Abstract
In addition to neurodevelopmental effects, alcohol consumption at high levels during pregnancy is associated with immunomodulation and premature birth. Premature birth, in turn, is associated with increased susceptibility to various infectious agents such as Respiratory Syncytial Virus (RSV). The initial line of pulmonary innate defense includes the mucociliary apparatus, which expels microorganisms trapped within the airway secretions. Surfactant proteins A and D (SP-A and SP-D, respectively) are additional components of pulmonary innate immunity and have an important role in pulmonary defense against inhaled pathogens. The purpose of this study was to determine if chronic alcohol consumption during the third trimester of pregnancy alters the function of the mucociliary apparatus and expression of SP-A and SP-D of fetal lung epithelia. Sixteen, date-mated ewes were assigned to two different groups; an ethanol exposed group in which ewes received ethanol through surgically implanted intra-abomasal cannula during the third trimester of pregnancy, and a control group in which ewes received the equivalent amount of water instead of ethanol. Within these two groups, ewes were further randomly assigned to a full-term group in which the lambs were naturally delivered, and a pre-term group in which the lambs were delivered prematurely via an abdominal incision and uterotomy. Ethanol was administered 5 times a week as a 40% solution at 1gr/kg of body weight. The mean maternal serum alcohol concentration (SAC) measured 6 hr post administration was 16.3 +/− 4.36 mg/dL. Tracheas from 6 full-term lambs were collected to assess ciliary beat frequency (CBF). The lung tissue from all (24) lambs was collected for immunohistochemistry (IHC) analysis of SP-A and SP-D protein production and fluorogenic real-time quantitative polymerase chain reaction analysis (qPCR) of SP-A and SP-D mRNA levels. Exposure to ethanol during pregnancy significantly blocked stimulated increase in CBF though ethanol-mediated desensitization of cAMP-dependant protein kinase (PKA). In addition, pre-term born/ethanol-exposed lambs showed significantly decreased SP-A m-RNA expression when compared to the pre-term born/control group (p=0.004); no significant changes were seen with SP-D. The full-term/ethanol exposed lambs had no significant alterations in mRNA levels, but had significantly less detectable SP-A protein when compared to the full-term/control lambs (p=0.02). These findings suggest that chronic maternal ethanol consumption during the third trimester of pregnancy alters innate immune gene expression in fetal lung. These alterations may underlie increased susceptibility of pre-term infants, exposed to ethanol in utero, to RSV and other microbial agents.
Keywords: alcohol, ethanol, innate immunity, maternal, newborn, lung, premature, surfactant protein A
Introduction
The innate immune response of lung epithelia represents the first line of defense against potentially pathogenic microorganisms present in inspired air. These responses can immediately prevent colonization and proliferation of microbes, thus minimizing the involvement of the adaptive immune response and allowing efficient gaseous exchange. The respiratory tract innate immune system prevents initial colonization of airborne microorganisms through at least three distinct mechanisms. First, microorganisms trapped in the respiratory mucus can be removed mechanically by mucociliary activity. Second, when invading microbes reach the deeper respiratory compartments, such as the alveolar lumen, cellular and secretory mechanisms are triggered. These include alveolar macrophages, intravascular macrophages and other cells with phagocytic and pro-inflammatory properties such as eosinophils (Aderem and Underhill, 1999; Underhill and Ozinsky, 2002). The third mechanism involves antimicrobial peptides, proteins and other molecules secreted by respiratory epithelia (Bals and Hiemstra, 2004; Brogden et al., 2003; Hiemstra and Bals, 2004). Ethanol exposure significantly alters at least some of these lung innate responses (Happel and Nelson, 2005) in adults. While each of these innate immunity mechanisms are important in the initial respiratory host defense, their activity in individuals born pre-term with or without the influence of intrauterine ethanol exposure is poorly understood.
Alveolar type II cells (ATII) as well as non-ciliated bronchial and bronchiolar epithelial cells (Clara cells) secrete lung surfactant. Surfactant is a mixture of phospholipids which primarily functions to prevent alveolar collapse during end-expiration (Ochs, 2006). However, in addition to this physiological function, surfactant has been found to have potent antimicrobial properties which are largely attributed to the hydrophilic, collectin-like surfactant protein A and D (Crouch et al., 2000; Grubor et al., 2006; Kingma and Whitsett, 2006; Kishore et al., 2006; LeVine and Whitsett, 2001; Sano and Kuroki, 2005). The secretion of SP-A and SP-D is regulated by developmental stage (Kawashima et al., 2006; King et al., 1975; Mescher et al., 1975), hormones and potentially, by environmental factors such as alcohol consumption.
Infants born prematurely exhibit increased susceptibility to and severity of infections with RSV (Rossi et al., 2007). Risk factors associated with premature birth include maternal alcohol consumption at high levels, smoking, drug abuse and poor nutrition (Albertsen et al., 2004; Hack et al., 2002; Kyrklund-Blomberg et al., 2005; Parazzini et al., 2003). Alcohol consumption during pregnancy has been associated with many harmful effects in the developing fetus. Most studies have focused on neurodevelopmental (Riley and McGee, 2005; West and Blake, 2005) and behavioral anomalies (Mattson et al., 2001; Sood et al., 2001) in infants born from mothers who drank during pregnancy. Recently it has been suggested that in addition to neurodevelopmental effects, ethanol may play a significant role in immunomodulation and consequential increase in susceptibility to various infectious in newborn infants (Gauthier et al., 2005a; Gauthier et al., 2004; Gauthier et al., 2005b). It is our hypothesis that ethanol consumption in moderation during pregnancy alters mucociliary clearance and expression of SP-A and SP-D by fetal lung epithelia. Moreover, we suspect that intrauterine fetal ethanol exposure may more severely affect those born pre-term than those born full-term since full-term infants have had additional time to allow for lung epithelial cell maturation. To do this we have developed an ovine model. Gestating ewes and their offspring are well-suited for these studies since: 1) alveolar development in lambs occurs pre-natally as in humans unlike rodents, 2) lambs can be derived pre-term and survive unlike many other animal models (Meyerholz, Grubor et al. 2004), 3) ovine respiratory epithelia of airways, distal bronchioles and alveoli are similar to those of human lung and the ovine lung has submucosal glands not present in some animal models (Meyerholz, Grubor et al, 2004; Meyerholz, Kawashima et al, 2006), 4) expression of SP-A and SP-D and other innate immune genes of ovine lung are well-characterize and very similar to human (Grubor, Meyerholz et al, 2006; Meyerholz, Kawashima et al 2006), and 5) pre-term lambs are susceptible to RSV, a known pathogen of premature infants and maternal ethanol consumption is a risk for pre-term birth (Meyerholz, Grubor et al, 2004). Finally, sheep have long been used for respiratory studies related to human disease as well as the effects of ethanol on fetal brain development (Mescher, Platzker et al, 1975).
