Abstract
Lysine epsilon-aminotransferase (LAT) in the beta-lactam-producing actinomycetes is considered to be the first step in the antibiotic biosynthetic pathway. Cloning of restriction fragments from Streptomyces clavuligerus, a beta-lactam producer, into Streptomyces lividans, a nonproducer that lacks LAT activity, led to the production of LAT in the host. DNA sequencing of restriction fragments containing the putative lat gene revealed a single open reading frame encoding a polypeptide with an approximately Mr 49,000. Expression of this coding sequence in Escherichia coli led to the production of LAT activity. Hence, LAT activity in S. clavuligerus is derived from a single polypeptide. A second open reading frame began immediately downstream from lat. Comparison of this partial sequence with the sequences of delta-(L-alpha-aminoadipyl)-L-cysteinyl-D valine (ACV) synthetases from Penicillium chrysogenum and Cephalosporium acremonium and with nonribosomal peptide synthetases (gramicidin S and tyrocidine synthetases) found similarities among the open reading frames. Since mapping of the putative N and C termini of S. clavuligerus pcbAB suggests that the coding region occupies approximately 12 kbp and codes for a polypeptide related in size to the fungal ACV synthetases, the molecular characterization of the beta-lactam biosynthetic cluster between pcbC and cefE (approximately 25 kbp) is nearly complete.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. E., Bird J. W., Field R. A., O'Callaghan N. M., Schofield C. J., Willis A. C. Isolation and partial characterisation of ACV synthetase from Cephalosporium acremonium and Streptomyces clavuligerus. Evidence for the presence of phosphopantothenate in ACV synthetase. J Antibiot (Tokyo) 1991 Feb;44(2):241–248. doi: 10.7164/antibiotics.44.241. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Findlay P. R., Johnson M. W. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene. 1984 Oct;30(1-3):157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran J. L., Leskiw B. K., Petrich A. K., Westlake D. W., Jensen S. E. Production of Streptomyces clavuligerus isopenicillin N synthase in Escherichia coli using two-cistron expression systems. J Ind Microbiol. 1990 Jun;5(4):197–206. doi: 10.1007/BF01569677. [DOI] [PubMed] [Google Scholar]
