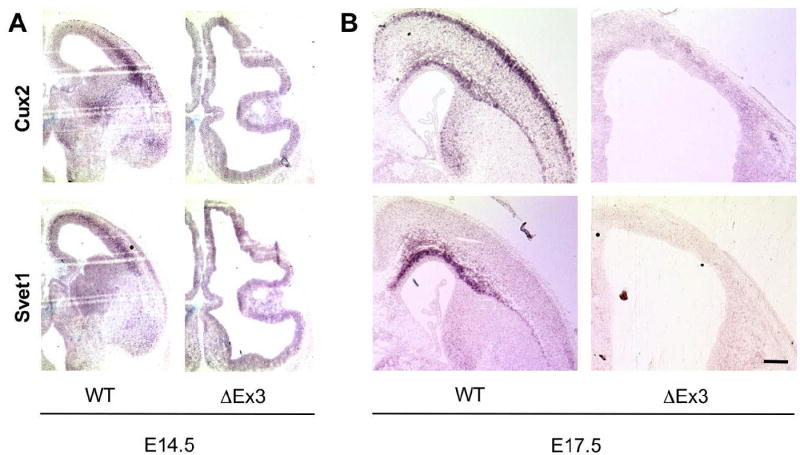
Transcripts specific to the SVZ and upper cortical layers are absent from ΔEx3 transgenic cortex. Cux2 or Svet1 antisense probes were hybridized to E14.5 (A) and E17.5 (B) coronal sections. Expression of each transcript was exclusively detected in the SVZ of wild-type cortices at E14.5; Cux2 was also expressed in the upper layers of wild-type cortices at E17.5. No signal was detected with a Cux2 sense probe. Bar = 500 μm.
β-catenin overexpression interferes with the development of superficial layer cortical neurons
A previous analysis of adult mice expressing an attenuated form of the Nestin-ΔN90-β-catenin transgene demonstrated a dramatic disorganization of cortical lamina; however, a molecular characterization of each individual neuronal layer was not conducted (Chenn and Walsh, 2003). Our observations that β-catenin perturbs the normal development of Tbr2-expressing intermediate progenitors in the SVZ suggests that β-catenin overexpression may furthermore have consequences for the development of upper layer cortical neurons. The developmental appearance of intermediate progenitors corresponds with the formation of the SVZ as a distinct secondary germinal zone, and accumulating evidence suggests that superficial layer neurons are derived from the SVZ (Martinez-Cerdeño et al., 2006; Tarabykin et al., 2001; Nieto et al., 2004). Correspondingly, in E17.5 wild-type brains, Cux2 expression was evident not only in the SVZ but also in the superficial layers of the cortical plate (Fig. 5B). In contrast, in addition to the developing ΔEx3 transgenic cortex lacking any definitive lamination, transgenic brains failed to develop Cux2 expression, suggesting that the defects in progenitor maturation that result from expression of stabilized β-catenin have specific consequences for the timing and rate of generation of upper layer neurons.
To determine whether persistent expression of stabilized β-catenin likewise has consequences for deeper layer cortical lamination, we characterized the expression patterns of the deep layer markers Tbr1 (subplate, layer VI; Bulfone et al., 1995) and FoxP2 (layers V-VI; Ferland et al., 2003). We found that both markers were present in ΔEx3 transgenic brains at E17.5 (Fig. 6A,B, center panels), consistent with the notion that deeper cortical layers arise directly from radial glial progenitors (Frantz et al., 1994; Miyata et al., 2001). However, lamination was markedly disorganized, correlating with the overall architectural abnormalities in the transgenic cortex at this age. We likewise probed Catnblox(Ex3);NesCre8 transgenic cortices for Tbr1 and FoxP2 and found a similarly widespread, disorganized pattern of expression (Fig. 6A,B, right panels), suggesting it is unlikely that deep layer neurons can only be observed in ΔEx3 transgenic embryos because they are specified prior to the onset of NesCre activity.