Materials and methods
Experimental design
Animal use and experimental procedures were approved by Iowa State University’s Animal Care and Use Committee according to the requirements of the NIH guide for the Care and Use of Laboratory Animals. Abomasal cannula was surgically implanted in 16, mixed breed (1/2Rambouillet, 1/4Dorset and1/4Finsheep), date-mated, pregnant ewes approximately 6 weeks prior to lambing dates. The ewes were randomly assigned to two groups, an ethanol-exposed and a control group. Within these groups, ewes were further randomly assigned to a full-term group in which the lambs were naturally delivered and a pre-term group in which the lambs were delivered prematurely via abdominal incision and uterotomy (day 138 of gestation, term=147). The ewes received either ethanol or water five days a week for three weeks. A total of twenty four lambs were obtained and assigned to experimental groups. Each group had six lambs (n=6 lambs). The full-term/control group (FTC) had four singlets and one set of twins. The full-term/alcohol exposed group (FTA) had three sets of twins. The pre-term/control group (PTC) had two singlets and two sets of twins. The pre-term/alcohol exposed group (PTA) had two singlets and two sets of twins. In addition, total of six lamb tracheae were obtained, three from FTC group (n=3 lambs) and three from FTA group (n=3 lambs) for CBF analysis.
Abomasal cannulation
The delivery of ethanol into the ovine abomasum allows bypass of the rumen and degradation by ruminal microflora. It also promotes hepatic bio-metabolism as the abomasum is the “true” stomach of ruminants and empties directly into duodenum. Pregnant ewes were deprived of water and food for 12 hr prior to induction of anesthesia. Anesthesia was induced by administration of Telazol (1–2 mg/kg, I.M; Fort Dodge, IA) and ewes were positioned in the dorsal recumbence. General anesthesia was maintained by inhalation of 2% isoflurane. The median abdominal and right paracostal regions were prepared for aseptic surgery. The initial, 10 cm incision was made from umbilicus to the epigastric region. Abomasum was identified, exteriorized, and isolated by towels moistened with sterile saline. A ½” polyethylene cannula (Bar Diamond, Parma, ID) was placed in the pyloric antrum and the abomasal incision was closed with purse string sutures. A second, 5 cm long incision was made in the right paracostal region and the cannula shaft was exteriorized. To secure the cannula placement, an additional 4 sutures through the cannula ring were added. Finally, both abdominal incisions were sutured closed (Figure 1).
Figure 1.
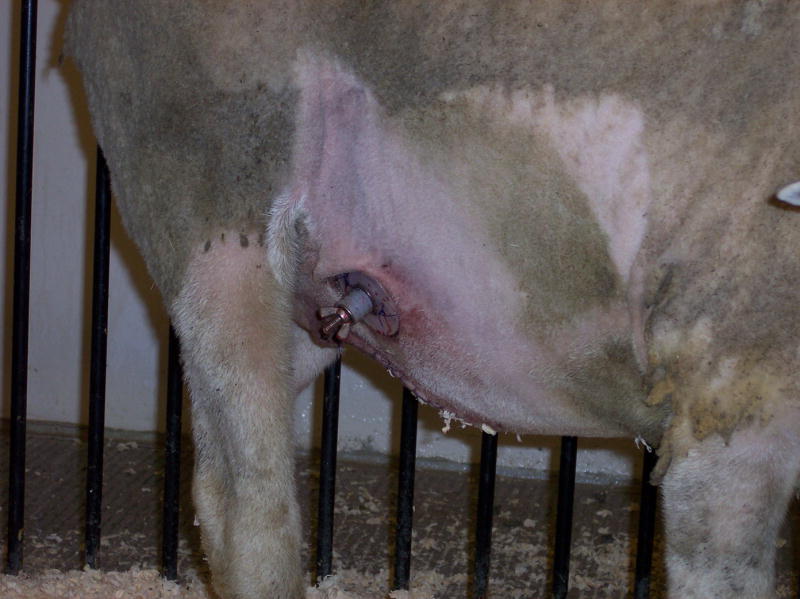
Intra-abomasal cannula for alcohol delivery. The canula is present along the ventral right paralumbar area. To deliver alcohol, the screw-cap is released and a catheter is inserted and alcohol enters by gravity flow.
Ethanol administration protocol
In 16 pregnant ewes, 40% ethanol at 1gr/kg, V/V (Sigma, St. Louis, MO) administration began 5 days after cannula insertion and was repeated 5 days a week during the last 5 weeks of gestation. Control ewes received the equivalent amount of water. After 4 weeks of ethanol administration (5 days/week) serum alcohol (ethanol) concentrations (SAC) were determined at 6, 12 and 24 hours post a single infusion by headspace gas chromatography analysis (University of Iowa Diagnostic Laboratories, Iowa City, IA).
Tissue collection
Within 24 hr of natural lambing or uterotomy, all lambs were euthanized by intravenous administration of sodium pentobarbital and lung tissue was collected immediately. The samples from the left and right cranial lobes were collected into cryovials, snap frozen in liquid nitrogen and stored at −80 °C until RNA extraction and qPCR analysis. The remaining tissue from the same lung lobes were fixed in 10% buffered formalin for IHC. The tracheas were collected into complete Dulbecco’s modified Eagle’s medium (complete DMEM), placed on ice and immediately shipped for mucociliary activity analysis (Department of Internal Medicine, University of Nebraska Medical Center, Pulmonary and Critical Care Medicine Section).
Ciliary Beat Frequency analysis
A total of 6 lamb tracheae were obtained, 3 from FTC group (n=3 lambs) and 3 from FTA group (n=3 lambs). Lamb tracheae were sliced into 2–5 mm rings and placed in M199 media in 35 mm tissue culture dishes (Falcon, Franklin Lakes, NJ). Tissues were maintained at room temperature (25°C ± 0.5°C) during assay procedures using a thermostatically-controlled heated stage. Cilia motion frequency was quantitated using the Sisson-Ammons Video Analysis (SAVA) system. Images of ciliated cells were visualized by inverted phase-contrast microscope, digital video images captured and analyzed for motion by SAVA using a process known as Whole Field Analysis (Sisson et al., 2003). The SAVA software analyzed each image containing 19,200 possible motile zones to determine the average frequency and the standard error of the mean for each field captured. For each experimental condition, a minimum of 6 separate fields were captured, analyzed and expressed as a data point.
Determination of cAMP-dependent Kinase Activity
PKA activity was determined in crude whole-cell fractions of tracheal epithelial cells. Tracheae were exposed to M199 (media control) or 100 μM isoprotrenol for 1 hour and the tracheal epithelial cells were scraped from the luminal surface of the tracheae using a sterile cell lifter. Cells were flash frozen on liquid nitrogen in a cell lysis buffer consisting of phosphate buffered saline (pH 7.4) containing protease inhibitors (1 μg/ml each of leupeptin, aprotinin, PMSF and chymostatin). Cell lysates were sonicated and particulates removed by centrifugation. Protein concentration was determined by the Bradford method (Schleicher and Wieland, 1978) with Bio-Rad protein reagent (Bio-Rad, Hercules, CA).