- Díez B., Gutiérrez S., Barredo J. L., van Solingen P., van der Voort L. H., Martín J. F. The cluster of penicillin biosynthetic genes. Identification and characterization of the pcbAB gene encoding the alpha-aminoadipyl-cysteinyl-valine synthetase and linkage to the pcbC and penDE genes. J Biol Chem. 1990 Sep 25;265(27):16358–16365. [PubMed] [Google Scholar]
- Fothergill J. C., Guest J. R. Catabolism of L-lysine by Pseudomonas aeruginosa. J Gen Microbiol. 1977 Mar;99(1):139–155. doi: 10.1099/00221287-99-1-139. [DOI] [PubMed] [Google Scholar]
- García-Domínguez M., Liras P., Martín J. F. Cloning and characterization of the isopenicillin N synthase gene of Streptomyces griseus NRRL 3851 and studies of expression and complementation of the cephamycin pathway in Streptomyces clavuligerus. Antimicrob Agents Chemother. 1991 Jan;35(1):44–52. doi: 10.1128/aac.35.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez S., Díez B., Montenegro E., Martín J. F. Characterization of the Cephalosporium acremonium pcbAB gene encoding alpha-aminoadipyl-cysteinyl-valine synthetase, a large multidomain peptide synthetase: linkage to the pcbC gene as a cluster of early cephalosporin biosynthetic genes and evidence of multiple functional domains. J Bacteriol. 1991 Apr;173(7):2354–2365. doi: 10.1128/jb.173.7.2354-2365.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins J. A., O'Callaghan N., Queener S. W., Cantwell C. A., Wood J. S., Chen V. J., Skatrud P. L. Gene disruption of the pcbAB gene encoding ACV synthetase in Cephalosporium acremonium. Curr Genet. 1990 Dec;18(6):523–530. doi: 10.1007/BF00327023. [DOI] [PubMed] [Google Scholar]
- Ingolia T. D., Queener S. W. Beta-lactam biosynthetic genes. Med Res Rev. 1989 Apr-Jun;9(2):245–264. doi: 10.1002/med.2610090206. [DOI] [PubMed] [Google Scholar]
- Jensen S. E., Wong A., Rollins M. J., Westlake D. W. Purification and partial characterization of delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine synthetase from Streptomyces clavuligerus. J Bacteriol. 1990 Dec;172(12):7269–7271. doi: 10.1128/jb.172.12.7269-7271.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern B. A., Hendlin D., Inamine E. L-lysine epsilon-aminotransferase involved in cephamycin C synthesis in Streptomyces lactamdurans. Antimicrob Agents Chemother. 1980 Apr;17(4):679–685. doi: 10.1128/aac.17.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic S., Miller J. R. Cloning and sequencing of the beta-lactam hydroxylase gene (cefF) from Streptomyces clavuligerus: gene duplication may have led to separate hydroxylase and expandase activities in the actinomycetes. J Bacteriol. 