While both NesCre and NesCre8 exhibit activity prior to the specification of the majority of cortical neurons, it is nonetheless possible that the deep layer neurons we observed were either specified prior to Cre induction or derived from progenitors in which recombination failed to occur. To confirm that sustained β-catenin activity is not prohibitive of deep layer neurogenesis, we crossed males bearing both the ROSA26LacZ reporter and the NesCre transgene to Catnblox(Ex3) females. We observed widespread labeling for β-galactosidase exclusively in Catnblox(Ex3)/+;ROSA26NesCre transgenic embryos (Fig. 6C,D). We detected extensive overlap of Tbr1 and β-galactosidase as well as FoxP2 and β-galactosidase expression in ΔEx3 transgenic embryos (Fig. 6C,D). We further observed a qualitative enlargement of the FoxP2-positive population upon persistent activation of β-catenin signaling, consistent.with a recent report showing that attenuation of Wnt signaling leads to a dimunition of TLE4-expressing deep layer neurons (Zhou et al., 2006).
Because disruptions in tissue architecture may lead to changes in cell proliferation and identity (Bilder et al., 2000; Peifer, 2000), we then used an in vivo electroporation approach to examine the cell-autonomous function of β-catenin in generation of laminar identity. Following electroporation of stabilized β-catenin (ΔN90β-catenin (Chenn and Walsh, 2002)) into E13.5 cortical progenitors and analysis at E19.5, we found that persistent activation of β-catenin caused a relative decrease in the production of upper-layer neurons (Fig. 7), defined by expression of the POU homeodomain protein Brn-1 (Sugitani et al., 2002). The fraction of Δ90βCat expressing cells positive for Brn1 (~24%) was significantly decreased when compared to GFP control (~53%, p= 0.0337). Similar to the cortices of ΔEx3 transgenic mice, we observed a relative increase in FoxP2-expressing cortical neurons (~53%) when compared to cells expressing GFP (~39%, p= 0.0177). These electroporation findings support the studies from transgenic ΔEx3 cortices indicating that β-catenin regulates the production of upper-layer cortical neurons and their progenitors.
Figure 7.
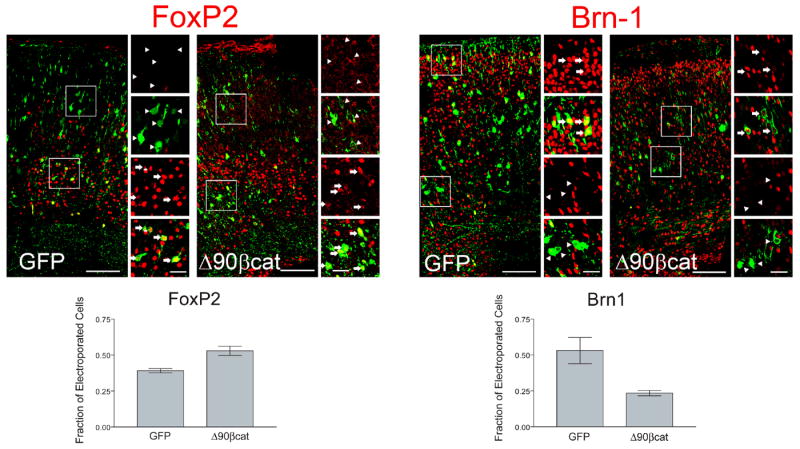
Expression of stabilized β-catenin by in utero electroporation increases the proportion of deep layer cortical neurons produced relative to superficial layer neurons. E13.5 embryos were microinjected with plasmid expression vectors for either GFP-fused stabilized β-catenin (Δ90βCat) or GFP control (GFP) and electroporated. Coronal sections of electroporated E19.5 cortex were immunostained for GFP and molecular markers specific for superficial (Brn1; layers II-IV) and deep layer neurons (FoxP2; layers V-VI). Boxes show areas of interest which are enlarged to the right. Arrows denote examples of cells co-positive for cortical layer markers (FoxP2 or Brn1) and GFP; arrowheads show examples of GFP positive cells that are negative for FoxP2 or Brn1. A significantly increased fraction of cells expressing Δ90βCat stained positive for FoxP2 (0.53) when compared to cells expressing GFP (0.39, p= 0.0177). Correspondingly, the fraction of Δ90βCat expressing cells positive for Brn1 (0.23) significantly decreased when compared to GFP control (0.53, p= 0.0337). Graphs show the results of 3 experiments (2144 cells analyzed total). Bar = 100 μm (large panels), 25 μm (small panels).