This PKA assay has been previously described in detail (Wyatt and Sisson, 2001). Kinase activity was expressed in relation to total cellular protein assayed and expressed in terms of picomoles of phosphate incorporated/min/mg of total protein assayed. All samples were assessed in triplicate and no less than three separate experiments were performed per unique parameter.
qPCR analysis
Our approach to this procedure has been previously described in detail (Gallup and Ackermann, 2006; Kawashima et al., 2006). Briefly, total RNA was isolated from whole lung tissue samples and assessed for quantity and purity by spectrophotometry. This procedure was followed by DNase treatment (TURBO DNA-free kit, Ambion, Austin, TX). Prior to qPCR analysis, a test plate was run to determine which RNA dilution ranges gave the best signal (lacked inhibition and exhibited LOG-linear behavior and amplification efficiencies >80%) for SP-A and SP-D targets. The DNase treated RNA isolates were diluted 1:10 with nuclease-free water, then diluted to their ideal ranges on a per-sample, per-target basis (according to what was learned from the test plate analysis), and further placed in duplicate into 96-well qPCR reaction plates (Applied Biosystems Incorporated, Forest City, CA). Each initially-prepared 30 μl reaction contained 7.8 μl of DNase treated, appropriately-diluted RNA isolate, 15 μl of a commercially available Applied Biosystems One-Step Master Mix, 1000 nM primers, 150 nM TaqMan (5′-6FAM, 3′-TAMRA-quenched) probe, and nuclease-free water. The negative, no-template control wells contained nuclease-free water (Ambion) instead of RNA isolate. 27 μl of each reaction was used in-well in the 96-well reaction plates. The plates were run in the GeneAmp 5700 Sequence Detection System (Applied Biosystems, Foster City, CA) for detection and relative quantification of SP-A and SP-D mRNA targets. The results were analyzed by using the GeneAmp 5700 software and departmentally available Excel files. In addition, the ribosomal protein S15 mRNA levels were used as internal control.
IHC analysis
This procedure and scoring system has been previously described in detail by our laboratory (Grubor et al., 2004). Briefly, sections of lung on glass slides were deparaffinized and rehydrated followed by antigen retrieval, a protein blocking procedure, SP-A specific primary antibody incubation (Mouse IgM anti-human SP-A antibody, Chemicon International, Inc., Temecula, CA), secondary antibody application (biotinylated Rat anti-Mouse Isotype Rat (LOU) IgG2a, κ, B.D. Pharmingen, San Diego, CA), streptavidin-conjugated horseradish peroxidase incubation (BioGenex, San Ramon, CA) and Nova Red substrate (Vector, Burlingame, CA) development. All slides were counter-stained with Shandon’s ¼-strength hematoxylin (Shandon-Lipshaw, Pittsburgh, PA), dehydrated through a series of graded solvents, and cover-slipped using Permount (Fisher, Hanover, IL) according to standard IHC protocol. For IHC scoring system, five fields of each lung section were assessed for intensity and distribution of SP-A stained epithelial cells in the terminal bronchioles and bronchiolar/alveolar junctions. Scoring was based on predetermined scale; 0, no staining of cells; 1, <30% of epithelial cells stained with minimal detectable intracytoplasmic staining; 2, 30–60% of epithelial cells stained with minimal detectable intracytoplasmic staining; 3, 30–60% of epithelial cells stained with >50% of cytoplasm stained; 4, >60% of epithelial cells stained with >50% of cytoplasm stained.
Statistical analysis
For CBF data, ANOVA was run on each data point. For determination of cAMP-dependent Kinase Activity data was analyzed for statistical significance using one-way ANOVA. For IHC and qPCR data, summary statistics (mean and standard error of the mean) were calculated for each experimental group. Student-t tests (Graph Pad Sigma software, San Diego, CA) were also performed on normalized qPCR and IHC-score data. The acceptable level of significance was p<.05
Results
Sheep serum alcohol (ethanol) concentration
The ethanol in the maternal serum was measured 6 hr post-infusion and ranged from undetectable (in one ewe) and 10–32 mg/dl (in other 7ewes), mean was16.3 +/− 4.36 mg/dL. Ethanol was undetectable at 12 and 24 hours in these ewes (table 1).
Table 1.
Serum alcohol concentrations in mg/dl, in acclimated (receiving daily ethanol for five days for three weeks) pregnant ewes at six, 12 and 24 hours after a single infusion (40% at 1 mg/kg, V/V).
| Ewe 1 | Ewe 2 | Ewe 3 | Ewe 4 | Ewe 5 | Ewe 6 | Ewe 7 | |
|---|---|---|---|---|---|---|---|
| 6hr | 0 | 23 | 11 | 10 | 10 | 28 | 32 |
| 12hr | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 24hr | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
CBF results
Ex vivo tracheal CBF was assayed in full-term lambs delivered to both control and ethanol-exposed pregnant ewes. In ethanol-exposed trachea, baseline CBF did not significantly differ from control trachea (Figure 2A). However, isoproterenol-stimulated CBF increases in control lamb tracheal rings were not observed in tracheal rings obtained from ethanol-exposed lambs. Because isoproterenol-stimulated CBF increases require the activation of PKA (Wyatt et al., 1998) epithelial cell PKA activity was also assayed in this study. Isoproterenol significantly increased PKA activity in control trachea, but failed to activate PKA in ethanol-exposed trachea (Figure 2B).
Figure 2.
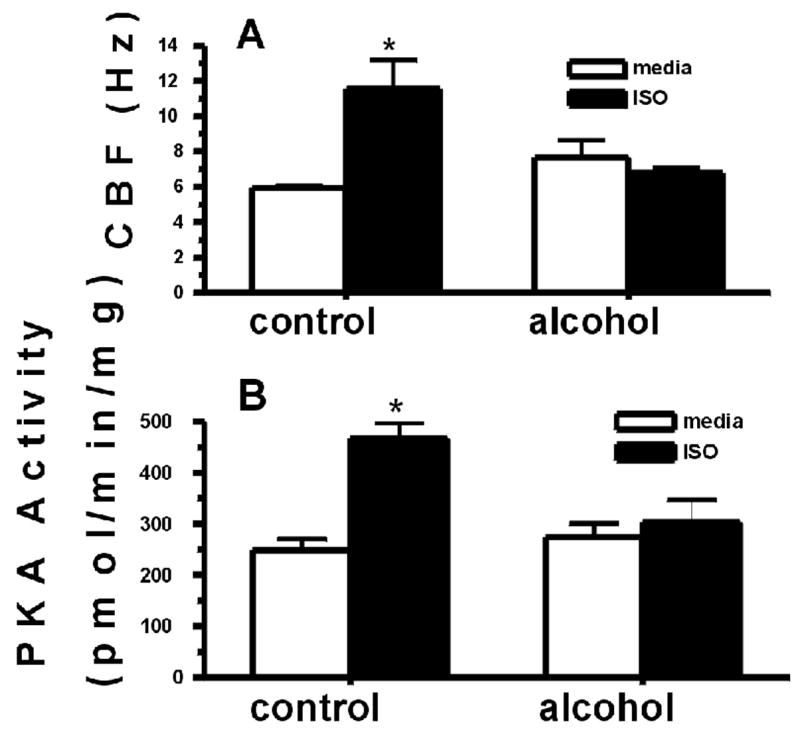
Ewes were exposed to alcohol as described. 6 full-term lambs were sacrificed and tracheal ring cilia were incubated with control media or 100 μM isoproterenol (ISO) for 30 min. CBF (panel A) and PKA (panel B) were assayed. ISO significantly stimulates increased lamb tracheal epithelial CBF and PKA from control ewes (*p<0.05; n=3 lambs). Maternal alcohol exposure desensitizes the lamb cilia to beta-agonist stimulated increases in CBF and PKA (n=3 lambs).