1991 Jan;173(1):398–400. doi: 10.1128/jb.173.1.398-400.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic S., Tobin M. B., Miller J. R. The beta-lactam biosynthesis genes for isopenicillin N epimerase and deacetoxycephalosporin C synthetase are expressed from a single transcript in Streptomyces clavuligerus. J Bacteriol. 1990 Jul;172(7):3952–3958. doi: 10.1128/jb.172.7.3952-3958.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic S., Weigel B. J., Tobin M. B., Ingolia T. D., Miller J. R. Cloning, characterization, and expression in Escherichia coli of the Streptomyces clavuligerus gene encoding deacetoxycephalosporin C synthetase. J Bacteriol. 1989 Feb;171(2):754–760. doi: 10.1128/jb.171.2.754-760.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
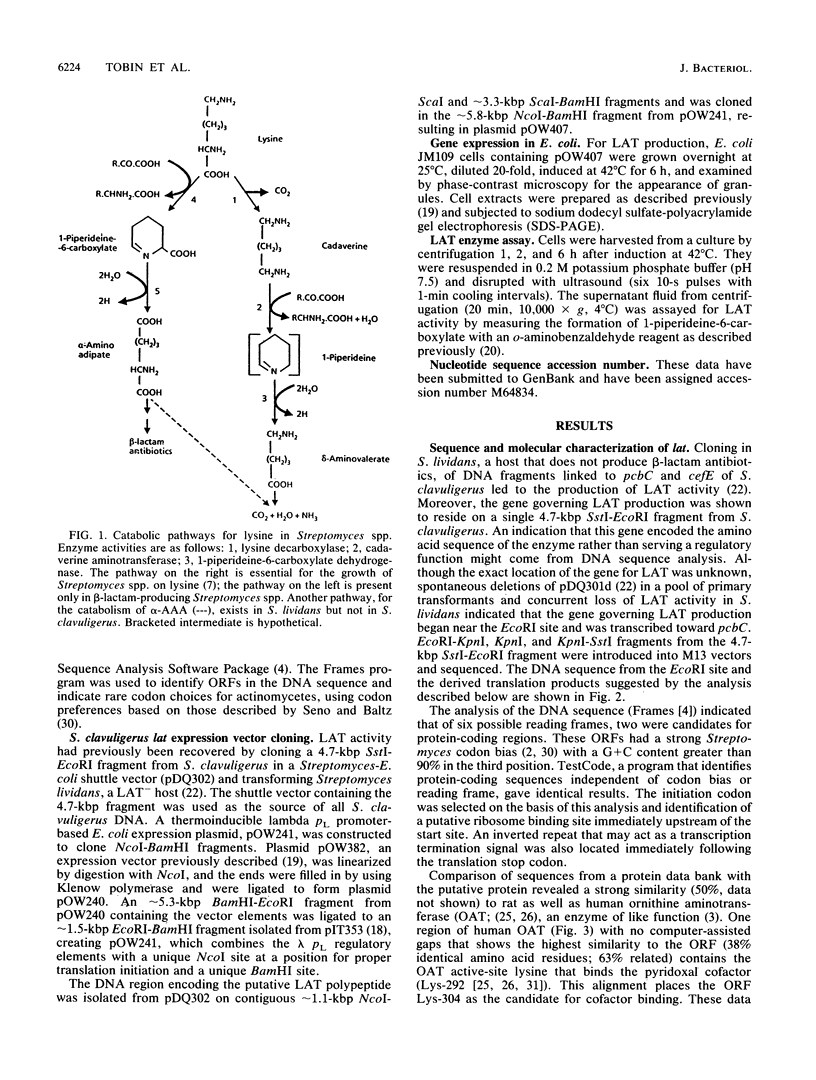
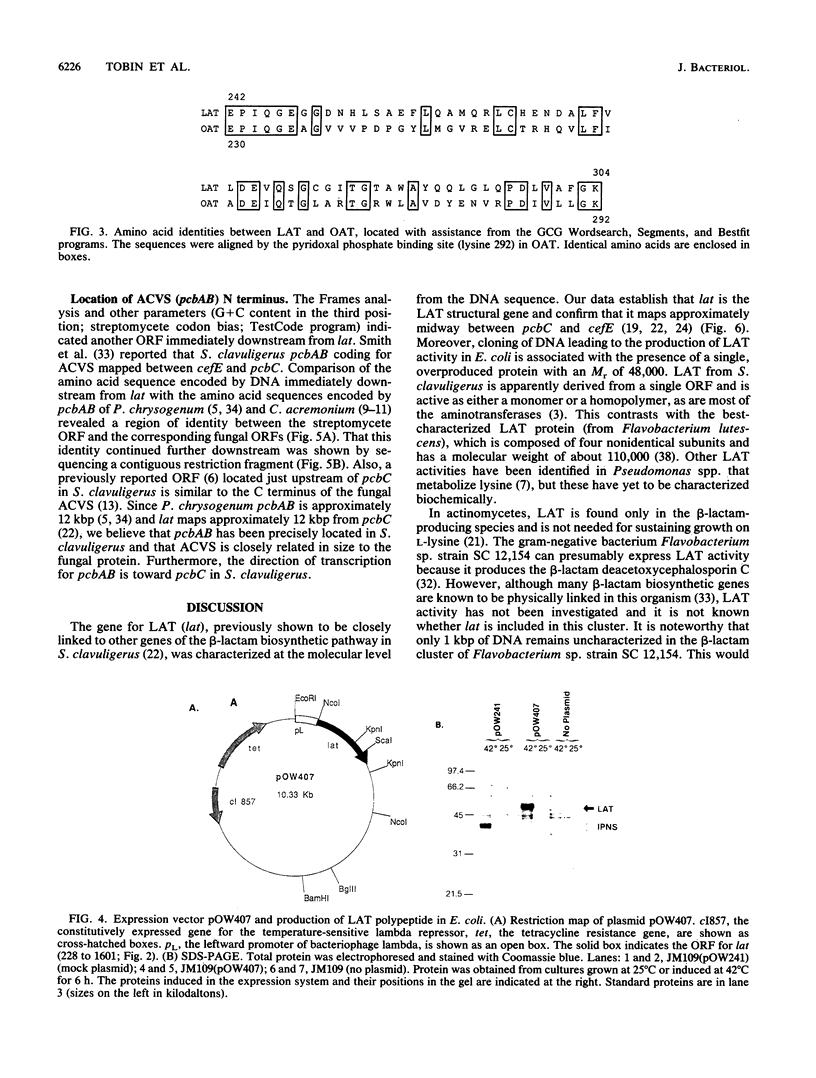
- Madduri K., Stuttard C., Vining L. C. Cloning and location of a gene governing lysine epsilon-aminotransferase, an enzyme initiating beta-lactam biosynthesis in Streptomyces spp. J Bacteriol. 1991 Feb;173(3):985–988. doi: 10.1128/jb.173.3.985-988.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madduri K., Stuttard C., Vining L. C. Lysine catabolism in Streptomyces spp. is primarily through cadaverine: beta-lactam producers also make alpha-aminoadipate. J Bacteriol. 1989 Jan;171(1):299–302. doi: 10.1128/jb.171.1.299-302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. A., Looney J. E., Brody L. C., Steel G., Suchanek M., Engelhardt J. F., Willard H. F., Valle D. Human ornithine-delta-aminotransferase. cDNA cloning and analysis of the structural gene. J Biol Chem. 1988 Oct 5;263(28):14288–14295. [PubMed] [Google Scholar]
- Mueckler M. M., Pitot H. C. Sequence of the precursor to rat ornithine aminotransferase deduced from a cDNA clone. J Biol Chem. 1985 Oct 25;260(24):12993–12997. [PubMed] [Google Scholar]
- Piret J., Resendiz B., Mahro B., Zhang J. Y., Serpe E., Romero J., Connors N., Demain A. L. Characterization and complementation of a cephalosporin-deficient mutant of Streptomyces clavuligerus NRRL 3585. Appl Microbiol Biotechnol. 1990 Feb;32(5):560–567. doi: 10.1007/BF00173728. [DOI] [PubMed] [Google Scholar]
- Simmaco M., John R. A., Barra D., Bossa F. The primary structure of ornithine aminotransferase. Identification of active-site sequence and site of post-translational proteolysis. FEBS Lett. 1986 Apr 7;199(1):39–42. doi: 10.1016/0014-5793(86)81219-4. [DOI] [PubMed] [Google Scholar]