Stabilized β-catenin impedes the acquisition of cortical ErbB4 expression and epidermal growth factor responsiveness
Signals mediated by receptors of the epidermal growth factor (EGF) family have been shown to be critical for the normal development of the embryonic and adult SVZ (Burrows et al., 1997; Anton et al., 2004). Neural progenitors initially responsive to fibroblast growth factor (FGF) acquire responsiveness to EGF during mid-corticogenesis (~E13.5); however, little is known about what factors regulate this transition (Tropepe et al., 1999; Ciccolini et al., 2001). Expression of each EGF family receptor (ErbB1-4) has been detected in discrete but overlapping patterns in the germinal zones of the developing rodent CNS, suggesting that changes in the levels of any single receptor could influence signaling outcomes in neural progenitors (Kornblum et al., 2000).
To examine whether the disruption in the development of Tbr2-expressing intermediate progenitors in ΔEx3 transgenic cortex is coupled with an alteration in the acquisition of EGF responsiveness, we first examined the expression of the ErbB receptors by immunohistochemistry and Western blot. Wild-type E17.5 coronal sections stained for ErbB4 demonstrated prominent expression throughout the SVZ/IZ as previously reported (Fig. 8A)(Kornblum et al., 2000). In contrast, the cortex of embryos expressing stabilized β-catenin showed negligible ErbB4 reactivity. Likewise, while expression of ErbB4 was detected in E17.5 wild-type cell lysates, it was absent from cells expressing stabilized β-catenin (Fig. 8B). These results indicate that in addition to the absence of the SVZ markers Svet1 and Cux2, transgenic cortices also fail to upregulate the ErbB4 receptor during development.
Figure 8.
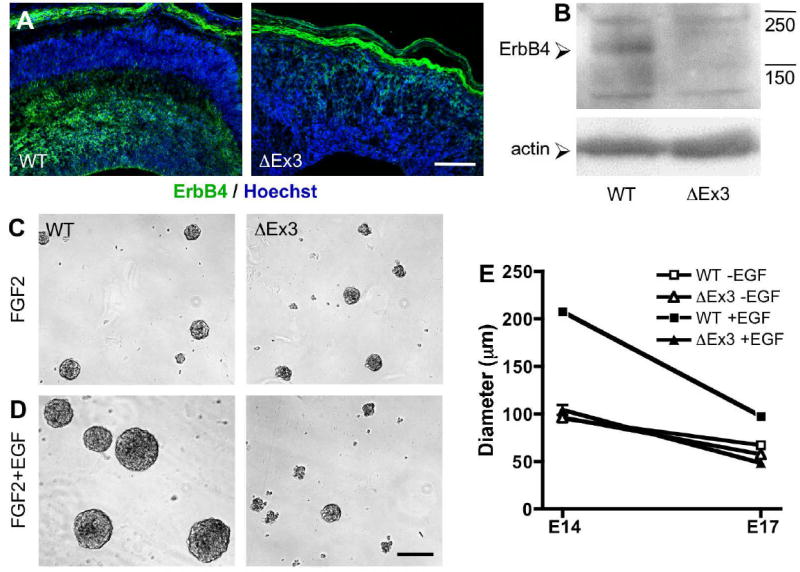
Stabilized β-catenin prevents induction of ErbB4 expression and the acquisition of a mitogenic responsiveness to EGF. (A) E17.5 coronal sections stained for ErbB4 (green) and Hoechst (blue). Bar = 150 μm. (B) Detection of ErbB4 (175 kDa) in E17.5 primary cortical lysates. Actin is shown as a control for equal protein loading. Phase images of E14.5 wild-type and ΔEx3 transgenic cortical cells after 6 days of neurosphere culture in the presence of 20 ng/mL FGF2 (C) or 20 ng/mL each FGF2 and EGF (D). Bar = 200 μm. (E) The diameter of a representative sample (n ≥ 30) of spheres generated from E14.5 or E17.5 cells was measured.