qPCR results
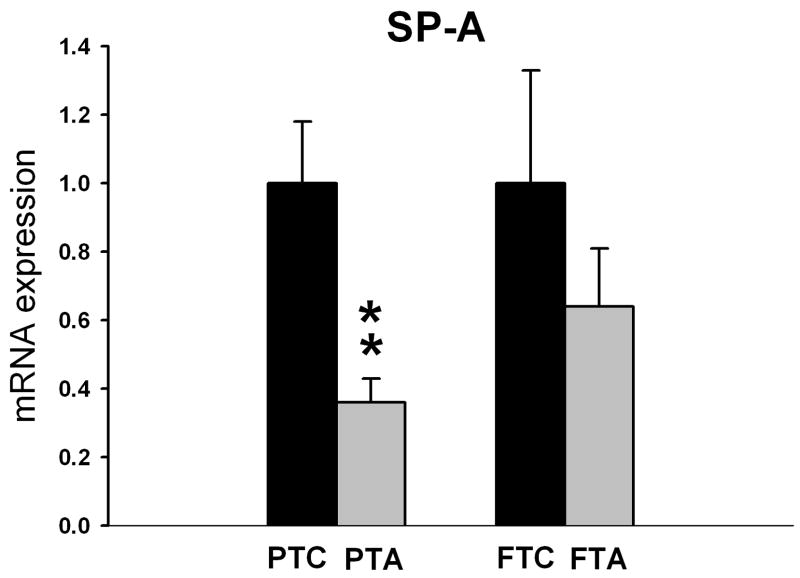
Pregnant ewes that received ethanol during the last 5 weeks of gestation showed significantly reduced SP-A mRNA expression in lungs of lambs delivered pre-term (p=0.004) compared to pre-term/control lambs not receiving ethanol (Figure 3). Although maternal ethanol administration exhibited a tendency toward decreased SP-D mRNA expression in pre-term lambs when compared to pre-term/control lambs, this difference was not statistically significant (p=0.4, Figure 4). In full-term lambs, there was no significant change in both SP-A and SP-D mRNA expression between full-term control and full-term/ethanol exposed lambs.
Figure 3.
The relative SP-A mRNA expression in pre-term control (PTC), and full-term control (FTC) lambs as well as pre-term alcohol-exposed (in utero) (PTA) and full-term alcohol-exposed (in utero) lambs (FTA). The FTC and PTC values are equal to one. The PTA and FTA values are presented relative to the corresponding controls. The SP-A mRNA expression is significantly reduced (p=0.004) in PTA lambs (n=6 lambs) when compared to PTC lambs (n=6lambs). There is no significant change in SP-A mRNA expression between FTA and FTC lambs.
Figure 4.
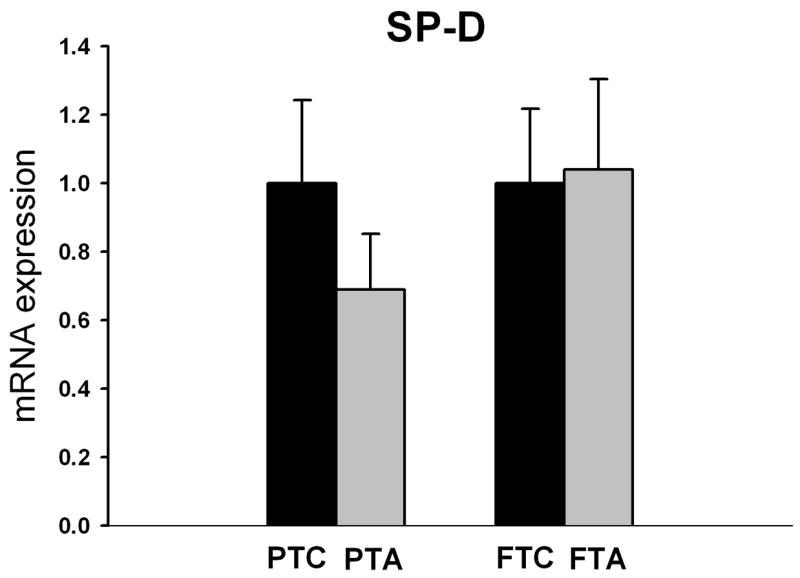
The relative SP-D mRNA expression in pre-term and full-term lambs with or without (control) maternal alcohol administration during gestation. Although maternal alcohol administration had a tendency to decrease the SP-D mRNA expression in the pre-term/alcohol exposed lambs (PTS), (n=6 lambs) when compared to the pre-term/control lambs (PTC), (n=6 lambs), this difference was not statistically significant (p=0.4). There is no significant change in SP-D mRNA expression between full-term/alcohol exposed and full-term/control lambs.
IHC analysis results
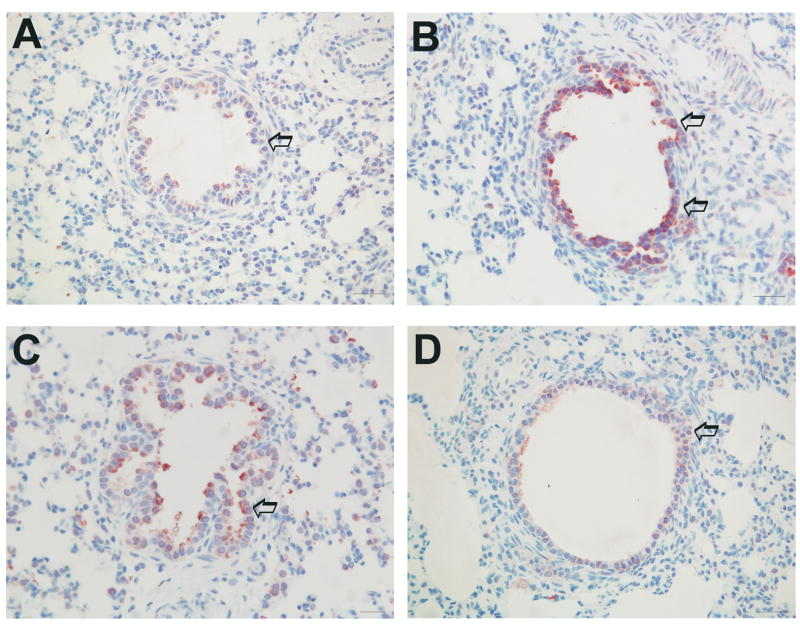
SP-A antigen was detected within the cytoplasm of terminal bronchiolar and bronchiolar/alveolar junction epithelial cells in all four groups of lambs. The mean score for all for groups was; PTC, 1.7 +/− 0.15; FTC, 2.88 +/−0.39; FTA, 1.66 +/−0.38 and PTA, 2.00 +/−0.41. The pre-term/control group (Figure 5D) had significantly less detectible SP-A-specific staining when compared to the full-term/control group (p=0.02, Figure 5B). The full-term/alcohol exposed group (Figure 5A) had significantly less detectible SP-A-specific staining (reduced SP-A protein expression) when compared to the full-term/control group (p=0.04, Figure 5B).