- Singh P. D., Ward P. C., Wells J. S., Ricca C. M., Trejo W. H., Principe P. A., Sykes R. B. Bacterial production of deacetoxycephalosporin C. J Antibiot (Tokyo) 1982 Oct;35(10):1397–1399. doi: 10.7164/antibiotics.35.1397. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Burnham M. K., Bull J. H., Hodgson J. E., Ward J. M., Browne P., Brown J., Barton B., Earl A. J., Turner G. Beta-lactam antibiotic biosynthetic genes have been conserved in clusters in prokaryotes and eukaryotes. EMBO J. 1990 Mar;9(3):741–747. doi: 10.1002/j.1460-2075.1990.tb08168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Earl A. J., Turner G. The multifunctional peptide synthetase performing the first step of penicillin biosynthesis in Penicillium chrysogenum is a 421,073 dalton protein similar to Bacillus brevis peptide antibiotic synthetases. EMBO J. 1990 Sep;9(9):2743–2750. doi: 10.1002/j.1460-2075.1990.tb07461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vining L. C., Shapiro S., Madduri K., Stuttard C. Biosynthesis and control of beta-lactam antibiotics: the early steps in the "classical" tripeptide pathway. Biotechnol Adv. 1990;8(1):159–183. doi: 10.1016/0734-9750(90)90011-y. [DOI] [PubMed] [Google Scholar]
- Whitney J. G., Brannon D. R., Mabe J. A., Wicker K. J. Incorporation of labeled precursors into A16886B, a novel -lactam antibiotic produced by Streptomyces clavuligerus. Antimicrob Agents Chemother. 1972 Mar;1(3):247–251. doi: 10.1128/aac.1.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T., Misono H., Kurihara N., Yamamoto T., Soda K. L-Lysine: 2-oxoglutarate 6-aminotransferase. Subunit structure composed of non-identical polypeptides and pyridoxal 5'-phosphate-binding subunit. J Biochem. 1980 May;87(5):1395–1402. doi: 10.1093/oxfordjournals.jbchem.a132880. [DOI] [PubMed] [Google Scholar]
- Zhang J. Y., Demain A. L. Purification from Cephalosporium acremonium of the initial enzyme unique to the biosynthesis of penicillins and cephalosporins. Biochem Biophys Res Commun. 1990 Jun 29;169(3):1145–1152. doi: 10.1016/0006-291x(90)92015-r. [DOI] [PubMed] [Google Scholar]
- van Liempt H., von Döhren H., Kleinkauf H. delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine synthetase from Aspergillus nidulans. The first enzyme in penicillin biosynthesis is a multifunctional peptide synthetase. J Biol Chem. 1989 Mar 5;264(7):3680–3684. [PubMed] [Google Scholar]