To determine the functional consequences of the absence of ErbB4 in ΔEx3 transgenic cortices, we examined the proliferation of neurospheres generated from wild-type and ΔEx3 transgenic cortical cells in the presence of either FGF2 alone or FGF2 and EGF. We found that only wild-type spheres grew significantly larger upon inclusion of EGF (Fig. 8C,D,E), resulting in a greater than two-fold increase in wild-type sphere diameter at E14.5 (E14.5 FGF2: WT, 95.2 ± 11.1 μm, ΔEx3, 96.6 ± 16.1 μm; FGF2/EGF: WT, 207.9 ± 59.4 μm, ΔEx3, 104.3 ± 17.9 μm); this difference was less pronounced at E17.5 (FGF2: WT, 67.3 ± 15.3 μm, ΔEx3, 57.9 ± 16.6 μm; FGF2/EGF: WT, 97.8 ± 34.5 μm, ΔEx3, 48.6 ± 11.1 μm). Collectively, these results suggest that in addition to delaying the normal maturation of radial glia to intermediate progenitors, β-catenin inhibits their acquisition of a proliferative response to EGF, and identify β-catenin as an important regulator of this developmental transition in progenitor growth factor responsiveness. Signals transduced by ErbB4 activation may likewise be critical to activate the expression of other factors characteristic of SVZ progenitors, including Cux2 and Svet1, which may function in the determination of neuronal subtype and/or cortical layer fate.
DISCUSSION
Conditional expression of stabilized β-catenin in neural progenitor cells causes an enlargement of cortical surface area resulting from expansion of the progenitor population (Chenn and Walsh, 2002). Here we find that the effect of enhanced β-catenin signaling on progenitor expansion is not homogeneous, but rather is characterized by an initial increase in the fraction of Pax6-positive radial glial progenitors and a corresponding delay in the generation of the Tbr2-expressing intermediate progenitors that comprise the SVZ. Furthermore, focal overexpression of stabilized β-catenin in cortical progenitors by in utero electroporation results in a relative decrease in upper layer vs. lower layer cortical neurons. Progenitors expressing stabilized β-catenin neither upregulate ErbB4 expression nor exhibit a mitogenic response to EGF. Collectively, these data suggest that β-catenin acts to maintain the population of radial glial progenitors at the expense of the generation of intermediate progenitors in the embryonic telencephalon, and represents a critical regulator of the capacity of developing progenitors to respond to specific mitogenic signals.
While we propose that persistently enhanced β-catenin activity delays the development of intermediate progenitors, it is clear that β-catenin has further consequences to progenitor maturation, as the Tbr2-expressing cells eventually generated in ΔEx3 transgenic cortices are markedly abnormal. In addition to being distributed in poorly-organized ectopic locations throughout the cortex, possibly indicative of defects in cell migration, these Tbr2-expressing cells do not express other markers characteristic of intermediate progenitors such as Svet1, Cux2, or ErbB4. Because ΔEx3 transgenic mice also die perinatally, we have not been able to examine whether the absence of these markers represents a transient or permanent delay in differentiation.
The mechanisms regulating progression of radial glial progenitors to intermediate progenitors remain poorly understood. As a population, neural progenitors transition from being exclusively responsive to FGF early in cortical development to being responsive to both EGF and FGF at approximately E13.5 (Ciccolini, 2001). Overexpression of EGFR in VZ progenitors leads them to adopt migratory and proliferative characteristics of their SVZ counterparts (Burrows et al., 1997). Furthermore, infusion of ErbB4 ligands selectively induces proliferation in the SVZ, and conditional deletion of ErbB4, which is primarily expressed in the transit-amplifying population, leads to a disruption of SVZ organization and migration patterns (Ghashghaei et al., 2006). Here we observed that absence of an EGF proliferative response is coupled with loss of the SVZ markers Svet1 and Cux2. Collectively, these observations lend support to the possibility that the level of EGFR/ErbB4 expression in cortical progenitors plays a critical role in regulating the transition to an intermediate phenotype, and that downregulation of β-catenin signaling may required for this transition to proceed normally.
While this transition correlates with the appearance of Tbr2-expressing progenitors in the SVZ, it remains to be determined whether the switch from Pax6 to Tbr2 expression is directly related to the acquisition of EGF responsiveness. While downregulation of Pax6 is necessary for corneal epithelial cells to proliferate in response to EGF (Li and Lu, 2005), EGF signaling can also function as an upstream antagonist of Pax6 in the Drosophila eye (Kumar and Moses, 2001). While our results suggest that a decrease in Pax6 expression and corresponding upregulation of Tbr2 alone are not sufficient to support the upregulation of ErbB4 and the development of a proliferative response to EGF through ErbB4 activation, they do not exclude the possibility that ErbB4 activity might further stimulate the production of Tbr2-expressing intermediate progenitors.