Figure 5.
Representative immunohistochemical detection of SP-A protein in bronchial/bronchiolar epithelium of the full-term/alcohol exposed lambs (A), full-term control lambs (B), pre-term/alcohol exposed lambs (C) and pre-term control lambs (D). High distribution and intensity of staining is seen in pre-term lambs receiving alcohol, suggesting that alcohol may affect SP-A protein release from epithelial cells. Arrows depict epithelial cells with SP-A protein expression.
Discussion
The finding that maternal ethanol exposure reduces the expression of SP-A mRNA and CBF in fetal lung demonstrates that ethanol has transplacental effects on key aspects of lung innate immunity. The decreased expression of SP-A mRNA in lungs of pre-term lambs and exposed to ethanol in utero when compared to pre-term born, control lambs lacking in utero ethanol exposure suggests that ethanol may even further hinder innate immunity beyond the level of a normal pre-term individual. Our previous studies have shown that innate immune gene expression in pre-term lambs is significantly reduced compared to full-term lambs (Hallman et al., 2001; Kawashima et al., 2006) and ethanol may exacerbate this alteration. In this study, pre-term/control (without ethanol exposure) SP-A mRNA levels tended to be reduced in comparison to full-term lambs. However, this reduction was not significant (p=0.9). This was expected since the gap in age between pre- and full-term lambs in this current study is less (9 days) than in our previous studies (17–32 days) (Meyerholz et al., 2006).
Although SP-A mRNA levels were reduced in the lungs of pre-term lambs exposed to ethanol in utero, the IHC results suggested that SP-A protein expression was not significantly altered. We suspect that this might be explained by decreased secretion of SP-A protein by the immature lung epithelial cells (Hallman et al., 2001). It is possible that ethanol may suppress SP-A transcriptional activity in immature epithelia and SP-A protein release into the airway.
In contrast to pre-term lambs, SP-A mRNA levels were not altered in full-term lambs with or without ethanol exposure in utero. We suspect that the additional gestation time may allow the lung epithelia to further mature and reach a critical point for essential SP-A transcription. The reduced SP-A protein detected in lungs of full-term/ethanol exposed lambs compared to the full-term/control lambs may suggest that while a mature level of SP-A gene transcription may occur at this developmental stage, there may be a time of “catch up” needed in newborn lambs to release mature protein into the airway lumen. Alternatively, ethanol may have a residual effect on post-translational intracellular mechanisms. However, IHC is a semiquantitative technique and to make a firm statement on lung SP-A protein level additional techniques e.g ELISA or Western Immunoblot are warranted. In addition, we have chosen to evaluate the IHC staining at the terminal bronchioles and broncho-alveolar junction, which is primarily composed of Clara cells for several reasons. The lung of pre-term and one day old lamb neonates is not fully developed and alveolar epithelial cells are flattened and difficult to identify. In our previous work we have shown that SP-A staining in the alveolar epithelium of these age groups is less intense than that of 15 days old lambs (Meyerholz, Kawashima et al, 2006). In that same study, we have shown that SP-A mRNA expression by epithelial cells at the broncho-alveolar junction (composed primarily of Clara cells and some ATII cells) parallels (e.g. is proportional to) that of ATII throughout different intrauterine and postnatal developmental stages. Also, SP-A mRNA expression by Clara cells was approximately 60% of that by ATII at each stage of gestation and postnatally, which makes IHC staining of Clara cells reliable evaluation criteria in pre-term and young neonatal lambs.
The decrease in stimulated trachea CBF in lambs exposed to ethanol is consistent with previous studies. Ethanol can have a detrimental effect on this lung protective mechanism depending on the length of exposure. When acutely-exposed to ethanol, the ciliary beat frequency in bovine bronchial epithelial cells was increased (Wyatt et al., 2003). Interestingly, when chronically exposed to ethanol, the effect was quite opposite and ciliary beating was decreased (Wyatt and Sisson, 2001). This chronic desensitization of cilia stimulation by ethanol has also been demonstrated in vivo in rat (Wyatt et al., 2004) and mouse (Elliott et al., 2006) models. Unique to our current study is the observation that maternal transmission of ethanol-mediated desensitization occurs in the full-term lamb as well. The combination of reduced SP-A and SP-D expression and decreased clearance due to desensitized CBF may underlie the increased susceptibility of the pre-term lung (exposed to maternal alcohol consumption) to infectious agents such as RSV.
The CBF and SP-A findings in fetal lung are consistent with studies that show an overall immunosuppressive effect of ethanol. For example, chronic alcoholics have an increased incidence and higher mortality rate related to bacterial pneumonia (de Roux et al., 2006; el-Ebiary et al., 1997; Jong et al., 1995) and a three-fold increase in incidence of infection and sepsis after surgery (von Dossow et al., 2004). In HIV patients, concurrent alcohol consumption increases the susceptibility to various infections (Justice et al., 2006). Maternal alcohol consumption during pregnancy and its effects on fetal immunity remains a largely unexplored area of clinical significance. Several studies have already demonstrated that newborns exposed to ethanol during prenatal life have a higher incidence of infection (Gauthier et al., 2004; Gauthier et al., 2005b). The exact mechanism by which this occurs is not fully understood but it is possible that reduction in tracheal CBF and SP-A may increase susceptibility to infections. Ethanol consumption has been shown to have a suppressive effect on cellular components of the innate immune system such as macrophages (Gauthier et al., 2005b) and/or neutrophils (Vinson et al., 1998), all of which may occur concurrently with reductions in CBF and SPA.
Although lung epithelia secrete many antimicrobial peptides, proteins and molecules, SP-A and SP-D are among the most extensively-studied pulmonary host defense/innate proteins. They are constitutively expressed and secreted by ATII cells and Clara cells (Crouch et al., 1992) and belong to a subgroup of collectin-containing mammalian C-type lectins, called collectins (Kishore et al., 2006). SP-A and SP-D can bind, aggregate and opsonize many microorganisms including Gram-negative and Gram-positive bacteria, enveloped viruses like Influenza A Virus (IAV), RSV, non-enveloped viruses like Rotavirus and fungal organisms in order to protect lung from possible infection (Grubor et al., 2006). In animal studies, the deficiency of SP-A, SP-D or both were associated with increased susceptibility to pulmonary Pseudomonas aeruginosa (LeVine et al., 1998) and Streptococcal infections(LeVine et al., 1997). Also, elevated levels of SP-A and SP-D mRNA in the lungs of neonatal lambs are associated with clearance of Parainfluenza virus-3 (PIV-3) (Grubor et al., 2004). Another study showed that SP-A and SP-D enhance mannose receptor mediated phagocytosis of Mycobacterium avium by macrophages (Kudo et al., 2004). Also, the reduced clearance of RSV in lambs is associated with prematurely and possibly inadequate expression in pulmonary collectins (Meyerholz et al., 2004) while SP-A enhances RSV clearance in mice (LeVine et al., 1999).
In addition, when SP-A and SP-D are administered to the lung in the murine model of invasive pulmonary aspergillosis, the mortality rate of infected animals was significantly reduced (Kishor et al., 2002). In rats, administration of SP-A reduces the oxidative damage of ventilated lungs (Bailey et al., 2006). In ventilated lambs, SP-A did not reduce inflammation indicators caused by inflammation (Ikegami and Jobe, 2002). However, there were no negative effects associated with SP-A administration.
In this study, we have shown that a moderate level of alcohol consumption during the third trimester of pregnancy significantly decreases the levels of SP-A gene expression in pre-term lambs while SP-D remained unaffected. The exact mechanism of this alcohol-related immunosuppressive effect is not clear but we suspect that ethanol may have a direct effect on lung epithelial cells, particularly ATII cells and Clara cells. For example, Brown et al. have previously shown that chronic ethanol ingestion potentiates apoptosis of ATII cells in rats (Brown et al., 2001). The results of this experiment suggest that chronic ethanol exposure decreases the bioavailability of glutathione (GSH) to mitochondria of ATII cells. As a consequence, ATII repair mechanisms are diminished resulting in a significant decrease in numbers of these SPA-producing cells. Also, the same investigators have previously published that chronic ethanol exposure and GSH depletion activates matrix metalloproteinases (MMP’s) which consequentially degrade lung epithelial cells (Lois et al., 1999). Although this might be a possible mechanism of ethanol-induced lung innate immunity impairment, other researchers have shown that prenatal exposure to ethanol effects maternal-fetal-hormonal interactions which in-turn substantially affects the functional immunity of offspring. Zhang at al. have demonstrated that alcohol consumption during pregnancy increases Hypothalamic-Pituitary-Adrenal (HPA) responsiveness in rat pups in such a way that ethanol-exposed neonates exhibit reduced increase responsiveness in early and pre-weaning life (Zhang et al., 2005). The exact nature and significance of this disturbed hormonal homeostasis still needs to be determined but it is possible that insufficient neonatal corticosteroid levels might be an underlying indicator for inadequate lung surfactant production. In addition, moderate levels of ethanol consumption are related to an increased incidence of premature birth (Albertsen et al., 2004; Parazzini et al., 2003). As previously demonstrated in our lab, pre-term birth is significantly correlated to decreased expression of SP-A and SP-D (Meyerholz et al., 2006).
In developing this ovine model, a cannula was placed into the abomasum for ethanol delivery. This allowed us to bypass the rumen and access a part of the ovine gastrointestinal tract that more accurately mimics the stomach of humans. The abomasum is the “true” stomach of ruminants out of which there is unimpeded flow of abomasal contents into the duodenum and small intestine – similar to gastric contents emptying into the small intestine of humans. The delivery of ethanol into the ovine abomasums in this manner allows for its absorption and passage into the portal veins draining into the liver thereby promoting hepatic bio-metabolism (Lieber et al., 1994), which is similar to the route of ethanol in humans.
As indicated, the levels of ethanol used in this study were similar to those of moderate, not excessive alcohol consumers (Brien et al., 1987). There is debate over “safe” levels of alcohol consumption during pregnancy, and many studies recommend complete abstinence from alcohol during gestation altogether (Martinez-Frias et al., 2004).
In conclusion, moderate alcohol consumption during the last trimester of pregnancy in sheep is associated with significant reduction of SP-A mRNA expression in pre-term lambs and CBF in full-term lambs. The IHC results are suggestive of SP-A protein alteration by maternal alcohol consumption however, additional work on SP-A protein levels is warranted. The deficiency in surfactant proteins, including SP-A, is associated with increased susceptibility to neonatal infections including RSV (Griese et al., 2002). This finding may have clinical significance and will allow us to further investigate RSV infection as a means to address the hypothesis that ethanol exposure during pregnancy may increase RSV susceptibility in human infants.
Acknowledgments
This work was funded, in part, by JG Salsbury Endowment and NIH NIAID grant R01AI062787. The authors would like to thank other individuals involved with the project including Drs. David Merkley and Linda Nelson, Rachel Derscheid, Pam Knake, Francis Zacharakis-Jutz, Beth Hafkey, and Laboratory Animal Resources, Iowa State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- Albertsen K, Andersen AM, Olsen J, Gronbaek M. Alcohol consumption during pregnancy and the risk of preterm delivery. Am J Epidemiol. 2004;159:155–161. doi: 10.1093/aje/kwh034. [DOI] [PubMed] [Google Scholar]
- Bailey TC, Maruscak AA, Petersen A, White S, Lewis JF, Veldhuizen RA. Physiological effects of oxidized exogenous surfactant in vivo: effects of high tidal volume and surfactant protein A. Am J Physiol Lung Cell Mol Physiol. 2006;291:L703–709. doi: 10.1152/ajplung.00538.2005. [DOI] [PubMed] [Google Scholar]
- Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J. 2004;23:327–333. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- Brien JF, Clarke DW, Smith GN, Richardson B, Patrick J. Disposition of acute, multiple-dose ethanol in the near-term pregnant ewe. Am J Obstet Gynecol. 1987;157:204–211. doi: 10.1016/s0002-9378(87)80381-2. [DOI] [PubMed] [Google Scholar]
- Brogden KA, Ackermann M, McCray PB, Jr, Tack BF. Antimicrobial peptides in animals and their role in host defences. Int J Antimicrob Agents. 2003;22:465–478. doi: 10.1016/s0924-8579(03)00180-8. [DOI] [PubMed] [Google Scholar]
- Brown LA, Harris FL, Guidot DM. Chronic ethanol ingestion potentiates TNF-alpha-mediated oxidative stress and apoptosis in rat type II cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L377–386. doi: 10.1152/ajplung.2001.281.2.L377. [DOI] [PubMed] [Google Scholar]
- Crouch E, Hartshorn K, Ofek I. Collectins and pulmonary innate immunity. Immunol Rev. 2000;173:52–65. doi: 10.1034/j.1600-065x.2000.917311.x. [DOI] [PubMed] [Google Scholar]
- Crouch E, Parghi D, Kuan SF, Persson A. Surfactant protein D: subcellular localization in nonciliated bronchiolar epithelial cells. Am J Physiol. 1992;263:L60–66. doi: 10.1152/ajplung.1992.263.1.L60. [DOI] [PubMed] [Google Scholar]
- de Roux A, Cavalcanti M, Marcos MA, Garcia E, Ewig S, Mensa J, Torres A. Impact of alcohol abuse in the etiology and severity of community-acquired pneumonia. Chest. 2006;129:1219–1225. doi: 10.1378/chest.129.5.1219. [DOI] [PubMed] [Google Scholar]
- el-Ebiary M, Sarmiento X, Torres A, Nogue S, Mesalles E, Bodi M, Almirall J. Prognostic factors of severe Legionella pneumonia requiring admission to ICU. Am J Respir Crit Care Med. 1997;156:1467–1472. doi: 10.1164/ajrccm.156.5.97-04039. [DOI] [PubMed] [Google Scholar]
- Elliott MK, Sisson JH, West WW, Wyatt TA. Differential in vivo effects of whole cigarette smoke exposure versus cigarette smoke extract on mouse ciliated tracheal epithelium. Exp Lung Res. 2006;32:99–118. doi: 10.1080/01902140600710546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallup JM, Ackermann MR. Addressing fluorogenic real-time qPCR inhibition using the novel custom Excel file system 'FocusField2-6GallupqPCRSet-upTool-001' to attain consistently high fidelity qPCR reactions. Biol Proced Online. 2006;8:87–152. doi: 10.1251/bpo122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier TW, Drews-Botsch C, Falek A, Coles C, Brown LA. Maternal alcohol abuse and neonatal infection. Alcohol Clin Exp Res. 2005a;29:1035–1043. doi: 10.1097/01.alc.0000167956.28160.5e. [DOI] [PubMed] [Google Scholar]
- Gauthier TW, Manar MH, Brown LA. Is maternal alcohol use a risk factor for early-onset sepsis in premature newborns? Alcohol. 2004;33:139–145. doi: 10.1016/j.alcohol.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Gauthier TW, Ping XD, Harris FL, Wong M, Elbahesh H, Brown LA. Fetal alcohol exposure impairs alveolar macrophage function via decreased glutathione availability. Pediatr Res. 2005b;57:76–81. doi: 10.1203/01.PDR.0000149108.44152.D3. [DOI] [PubMed] [Google Scholar]
- Griese M, Maderlechner N, Ahrens P, Kitz R. Surfactant proteins A and D in children with pulmonary disease due to gastroesophageal reflux. Am J Respir Crit Care Med. 2002;165:1546–1550. doi: 10.1164/rccm.2107147. [DOI] [PubMed] [Google Scholar]
- Grubor B, Gallup JM, Meyerholz DK, Crouch EC, Evans RB, Brogden KA, Lehmkuhl HD, Ackermann MR. Enhanced surfactant protein and defensin mRNA levels and reduced viral replication during parainfluenza virus type 3 pneumonia in neonatal lambs. Clin Diagn Lab Immunol. 2004;11:599–607. doi: 10.1128/CDLI.11.3.599-607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubor B, Meyerholz DK, Ackermann MR. Collectins and cationic antimicrobial peptides of the respiratory epithelia. Vet Pathol. 2006;43:595–612. doi: 10.1354/vp.43-5-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack M, Flannery DJ, Schluchter M, Cartar L, Borawski E, Klein N. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002;346:149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- Hallman M, Glumoff V, Ramet M. Surfactant in respiratory distress syndrome and lung injury. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:287–294. doi: 10.1016/s1095-6433(01)00324-5. [DOI] [PubMed] [Google Scholar]
- Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2:428–432. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- Hiemstra PS, Bals R. Series introduction: Innate host defense of the respiratory epithelium. J Leukoc Biol. 2004;75:3–4. doi: 10.1189/jlb.0903410. [DOI] [PubMed] [Google Scholar]
- Ikegami M, Jobe AH. Injury responses to different surfactants in ventilated premature lamb lungs. Pediatr Res. 2002;51:689–695. doi: 10.1203/00006450-200206000-00005. [DOI] [PubMed] [Google Scholar]
- Jong GM, Hsiue TR, Chen CR, Chang HY, Chen CW. Rapidly fatal outcome of bacteremic Klebsiella pneumoniae pneumonia in alcoholics. Chest. 1995;107:214–217. doi: 10.1378/chest.107.1.214. [DOI] [PubMed] [Google Scholar]
- Justice AC, Lasky E, McGinnis KA, Skanderson M, Conigliaro J, Fultz SL, Crothers K, Rabeneck L, Rodriguez-Barradas M, Weissman SB, et al. Medical disease and alcohol use among veterans with human immunodeficiency infection: A comparison of disease measurement strategies. Med Care. 2006;44:S52–60. doi: 10.1097/01.mlr.0000228003.08925.8c. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Meyerholz DK, Gallup JM, Grubor B, Lazic T, Lehmkuhl HD, Ackermann MR. Differential expression of ovine innate immune genes by preterm and neonatal lung epithelia infected with respiratory syncytial virus. Viral Immunol. 2006;19:316–323. doi: 10.1089/vim.2006.19.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RJ, Ruch J, Gikas EG, Platzker AC, Creasy RK. Appearance of paoproteins of pulmonary surfactant in human amniotic fluid. J Appl Physiol. 1975;39:735–741. doi: 10.1152/jappl.1975.39.5.735. [DOI] [PubMed] [Google Scholar]
- Kingma PS, Whitsett JA. In defense of the lung: surfactant protein A and surfactant protein D. Curr Opin Pharmacol. 2006;6:277–283. doi: 10.1016/j.coph.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Kishor U, Madan T, Sarma PU, Singh M, Urban BC, Reid KB. Protective roles of pulmonary surfactant proteins, SP-A and SP-D, against lung allergy and infection caused by Aspergillus fumigatus. Immunobiology. 2002;205:610–618. doi: 10.1078/0171-2985-00158. [DOI] [PubMed] [Google Scholar]
- Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, Bernal AL, Reid KB, Madan T, Chakraborty T. Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol. 2006;43:1293–1315. doi: 10.1016/j.molimm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Kudo K, Sano H, Takahashi H, Kuronuma K, Yokota S, Fujii N, Shimada K, Yano I, Kumazawa Y, Voelker DR, et al. Pulmonary collectins enhance phagocytosis of Mycobacterium avium through increased activity of mannose receptor. J Immunol. 2004;172:7592–7602. doi: 10.4049/jimmunol.172.12.7592. [DOI] [PubMed] [Google Scholar]
- Kyrklund-Blomberg NB, Granath F, Cnattingius S. Maternal smoking and causes of very preterm birth. Acta Obstet Gynecol Scand. 2005;84:572–577. doi: 10.1111/j.0001-6349.2005.00848.x. [DOI] [PubMed] [Google Scholar]
- LeVine AM, Bruno MD, Huelsman KM, Ross GF, Whitsett JA, Korfhagen TR. Surfactant protein A-deficient mice are susceptible to group B streptococcal infection. J Immunol. 1997;158:4336–4340. [PubMed] [Google Scholar]
- LeVine AM, Gwozdz J, Stark J, Bruno M, Whitsett J, Korfhagen T. Surfactant protein-A enhances respiratory syncytial virus clearance in vivo. J Clin Invest. 1999;103:1015–1021. doi: 10.1172/JCI5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine AM, Kurak KE, Bruno MD, Stark JM, Whitsett JA, Korfhagen TR. Surfactant protein-A-deficient mice are susceptible to Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol. 1998;19:700–708. doi: 10.1165/ajrcmb.19.4.3254. [DOI] [PubMed] [Google Scholar]
- LeVine AM, Whitsett JA. Pulmonary collectins and innate host defense of the lung. Microbes Infect. 2001;3:161–166. doi: 10.1016/s1286-4579(00)01363-0. [DOI] [PubMed] [Google Scholar]
- Lieber CS, Gentry RT, Baraona E. First pass metabolism of ethanol. Alcohol Alcohol Suppl. 1994;2:163–169. [PubMed] [Google Scholar]
- Lois M, Brown LA, Moss IM, Roman J, Guidot DM. Ethanol ingestion increases activation of matrix metalloproteinases in rat lungs during acute endotoxemia. Am J Respir Crit Care Med. 1999;160:1354–1360. doi: 10.1164/ajrccm.160.4.9811060. [DOI] [PubMed] [Google Scholar]
- Martinez-Frias ML, Bermejo E, Rodriguez-Pinilla E, Frias JL. Risk for congenital anomalies associated with different sporadic and daily doses of alcohol consumption during pregnancy: a case-control study. Birth Defects Res A Clin Mol Teratol. 2004;70:194–200. doi: 10.1002/bdra.20017. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Res Health. 2001;25:185–191. [PMC free article] [PubMed] [Google Scholar]
- Mescher EJ, Platzker AC, Ballard PL, Kitterman JA, Clements JA, Tooley WH. Ontogeny of tracheal fluid, pulmonary surfactant, and plasma corticoids in the fetal lamb. J Appl Physiol. 1975;39:1017–1021. doi: 10.1152/jappl.1975.39.6.1017. [DOI] [PubMed] [Google Scholar]
- Meyerholz DK, Grubor B, Fach SJ, Sacco RE, Lehmkuhl HD, Gallup JM, Ackermann MR. Reduced clearance of respiratory syncytial virus infection in a preterm lamb model. Microbes Infect. 2004;6:1312–1319. doi: 10.1016/j.micinf.2004.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerholz DK, Kawashima K, Gallup JM, Grubor B, Ackermann MR. Expression of select immune genes (surfactant proteins A and D, sheep beta defensin 1, and toll-like receptor 4) by respiratory epithelia is developmentally regulated in the preterm neonatal lamb. Dev Comp Immunol. 2006;30:1060–1069. doi: 10.1016/j.dci.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs M. A brief update on lung stereology. J Microsc. 2006;222:188–200. doi: 10.1111/j.1365-2818.2006.01587.x. [DOI] [PubMed] [Google Scholar]
- Parazzini F, Chatenoud L, Surace M, Tozzi L, Salerio B, Bettoni G, Benzi G. Moderate alcohol drinking and risk of preterm birth. Eur J Clin Nutr. 2003;57:1345–1349. doi: 10.1038/sj.ejcn.1601690. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Rossi GA, Medici MC, Arcangeletti MC, Lanari M, Merolla R, Paparatti UD, Silvestri M, Pistorio A, Chezzi C. Risk factors for severe RSV-induced lower respiratory tract infection over four consecutive epidemics. Eur J Pediatr. 2007 doi: 10.1007/s00431-007-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Kuroki Y. The lung collectins, SP-A and SP-D, modulate pulmonary innate immunity. Mol Immunol. 2005;42:279–287. doi: 10.1016/j.molimm.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Schleicher E, Wieland OH. Evaluation of the Bradford method for protein determination in body fluids. J Clin Chem Clin Biochem. 1978;16:533–534. [PubMed] [Google Scholar]
- Sisson JH, Stoner JA, Ammons BA, Wyatt TA. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc. 2003;211:103–111. doi: 10.1046/j.1365-2818.2003.01209.x. [DOI] [PubMed] [Google Scholar]
- Sood B, Delaney-Black V, Covington C, Nordstrom-Klee B, Ager J, Templin T, Janisse J, Martier S, Sokol RJ. Prenatal alcohol exposure and childhood behavior at age 6 to 7 years: I. dose-response effect. Pediatrics. 2001;108:E34. doi: 10.1542/peds.108.2.e34. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- Vinson RB, Carroll JL, Pruett SB. Mechanism of suppressed neutrophil mobilization in a mouse model for binge drinking: role of glucocorticoids. Am J Physiol. 1998;275:R1049–1057. doi: 10.1152/ajpregu.1998.275.4.R1049. [DOI] [PubMed] [Google Scholar]
- von Dossow V, Schilling C, Beller S, Hein OV, von Heymann C, Kox WJ, Spies CD. Altered immune parameters in chronic alcoholic patients at the onset of infection and of septic shock. Crit Care. 2004;8:R312–321. doi: 10.1186/cc2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JR, Blake CA. Fetal alcohol syndrome: an assessment of the field. Exp Biol Med (Maywood) 2005;230:354–356. doi: 10.1177/15353702-0323006-02. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Forget MA, Sisson JH. Ethanol stimulates ciliary beating by dual cyclic nucleotide kinase activation in bovine bronchial epithelial cells. Am J Pathol. 2003;163:1157–1166. doi: 10.1016/S0002-9440(10)63475-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt TA, Gentry-Nielsen MJ, Pavlik JA, Sisson JH. Desensitization of PKA-stimulated ciliary beat frequency in an ethanol-fed rat model of cigarette smoke exposure. Alcohol Clin Exp Res. 2004;28:998–1004. doi: 10.1097/01.ALC.0000130805.75641.F4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt TA, Sisson JH. Chronic ethanol downregulates PKA activation and ciliary beating in bovine bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L575–581. doi: 10.1152/ajplung.2001.281.3.L575. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Spurzem JR, May K, Sisson JH. Regulation of ciliary beat frequency by both PKA and PKG in bovine airway epithelial cells. Am J Physiol. 1998;275:L827–835. doi: 10.1152/ajplung.1998.275.4.L827. [DOI] [PubMed] [Google Scholar]
- Zhang X, Sliwowska JH, Weinberg J. Prenatal alcohol exposure and fetal programming: effects on neuroendocrine and immune function. Exp Biol Med (Maywood) 2005;230:376–388. doi: 10.1177/15353702-0323006-05. [DOI] [PubMed] [Google Scholar]