ErbB4 signaling is instrumental in the development of the SVZ, as its deletion in either the GFAP-positive or nestin-positive neural progenitor populations leads to a disruption of SVZ structural organization (Anton et al., 2004). Moreover, ventricular infusion of the ErbB4 ligand neuregulin-2 exclusively promotes the proliferation of SVZ progenitors, suggesting it may be critical for the normal expansion of this population (Ghashghaei et al., 2006). Intriguingly, ErbB4 may be uniquely poised to impact the expression of factors critical for the differentiation of VZ to SVZ progenitors and subsequently into upper layer neurons, as it is the only member of the EGF receptor family known to undergo intramembrane cleavage, releasing a C-terminal fragment that translocates to the nucleus (Ni et al., 2001). The ErbB4 cytoplasmic fragment was recently shown to antagonize the Eto2 transcriptional corepressor, a function that may be of importance to normal epithelial differentiation (Linggi and Carpenter, 2006).
While symmetric proliferative divisions in the VZ serve to expand the radial glia progenitor pool, recent studies demonstrate that symmetric terminal divisions in the SVZ contribute to neuronal production during mid-to-late cortical development (Kornack and Rakic, 1995; Mione et al., 1997; Haubensak et al., 2004; Noctor et al., 2004). It has been proposed that a two-step pattern of neurogenesis, consisting of asymmetric radial glial divisions in the VZ followed by symmetric terminal divisions in the SVZ, underlies a sustained period of neuron production and the evolutionary expansion of cortical surface area (Kriegstein et al., 2006). Our data suggest that persistent β-catenin signaling may underlie a selective expansion of the radial glial population while delaying the onset of the second stage of abventricular neurogenesis, and that downregulation of β-catenin activity may be a critical prerequisite for this progression.
Although our methods do not enable us to examine directly whether β-catenin expression regulates the specific mitotic outcome of radial glial division, our results support a model where the level of β-catenin signaling can prolong the first phase of progenitor expansion by increasing the relative frequency of symmetric, proliferative divisions versus the secondary mode of self-renewing asymmetric divisions that give rise to one radial glial cell and one intermediate progenitor (Fig. 9). Subsequent modulation of progenitor responsiveness to environmental stimuli, including secreted growth factors such as EGF, may be required for symmetric, proliferative divisions of intermediate progenitors. Changes in the time of onset of the second stage of neurogenesis could have further consequences for the induction of factors involved in the determination of specific layer fates, as illustrated by the absence of Cux2 and Svet1 in the ΔEx3 transgenic cortex.
Figure 9.
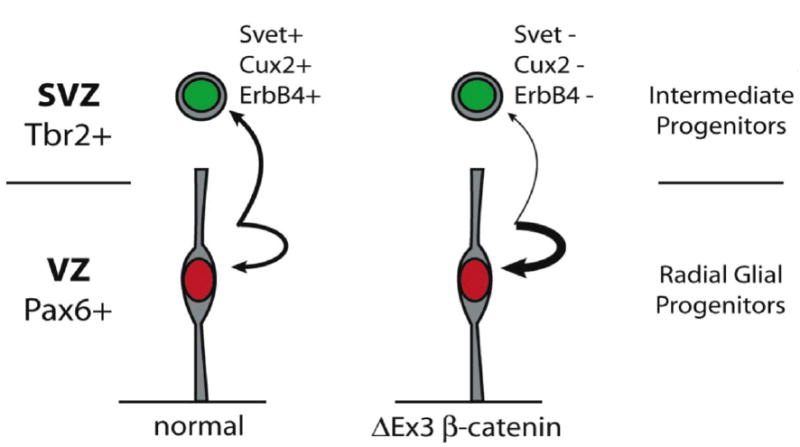
Model illustrating how persistent expression of stabilized β-catenin may delay progenitor maturation. β-catenin signaling prolongs the expansion of Pax6-positive radial glia progenitors by increasing the relative frequency of symmetric self-renewing divisions versus the asymmetric divisions that give rise to Tbr2-positive intermediate progenitors. The resulting expansion of the progenitor pool ultimately leads to a dramatic increase in cortical surface area. The failure of ΔEx3 transgenic intermediate progenitors to induce expression of ErbB4 and the absence of SVZ markers Cux2 and Svet1 in the transgenic cortex are suggestive of additional defects in progenitor maturation.
While Pax6 has not been reported as a direct transcriptional target of β-catenin, its 5’ untranslated region contains multiple putative TCF/LEF binding sites as identified by the Patch 1.0 web-based algorithm (unpublished observations). A previous report demonstrated a ventral expansion of Pax6 expression upon NesCre-mediated conditional expression of stabilized β-catenin (Backman et al., 2005). The converse was true when β-catenin was deleted, suggesting its activity is necessary to achieve appropriate regional patterning of Pax6 expression; in contrast, the pattern of Emx2 expression was unaffected (Backman et al., 2005). These two factors have been shown to play antagonistic roles in the specification of rostral and caudal areas in the developing neocortex, a relationship that was revealed only upon ablation of one or the other factor (Bishop et al., 2000). Therefore the ability of β-catenin to sustain Pax6 expression may not be sufficient to impact cortical regionalization along this axis. Furthermore, since explant studies have revealed that the specification of cortical and hippocampal fields is initiated prior to E10.5 (Grove and Tole, 1999), the timing of the Cre-mediated activation of β-catenin in our current model may not be suited to such an analysis. Our findings nonetheless suggest that an improved understanding of the interrelationship between β-catenin and Pax6 activities will lend valuable insight into the mechanisms that regulate regional patterning of the neocortex as well as the timing and generation of secondary progenitor populations during cortical development.
Acknowledgments
We are grateful to J.A. Kessler (Northwestern University, Chicago, IL), E. S. Monuki (University of California, Irvine, CA), E. C. Olson (State University of New York, Upstate, NY), and members of the Chenn lab for helpful comments on this manuscript. We thank R.T. Moon (University of Washington, Seattle, WA) for providing the Super(8x)TOPFLASH and Super(8x)FOPFLASH constructs and C. Gottardi (Northwestern) for pCDNA3-ICAT, and are grateful to Urs Berger (UB In Situ, Wellesley, MA) for technical assistance with the in situ hybridizations. This work was supported by NIH 1 RO1 NS047191 and a Sontag Foundation Distinguished Scientist Award to A.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, Lloyd KC, Lai C. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–28. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- Backman M, Machon O, Mygland L, van den Bout CJ, Zhong W, Taketo MM, Krauss S. Effects of canonical Wnt signaling on dorso-ventral specification of the mouse telencephalon. Dev Biol. 2005;279:155–68. doi: 10.1016/j.ydbio.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Barth AI, Pollack AL, Altschuler Y, Mostov KE, Nelson WJ. NH2-terminal deletion of beta-catenin results in stable colocalization of mutant beta-catenin with adenomatous polyposis coli protein and altered MDCK cell adhesion. J Cell Biol. 1997;136:693–706. doi: 10.1083/jcb.136.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder D, Li M, Perrimon N. Cooperative Regulation of Cell Polarity and Growth by Drosophila Tumor Suppressors. Science. 2000;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Goudreau G, O’Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–9. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- Bulfone A, Smiga SM, Shimamura K, Peterson A, Puelles L, Rubenstein JL. T-brain-1: a homolog of Brachyury whose expression defines molecularly distinct domains within the cerebral cortex. Neuron. 1995;15:63–78. doi: 10.1016/0896-6273(95)90065-9. [DOI] [PubMed] [Google Scholar]
- Burrows RC, Wancio D, Levitt P, Lillien L. Response diversity and the timing of progenitor cell maturation are regulated by developmental changes in EGFR expression in the cortex. Neuron. 1997;19:251–67. doi: 10.1016/s0896-6273(00)80937-x. [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–75. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Chanas-Sacre G, Thiry M, Pirard S, Rogister B, Moonen G, Mbebi C, Verdiere-Sahuque M, Leprince P. A 295-kDA intermediate filament-associated protein in radial glia and developing muscle cells in vivo and in vitro. Dev Dyn. 2000;219:514–25. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1078>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–9. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Increased neuronal production, enlarged forebrains and cytoarchitectural distortions in beta-catenin overexpressing transgenic mice. Cereb Cortex. 2003;13:599–606. doi: 10.1093/cercor/13.6.599. [DOI] [PubMed] [Google Scholar]
- Ciccolini F. Identification of two distinct types of multipotent neural precursors that appear sequentially during CNS development. Mol Cell Neurosci. 2001;17:895–907. doi: 10.1006/mcne.2001.0980. [DOI] [PubMed] [Google Scholar]
- Clevers H, van de Wetering M. TCF/LEF factor earn their wings. Trends Genet. 1997;13:485–9. doi: 10.1016/s0168-9525(97)01305-x. [DOI] [PubMed] [Google Scholar]
- Edlund T, Jessell TM. Progression from extrinsic to intrinsic signaling in cell fate specification: a view from the nervous system. Cell. 1999;96:211–24. doi: 10.1016/s0092-8674(00)80561-9. [DOI] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–51. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland RJ, Cherry TJ, Preware PO, Morrisey EE, Walsh CA. Characterization of Foxp2 and Foxp1 mRNA and protein in the developing and mature brain. J Comp Neurol. 2003;460:266–79. doi: 10.1002/cne.10654. [DOI] [PubMed] [Google Scholar]
- Frantz GD, Weimann JM, Levin ME, McConnell SK. Otx1 and Otx2 define layers and regions in developing cerebral cortex and cerebellum. J Neurosci. 1994;14:5725–40. doi: 10.1523/JNEUROSCI.14-10-05725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Weber J, Pevny L, Schmid R, Schwab MH, Lloyd KC, Eisenstat DD, Lai C, Anton ES. The role of neuregulin-ErbB4 interactions on the proliferation and organization of cells in the subventricular zone. Proc Natl Acad Sci U S A. 2006;103:1930–5. doi: 10.1073/pnas.0510410103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi CJ, Gumbiner BM. Role for ICAT in beta-catenin-dependent nuclear signaling and cadherin functions. Am J Physiol Cell Physiol. 2004;286:C747–56. doi: 10.1152/ajpcell.00433.2003. [DOI] [PubMed] [Google Scholar]
- Gritti A, Frolichsthal-Schoeller P, Galli R, Parati EA, Cova L, Pagano SF, Bjornson CR, Vescovi AL. Epidermal and fibroblast growth factors behave as mitogenic regulators for a single multipotent stem cell-like population from the subventricular region of the adult mouse forebrain. J Neurosci. 1999;19:3287–97. doi: 10.1523/JNEUROSCI.19-09-03287.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove EA, Tole S. Patterning events and specification signals in the developing hippocampus. Cereb Cortex. 1999;9:551–61. doi: 10.1093/cercor/9.6.551. [DOI] [PubMed] [Google Scholar]
- Gunhaga L, Marklund M, Sjodal M, Hsieh JC, Jessell TM, Edlund T. Specification of dorsal telencephalic character by sequential Wnt and FGF signaling. Nat Neurosci. 2003;6:701–7. doi: 10.1038/nn1068. [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. Embo J. 1999;18:5931–42. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci U S A. 2004;101:3196–201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, Gotoh Y. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- Holt CE, Lemaire P, Gurdon JB. Cadherin-mediated cell interactions are necessary for the activation of MyoD in Xenopus mesoderm. Proc Natl Acad Sci U S A. 1994;91:10844–8. doi: 10.1073/pnas.91.23.10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israsena N, Hu M, Fu W, Kan L, Kessler JA. The presence of FGF2 signaling determines whether beta-catenin exerts effects on proliferation or neuronal differentiation of neural stem cells. Dev Biol. 2004;268:220–31. doi: 10.1016/j.ydbio.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Radial and horizontal deployment of clonally related cells in the primate neocortex: relationship to distinct mitotic lineages. Neuron. 1995;15:311–21. doi: 10.1016/0896-6273(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Kornblum HI, Yanni DS, Easterday MC, Seroogy KB. Expression of the EGF receptor family members ErbB2, ErbB3, and ErbB4 in germinal zones of the developing brain and in neurosphere cultures containing CNS stem cells. Dev Neurosci. 2000;22:16–24. doi: 10.1159/000017423. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Noctor S, Martinez-Cerdeno V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–90. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- Kumar JP, Moses K. EGF receptor and Notch signaling act upstream of Eyeless/Pax6 to control eye specification. Cell. 2001;104:687–97. doi: 10.1016/s0092-8674(01)00265-3. [DOI] [PubMed] [Google Scholar]
- Li T, Lu L. Epidermal growth factor-induced proliferation requires down-regulation of Pax6 in corneal epithelial cells. J Biol Chem. 2005;280:12988–95. doi: 10.1074/jbc.M412458200. [DOI] [PubMed] [Google Scholar]
- Linggi B, Carpenter G. ErbB-4 s80 intracellular domain abrogates ETO2-dependent transcriptional repression. J Biol Chem. 2006;281:25373–80. doi: 10.1074/jbc.M603998200. [DOI] [PubMed] [Google Scholar]
- Lothian C, Lendahl U. An evolutionarily conserved region in the second intron of the human nestin gene directs gene expression to CNS progenitor cells and to early neural crest cells. Eur J Neurosci. 1997;9:452–62. doi: 10.1111/j.1460-9568.1997.tb01622.x. [DOI] [PubMed] [Google Scholar]
- Machon O, van den Bout CJ, Backman M, Kemler R, Krauss S. Role of beta-catenin in the developing cortical and hippocampal neuroepithelium. Neuroscience. 2003;122:129–43. doi: 10.1016/s0306-4522(03)00519-0. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–63. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Noctor SC, Kriegstein AR. The role of intermediate progenitor cells in the evolutionary expansion of the cerebral cortex. Cereb Cortex. 2006;16(Suppl 1):i152–61. doi: 10.1093/cercor/bhk017. [DOI] [PubMed] [Google Scholar]
- Mione MC, Cavanagh JF, Harris B, Parnavelas JG. Cell fate specification and symmetrical/asymmetrical divisions in the developing cerebral cortex. J Neurosci. 1997;17:2018–29. doi: 10.1523/JNEUROSCI.17-06-02018.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–41. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron. 1989;2:1547–58. doi: 10.1016/0896-6273(89)90043-3. [DOI] [PubMed] [Google Scholar]
- Ni CY, Murphy MP, Golde TE, Carpenter G. gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–81. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- Nieto M, Monuki ES, Tang H, Imitola J, Haubst N, Khoury SJ, Cunningham J, Gotz M, Walsh CA. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J Comp Neurol. 2004;479:168–80. doi: 10.1002/cne.20322. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–9. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–44. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Peifer M. Cell biology. Travel bulletin--traffic jams cause tumors. Science. 2000;289:67–9. doi: 10.1126/science.289.5476.67. [DOI] [PubMed] [Google Scholar]
- Petersen PH, Zou K, Hwang JK, Jan YN, Zhong W. Progenitor cell maintenance requires numb and numblike during mouse neurogenesis. Nature. 2002;419:929–34. doi: 10.1038/nature01124. [DOI] [PubMed] [Google Scholar]
- Seaberg RM, Smukler SR, van der Kooy D. Intrinsic differences distinguish transiently neurogenic progenitors from neural stem cells in the early postnatal brain. Dev Biol. 2005;278:71–85. doi: 10.1016/j.ydbio.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Sugitani Y, Nakai S, Minowa O, Nishi M, Jishage K, Kawano H, Mori K, Ogawa M, Noda T. Brn-1 and Brn-2 share crucial roles in the production and positioning of mouse neocortical neurons. Genes Dev. 2002;16:1760–5. doi: 10.1101/gad.978002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Goto T, Miyama S, Nowakowski RS, Caviness VS., Jr Sequence of neuron origin and neocortical laminar fate: relation to cell cycle of origin in the developing murine cerebral wall. J Neurosci. 1999;19:10357–71. doi: 10.1523/JNEUROSCI.19-23-10357.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabykin V, Stoykova A, Usman N, Gruss P. Cortical upper layer neurons derive from the subventricular zone as indicated by Svet1 gene expression. Development. 2001;128:1983–93. doi: 10.1242/dev.128.11.1983. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF, van der Kooy D. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol. 1999;208:166–88. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–5. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- Woodhead GJ, Mutch CA, Olson EC, Chenn A. Cell-autonomous beta-catenin signaling regulates cortical precursor proliferation. J Neurosci. 2006;26:12620–30. doi: 10.1523/JNEUROSCI.3180-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner D, Fujita Y, Hulsken J, Muller T, Walther I, Taketo MM, Crenshaw EB, 3rd, Birchmeier W, Birchmeier C. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–18. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Zhou CJ, Borello U, Rubenstein JL, Pleasure SJ. Neuronal production and precursor proliferation defects in the neocortex of mice with loss of function in the canonical Wnt signaling pathway. Neuroscience. 2006;142:1119–31. doi: 10.1016/j.neuroscience.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Zimmer C, Tiveron MC, Bodmer R, Cremer H. Dynamics of Cux2 expression suggests that an early pool of SVZ precursors is fated to become upper cortical layer neurons. Cereb Cortex. 2004;14:1408–20. doi: 10.1093/cercor/bhh102. [DOI] [PubMed] [Google Scholar]


